Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jakub Kiepś | -- | 4139 | 2022-08-16 13:28:00 | | | |
| 2 | Jakub Kiepś | -143 word(s) | 3996 | 2022-08-16 13:30:52 | | | | |
| 3 | Conner Chen | + 20 word(s) | 4016 | 2022-08-18 02:25:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kiepś, J.; Dembczyński, R. Drying Methods of Probiotic Formulations. Encyclopedia. Available online: https://encyclopedia.pub/entry/26192 (accessed on 03 March 2026).
Kiepś J, Dembczyński R. Drying Methods of Probiotic Formulations. Encyclopedia. Available at: https://encyclopedia.pub/entry/26192. Accessed March 03, 2026.
Kiepś, Jakub, Radosław Dembczyński. "Drying Methods of Probiotic Formulations" Encyclopedia, https://encyclopedia.pub/entry/26192 (accessed March 03, 2026).
Kiepś, J., & Dembczyński, R. (2022, August 16). Drying Methods of Probiotic Formulations. In Encyclopedia. https://encyclopedia.pub/entry/26192
Kiepś, Jakub and Radosław Dembczyński. "Drying Methods of Probiotic Formulations." Encyclopedia. Web. 16 August, 2022.
Copy Citation
Preparations containing probiotic strains of bacteria have a beneficial effect on human and animal health. Concentrated probiotic bacteria used in animal nutrition and consumed by humans most commonly occur in the form of dried biomass. The benefits of probiotics translate into an increased interest in techniques for the preservation of microorganisms.
lactic acid bacteria
spray drying
freeze drying
vacuum drying
1. Drying Methods
Cryopreservation is one of the most commonly used methods of preserving and storing live cultures of microorganisms for a long time; this approach is used in microbiological laboratories. From a commercial point of view, this method has disadvantages, which include high levels of energy consumption and the need to maintain and transport the samples at temperatures below zero. In addition, freezing and thawing the cells of microorganisms can damage them. When large amounts of probiotic cultures are produced, it is preferred to use other methods of preservation, such as different drying techniques [1].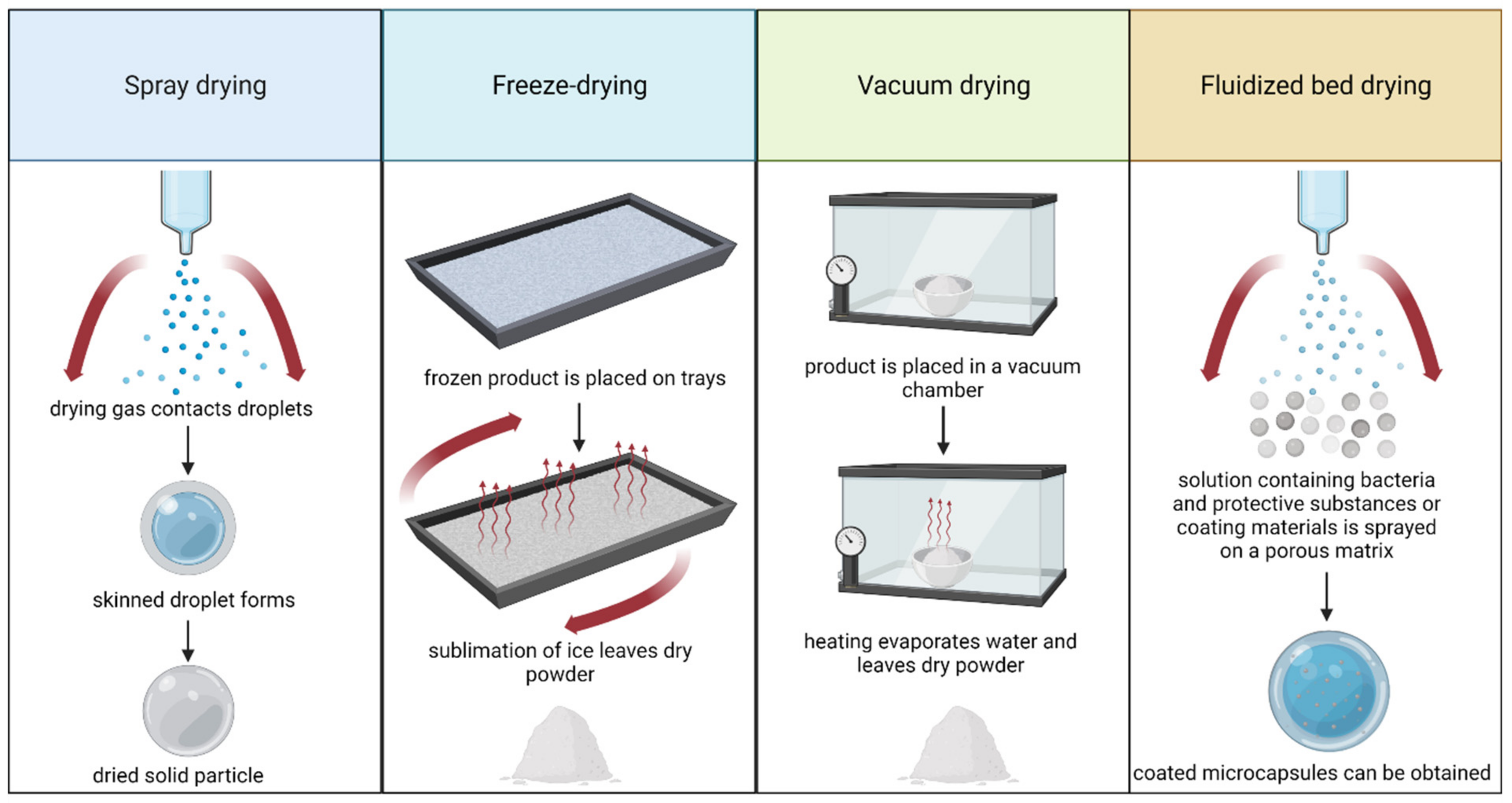

Figure 1. Summary of the basic principles for the most commonly applied drying techniques. Created with Biorender.com (accessed on 27 July 2022).
2. Traditional Drying Methods
2.1. Spray Drying
Spray drying is a fast and relatively inexpensive technique that makes it possible to obtain dry, mostly spherical powder particles with good flow properties, uniform shape, and particle size distribution [2]. The drying process occurs in four stages. In the first stage, the microorganism suspension is sprayed into small droplets. Then, the droplets are carried by hot air, with three different methods for the air to contact the droplets: co-current, counter-current, and mixed [3]. Because probiotic bacteria are sensitive to high temperatures, the co-current flow is usually applied. In this way, drops with a high water content contact the high-temperature inlet air, and the dry particles contact the lowest-temperature exhaust air, which reduces the risk of damage to microorganisms. For example, in previous works, inlet air temperatures for the spray drying of L. rhamnosus were 130–150 °C [4]; for probiotic almond milk powder containing L. plantarum, temperatures of 170–190 °C were used [5]. The third stage of the spray-drying process is the drying of the droplets and the formation of dry particles. It is at this stage that the microorganisms are most susceptible to thermal inactivation [6].
In comparison with other methods, spray drying has several advantages. These include a short drying time, the capacity for continuous operation, and low cost; these factors translate into the possibility of drying large volumes of suspension in a relatively short time. Additionally, it is possible to influence the characteristics of the powder, and the process is relatively easy to scale up [7].
Spray freeze drying is a method that combines the advantages of spray drying and freeze drying. It is conducted in three stages: the atomization of a cell suspension in a spray dryer, freezing (over liquid nitrogen), and freeze drying. In this process, the cells are first atomized with the addition of a protective material (such as WPI) over liquid nitrogen, which allows the droplets to quickly freeze. They are then additionally freeze dried. Studies conducted using spray freeze drying showed an encapsulation efficiency of 88–95% for L. plantarum MTCC 5422 with various wall materials. The overall encapsulation efficiency was, however, lower than in regular freeze drying due to the additional stress factors that occurred during atomization and freezing [8].
2.2. Freeze Drying
Freeze drying, or lyophilization, is a common method of removing water from probiotic bacterial cells to ensure their storage stability. The dryer consists of a vacuum chamber with a freezing system, a system for removing water vapor, and heating elements that are necessary to supply heat for sublimation. The freeze-drying process occurs in three stages: the freezing of the cell culture, sublimation, and final drying, the first of which is often carried out outside the dryer. In the next stage of the process, the frozen water is removed in the sublimation process under reduced pressure, and in the last stage, non-frozen water is removed in the process of desorption in order to attain the final water content [9]). Because the conditions of the freeze-drying process are milder than those of the spray-drying process, probiotic cultures dried by this method usually show higher rates of survival [10].
Despite the frequent use of this method of drying microorganisms, including probiotics, freeze drying has several disadvantages. It is an expensive and lengthy batch process, and the final product is often compact and hard. Regardless of this, freeze drying is a useful and widely used drying technique with several strategies already developed to maximize the viability of probiotic cultures. Among such modifications is pulse-spouted microwave freeze drying [11][12]. It aims to shorten the drying time in comparison with the traditional variant of freeze drying.
2.3. Vacuum Drying
A vacuum dryer consists of a chamber in which heated shelves are located. Trays containing wet biomass are placed on the shelves, and the water vapor is removed using a vacuum pump and condensed in a condenser. During freeze drying, the cells are frozen before the water is removed, while in vacuum drying, they remain in liquid form. Moreover, vacuum dryers operate at a higher temperature and pressure, and the energy consumption is 40% lower compared to freeze drying [13]. Typical pressures for vacuum drying are above 30–60 mbar, which corresponds to a boiling point of water of 25–30 °C; for freeze drying, the pressure is lower than 6 mbar [1][14]. The main disadvantage of vacuum drying, compared to spray or fluid bed drying, is the long processing time, ranging from 20 to 100 h [15].
New developments in the field of vacuum drying include the use of pulse-spouted microwave vacuum drying (PSMVD) [11]. Banana cubes dried by PSMVD showed an expansion trend, resulting in a better structure and rehydration ratio. PSMVD-dried cubes also provided better nutritional value as measured by the content of ascorbic acid, which reached 7.96 mg 100 g−1 (compared to 4.23 mg 100 g−1 for the traditional variant of vacuum drying).
2.4. Fluidized Bed Drying
Fluidized bed drying is a process in which heated gas, usually air, flows at a certain speed through a layer of solid particles, causing them to reach a fluidized flow state. Because the fluidization process has very good heat and mass exchange conditions, water is quickly evaporated from the dried material. The time required for fluidized bed drying (1 min to 2 h) is shorter than that of freeze drying and comparable to that of spray drying. The relatively low drying temperature does not cause thermal stress [15]. The cell biomass is not dried on its own but mixed with additional material that acts as a carrier or matrix to which the cells adhere. In practice, many loose and powdered materials have been used for this purpose, such as wheat flour, skimmed milk powder, casein, maltodextrin, starch, microcrystalline cellulose, inulin, and NaCl [15][16][17][18]. Usually, the matrix material is first placed in the dryer chamber and fluidized; then, the bacterial suspension is sprayed onto the fluidized matrix via a nozzle. Another method is to prepare the granulate first; after mixing the wet biomass with the matrix material and forming the granules using a sieve, pellet mill, or drum granulator, they are then dried in a fluidized bed [19][20]. It is important to consider the purpose of the dried bacterial product when selecting the matrix material as it can have variable technological characteristics or provide additional properties (e.g., prebiotic inulin) [16].
3. Novel Immobilization Methods
New methods of probiotic cell immobilization are also emerging as an alternative to drying processes and microencapsulation. Škrlec et al. [21]. have developed two types of electrospun nanofibers (Figure 2): monolithic poly(ethylene oxide) and composite poly(ethylene oxide)/lyoprotectant. L. plantarum cells were applied to these nanofibers and achieved high cell concentrations (up to 7.6 × 108 cfu/mg). Moreover, their survival during storage at 25 °C was promising, with a 1.83 log decrease in viability over 24 weeks (from 8.51 to 6.68 cfu/mg). For comparison, lyophilized samples showed a 1.70 log decrease in viability over the same period (from 8.97 to 7.27 cfu/mg). The differences between nanofibers and lyophilized samples stored at 4 °C were also minimal. The release time was also measured. Nearly all (>90%) L. plantarum cells were released from the nanofibers within the first 30 min of the experiment. This was confirmed by both plate cell counts and fluorescence measurements of the mCherry protein and provided insight into possible applications of probiotic-loaded nanofibers. The 30 min release period allowed for the controlled delivery of probiotic bacteria and was sufficient for their adhesion to the intestinal epithelium and mucosa.
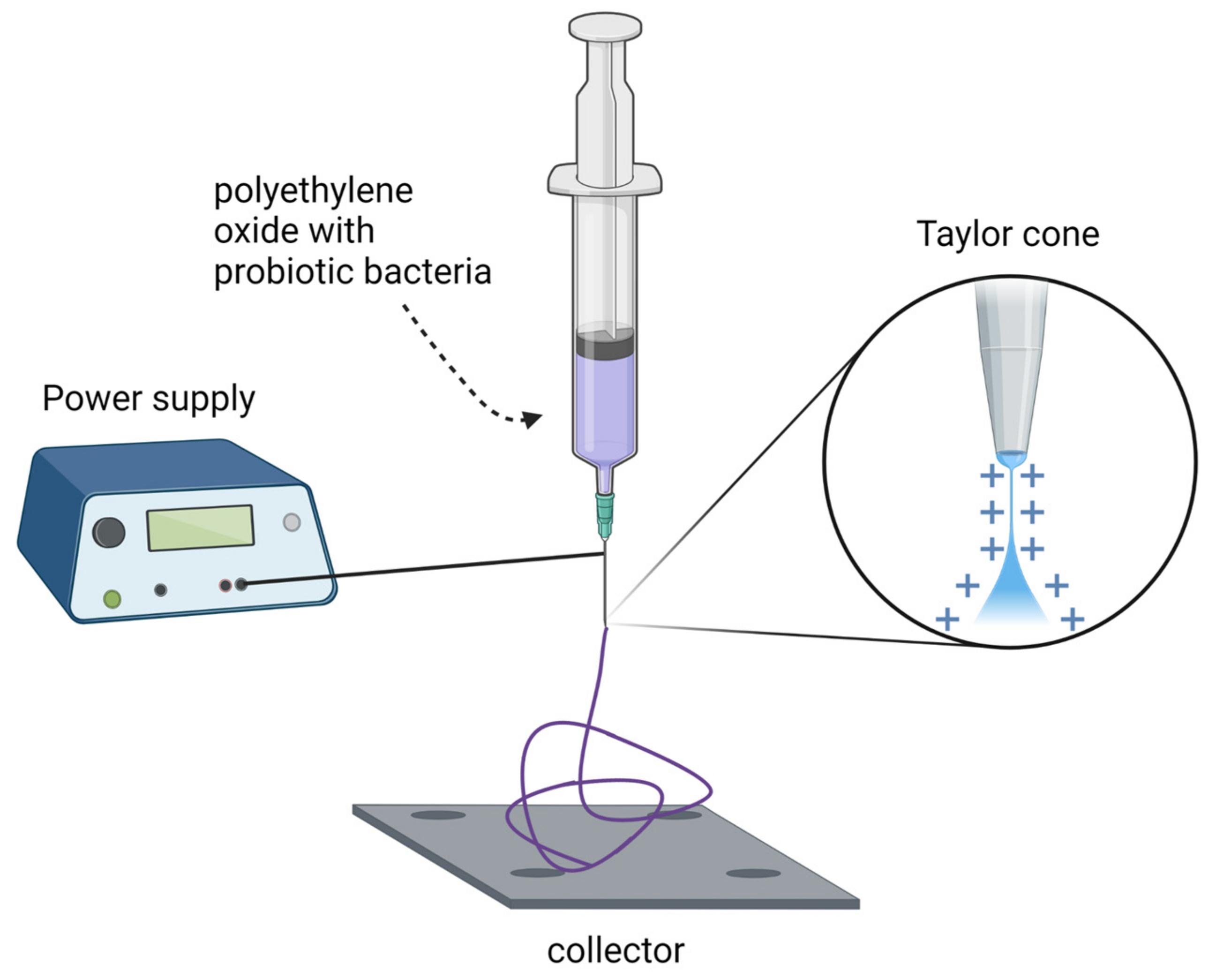
Figure 2. Principal elements of nanofiber fabrication by electrospinning. Created with Biorender.com (accessed on 1 June 2022).
3D printing is a novel method used in food industry applications that allows for the design of customized products. Yoha et al. [22] studied the effects of the 3D printing of probiotic encapsulates on their viability. To prepare the base for the 3D printing of L. plantarum (NCIM 2083), cells were dried using four encapsulation techniques (spray drying, freeze drying, spray freeze drying, and refractance window drying) with the addition of a prebiotic matrix (fructooligosaccharide, whey protein, and maltodextrin in a ratio 4:1:1, respectively). Dried microcapsules were then used for 3D printing with a composite flour formulation using the food 3D printer CARK [23]. Yoha et al. (2021) reported that the 3D printing process did not lower the viability of probiotic bacteria. They also determined that freeze drying yielded the highest level of cell viability, i.e., 8.23 ± 0.21 log10 CFU/g, followed by spray freeze drying (8.18 ± 0.16 log10 CFU/g). Under in vitro digestion, freeze-dried probiotics showed a lower level of viability (6.12 ± 0.29 log10 CFU/mL) than spray-freeze-dried samples (6.43 ± 0.29 log10 CFU/mL).
4. Auxiliary Methods
The standard drying methods, by themselves, are well-established and optimized. They can be, however, improved upon by the introduction of auxiliary methods, such as fluid bed coating. The combination of two different techniques allows the use of the most popular methods, such as spray drying or freeze drying, in the first step; then, the stability of the preparation is improved upon by fluid bed coating with different protective substances.
In their research, Jacobsen et al. [19] showed that the viability of freeze-dried probiotics after granulation and fluid bed coating was only slightly reduced. Freeze-dried L. reuteri LR92 was used to produce probiotic pellets by granulation, extrusion, and spheronization. The obtained probiotic pellets were then subjected to fluid coating with the Eudragit S100 and Eudragit FS30D coating suspensions to achieve delayed release. To evaluate the targeted delivery of coated probiotic pellets, an in vitro model simulating the conditions of the human gastric system, duodenum/jejunum, and ileum was developed. The release of active substances from the coated pellets was determined by the quantification of the released marker riboflavin with HPLC. The results show that the coated probiotic pellets achieved the desired release profile (release in the ileum) based on the release of riboflavin.
Fluidized bed drying was also used to prepare dried probiotic apple snacks. The apple cubes were first dried in a fluidized bed at 50 °C until they reached a level of water activity lower than 0.5 and a moisture content below 15%. Then, the dried apple cubes were coated with a solution of hydroxyethyl cellulose and polyethylene glycol mixed with washed Bacillus coagulans spores. The optimal ratio of coating substances was established at 0.125 g of HEC to 11.7 uL of PEG. Coating with the optimal coating mixture resulted in achieving a 77.7% coverage of the sample area. The achieved product was microbiologically stable during storage at room temperature for 90 days and was able to maintain at least an 8 log CFU/30 g portion. Reductions in enzymatic activity, specifically the activity of polyphenol oxidase (by 86%) and of peroxidase (by 92%), represented an additional improvement [24].
5. Factors Affecting the Viability of Probiotics during Drying
During drying, probiotic microorganisms are exposed to various stress factors, such as excessive dehydration and thermal, mechanical, osmotic, and oxidative stresses [25]. Probiotic microorganisms belong to the group of products with low thermal stability; at the same time, there is a certain critical water content that must be maintained. A reduction in water content below this critical value may cause the dehydration of the cells and, therefore, their inactivation. Thermal stress and dehydration are considered the main causes of losses of the viability of probiotic bacteria during spray drying. Stress factors that affect probiotic bacteria during different processing stages are presented in Figure 3.
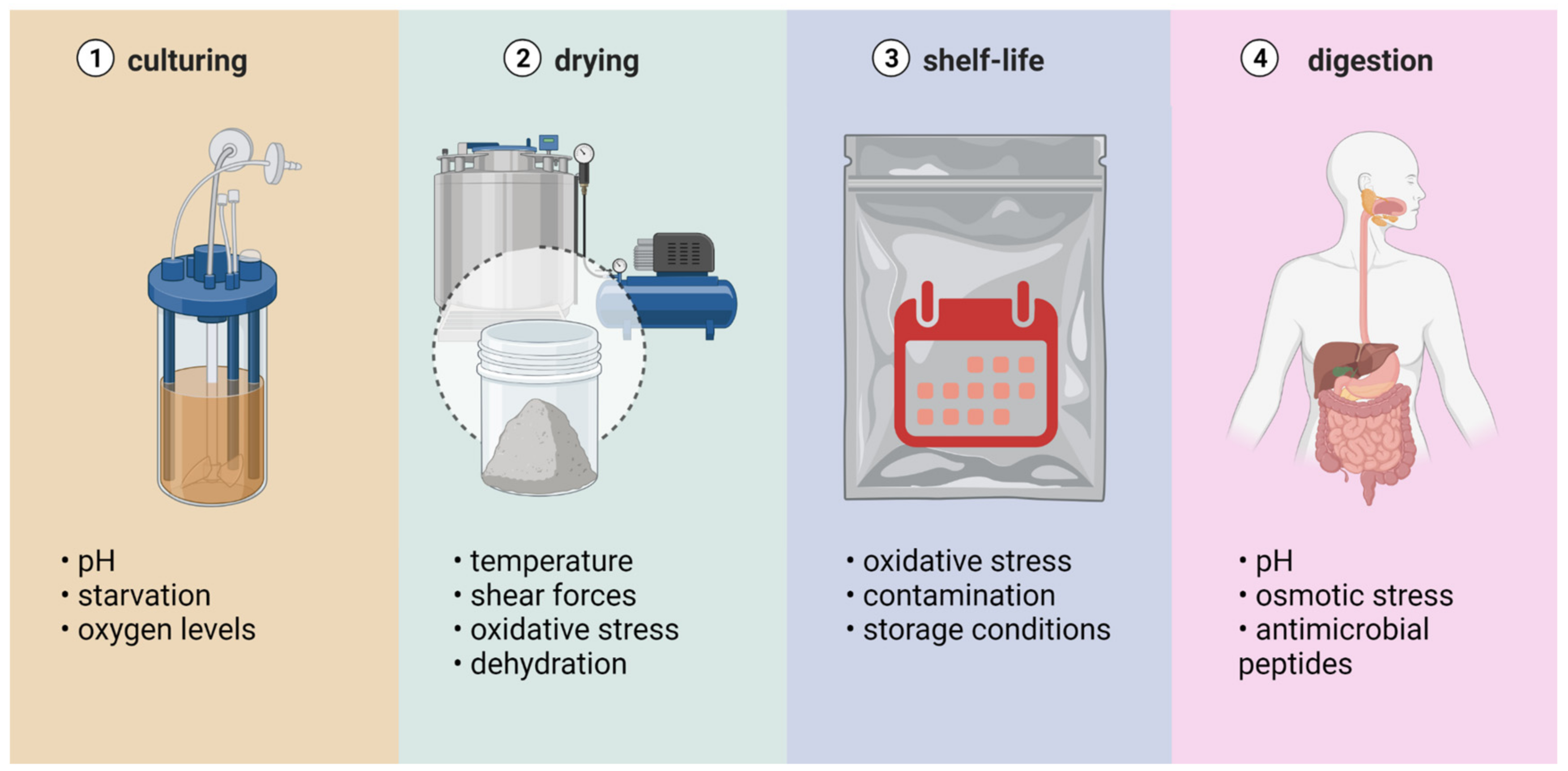
Figure 3. Stress factors affecting probiotics during different stages of their preparation and administration. Created with Biorender.com (accessed on 27 July 2022).
Thermal stress, i.e., the heat inactivation of microorganisms, is a significant risk in the second stage of drying, according to various authors. In this phase of the process, microorganisms can reach the temperature of the drying air, especially since the dried particles often remain in the dryer until the entire process is completed. Not all bacteria are equally prone to thermal inactivation. For example, L. acidophilus has shown better survival rates under various drying temperatures than E. coli K12. This can be explained by differences in the thickness of the cell wall, which is thicker in the case of Gram-positive bacteria (e.g., L. acidophilus). Moreover, drying in a medium containing nutrient broth yielded better survival rates than drying in a medium without broth components [26].
High temperatures can denature intracellular proteins and destabilize cell membranes, which in turn leads to cell death. At the same time, higher temperatures cause a decrease in the water activity of the dried samples, which translates into increased storage stability. Therefore, when choosing spray drying parameters for probiotic microorganisms, it is important to determine an optimal outlet air temperature that is high enough for the dried samples to have low water activity and, on the other hand, low enough to prevent cell damage [14]. Air temperature also significantly affects the bulk density of dried probiotic powders. As the temperature increases, evaporation rates also increase. The powder dries to a more porous structure and is more prone to forming hollow particles [27].
The inactivation of microorganisms caused by dehydration often occurs simultaneously with heat damage. During drying, water molecules are removed from the cells, which limits chemical reactions and metabolic activity. Because water is essential for the stabilization of various components of the cell, its removal may result in a loss of cell integrity, changes in cell structure, and damage to the enzyme system [1]. This applies to, among other things, changes in the lipid bilayer of the cell membrane that can cause the leakage of intracellular fluid and, consequently, cell death [28]. In experiments on drying single droplets of an L. plantarum suspension, it was shown that at an outlet air temperature below 45 °C, inactivation due to dehydration was dominant, while above this temperature, dehydration and temperature stress occurred simultaneously [29]. The authors of their study also believed that the longer the drying time, the more cells would undergo dehydrative inactivation.
Osmotic stress during drying occurs as a result of cells losing water to the environment, which increases the molarity of the intracellular solution and reduces the volume of the cytoplasm. A loss of cellular turgor occurs, and the cell undergoes plasmolysis, which, as a consequence, leads to a loss of viability [1].
Oxidative stress is caused by oxygen contained in the air and dissolved in an aqueous suspension of microorganisms [30]. Probiotic bacterial tolerance to oxygen is varied, with most Bifidobacterium species requiring strict anaerobic conditions and many strains of lactic acid bacteria tolerating oxygen. Oxidative stress is caused by reactive oxygen particles interacting with proteins, nucleic acids, and lipids. As a result, protein denaturation and lipid oxidation occur, leading to cell membrane damage and cell death [25].
The cells of probiotic microorganisms can also be inactivated by shear forces as their suspension is sprayed into the dryer head. Several studies have shown a relationship between the pressure of the suspension administered through atomizing nozzles and the survival of probiotic bacteria [30].
Similar to other methods of drying probiotic bacteria, in fluidized bed drying, certain factors cause losses of cell viability, mainly by osmotic stress, excessive dehydration, and oxidative stress [14]. It is believed that the threat of thermal shock at the temperatures used in the fluidized bed drying of microorganisms is insignificant up to a material moisture level of 15% and increases as the water activity of the dried material decreases [1]. Additionally, the pressure in the atomizing nozzle can affect the viability of the cells. An increase in nozzle pressure above 1.5 bar reduced the viability of Enterococcus faecium cells by 4 log cycles [18].
In the process of freeze drying, microorganisms are exposed to various stress factors caused by freezing and the sublimation of ice, leading to changes in the cell. These include deformation, mechanical damage to the cellular structure by ice crystals formed during the process, the loss of semipermeable properties of cell membranes, changes in the structure of membrane lipids, and the denaturation of protein components due to an increase in the concentration of intracellular compounds [1].
Ice crystals form at the biomass-freezing stage. The crystal growth depends on the freezing rate and temperature. Rapid freezing is recommended because the ice crystals reach small dimensions and do not damage the microbial cells. In addition, slowly lowering the temperature leads to ice forming mainly outside the cells, which leads to excessive dehydration. The formation of ice crystals is not the only threat to cell viability. During ice crystallization, the intracellular solution thickens, which can lead to osmotic stress. Moreover, the removal of water bound to the cells may damage surface proteins, the cell wall, and the cytoplasmic membrane. The lipid fraction of the bilayer cell membrane, where the structure of polar phospholipid parts may change, is particularly vulnerable to damage during dehydration [28].
Because vacuum drying occurs at a temperature higher than freeze drying but lower than spray drying, it is a milder process in terms of the effects of high or low temperature on the cells of microorganisms. Furthermore, the lack of oxygen in the drying environment can reduce oxidative stress, especially when drying oxygen-sensitive bacteria, e.g., Bifidobacteria [25]. Dehydration stress, however, is considered a major threat to cell viability during this process. For example, during vacuum drying, cell damage is observed mainly in the cell membrane [31].
The described stress factors, when introduced in a controlled manner in the culturing stage, can also be used to prevent the loss of viability during drying and storage. Research by Hernández et al. [32] confirms that fermentation parameters, such as pH and temperature, influence the stress resilience of L. reuteri DSM 17,938 during freeze drying. Simultaneous exposure to mild heat (50 °C) and osmotic stress (0.6 M of NaCl) also significantly improved the storage stability of L. casei CRL 431 when compared with bacteria exposed to just one of those stress factors [33].
6. Prevention of Stress Factors
The prevention of stress factors in the spray drying of probiotic microorganisms can be achieved by careful selection of the appropriate drying strategy [1][7]:
- The addition of protective substances;
- The proper selection of process parameters;
- The adaptation of cells to stress factors before drying.
These preventative measures affect the survival of probiotic bacteria directly during drying, as well as after drying during storage. The following substances are considered protectants and additives used to improve the survival rate of probiotics during spray drying: saccharides, skimmed milk, whey proteins, inulin, trehalose, and oligosaccharides, as well as polymers, such as gum arabic [13][27][28][34][35]. In the scientific literature, several hypotheses have been put forward explaining the protective effect of these substances on the cell membrane and intracellular proteins. These include the theory of vitrification, the hypothesis about the exchange of water molecules in the hydration layer of proteins and the cell membrane with a protective substance (the “water replacement hypothesis”), and the hypothesis of hydration force (the “hydration force hypothesis”) [1].
The activities aimed at optimizing the spray-drying process parameters for probiotic bacteria include, first and foremost, the correct selection of the chamber inlet and outlet air temperatures, the appropriate spray nozzle configuration, the atomizing pressure, and the flow volume of the suspension fed to the dryer [13][15]. A significant improvement in the viability of spray-dried L. lactis after replacing air with nitrogen has also been demonstrated [30].
To increase the survival of probiotic cells during spray drying, they can also be exposed to stress conditions during culturing. These stress conditions include exposure to low pH, thermal shock, culturing microorganisms without additional nutrition, exposure to sodium chloride and monosodium glutamate, and culturing with the addition of saccharides, such as mannose and sucrose. In general, it is also believed that cellular biomass derived from the stationary culture phase has better survival rates than that derived from the logarithmic growth phase [14].
There are numerous measures that were developed to improve the viability of freeze-dried bacteria. These include the addition of protective substances to bacterial suspensions, the appropriate control of process parameters during bacteria cultivation, and the adaptation of cells to stress factors before the drying process. The effectiveness of these operations may vary depending on the species of the microorganism [1]. These actions are, in many cases, similar to those used for spray drying.
The most common way to prevent stress factors in freeze-dried probiotic bacteria is to use cryo- and lyoprotectants. Cryoprotectants are water-soluble chemical compounds that lower the melting point of ice. When ice crystals form in the first stage of the process, bacterial cells cluster in the non-frozen fraction. The addition of cryoprotectants increases the volume of the non-frozen fraction of the solution, which increases the space occupied by cells, which in turn reduces cell damage as a result of mechanical and osmotic stresses. These cryoprotectants include polyols, polysaccharides, mono- and disaccharides, amino acids, proteins, minerals, organic acid salts, and complex vitamins [9][36]. In turn, lyoprotectants protect probiotic bacteria at the stage of water removal from the cell. The types and mechanisms of action of lyoprotectants are similar to those described for spray drying. Some sugars, such as sucrose and trehalose, act as both cryo- and lyoprotectants, which translates into their high effectiveness in ensuring the survival of probiotics after freeze drying [17].
The main strategies for protecting probiotic bacteria against stress factors in vacuum drying include the use of protective substances and the selection of process parameters [1]. Among the protective substances most commonly used in the vacuum drying of probiotics are sugars and polyalcohols, such as trehalose and sorbitol. The protective mechanism of these substances is the same as for spray and freeze drying [14].
The relatively low temperature of vacuum drying enables the dehydration of biomass prepared in a semisolid state, such as pellets mixed with a protective substance. The drying efficiency is increased compared to other methods because less water is removed; therefore, a smaller quantity of protective substances can be used [37]. This was proved by vacuum drying (100 mbar, 43 °C, 12 h) L. bulgaricus bacteria in the form of a pellet with the addition of powdered lactose, sorbitol, inulin, and xanthan gum [38]. An improvement in the rate of cell survival was found only for samples with 1% sorbitol as the protective substance. The protective effects of sorbitol are due to its ability to lower membrane phase transition temperatures via the interaction with phosphate groups in the membrane [39].
Drying time and temperature are the most important process parameters to be taken into account when optimizing the drying process. The shorter the drying time and the lower the temperature, the higher the survival rate of the dried cells [1]. For example, for L. delbrueckii subsp. bulgaricus dried at 30, 45, and 70 °C (13.3 mbar, 10 min), damage to the cell membrane was higher with decreases in water activity and increases in drying temperature [37].
The countermeasures used against threats to the viability of fluid-dried bacteria are essentially the same as those used in other drying techniques and include the addition of protective substances, control of process parameters, and the adaptation of cells to stress factors before drying.
The addition of various protective substances to dried probiotic bacteria is the most commonly used protective method. The viability of fluidized-bed-dried probiotic bacteria is also highly dependent on their final humidity. The authors of [15] believed that the critical level of humidity that threatens the viability of L. helveticus cells was 1–3%. It was indicated that this might depend on the bacterial species; for L. salivarius, for example, the critical humidity was in the range of 5–6%.
There are also certain factors that can influence the viability and survival of probiotic bacteria during storage. To increase the shelf-life of dried probiotics, various protective measures can be used. These include the addition of antioxidants, such as 0.5% (w/w) vitamin E; they protect the final formulation against oxidative stress [33]. Storage at a lower temperature, i.e., 4 °C, can also result in an improvement in the survival rate of dried probiotic powder compared to the results when stored at 22 °C or 35 °C [17].
References
- Broeckx, G.; Vandenheuvel, D.; Claes, I.J.J.; Lebeer, S.; Kiekens, F. Drying Techniques of Probiotic Bacteria as an Important Step towards the Development of Novel Pharmabiotics. Int. J. Pharm. 2016, 505, 303–318.
- dos Santos, R.C.S.; Finkler, L.; Finkler, C.L.L. Microencapsulation of Lactobacillus Casei by Spray Drying. J. Microencapsul. 2014, 31, 759–767.
- Sosnik, A.; Seremeta, K. Advantages and Challenges of the Spray-Drying Technology for the Production of Pure Drug Particles and Drug-Loaded Polymeric Carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54.
- Arepally, D.; Reddy, R.S.; Goswami, T.K. Studies on Survivability, Storage Stability of Encapsulated Spray Dried Probiotic Powder. Curr. Res. Food Sci. 2020, 3, 235–242.
- Lipan, L.; Rusu, B.; Sendra, E.; Hernández, F.; Vázquez-Araújo, L.; Vodnar, D.C.; Carbonell-Barrachina, Á.A. Spray Drying and Storage of Probiotic-enriched Almond Milk: Probiotic Survival and Physicochemical Properties. J. Sci. Food Agric. 2020, 100, 3697–3708.
- Golman, B.; Julklang, W. Analysis of Drying Kinetics of a Slurry Droplet in the Falling Rate Period of Spray Drying. Int. J. Chem. Mol. Eng. 2013, 7, 5.
- Huang, S.; Vignolles, M.-L.; Chen, X.D.; Le Loir, Y.; Jan, G.; Schuck, P.; Jeantet, R. Spray Drying of Probiotics and Other Food-Grade Bacteria: A Review. Trends Food Sci. Technol. 2017, 63, 1–17.
- Rajam, R.; Anandharamakrishnan, C. Spray Freeze Drying Method for Microencapsulation of Lactobacillus Plantarum. J. Food Eng. 2015, 166, 95–103.
- Gaidhani, K.A.; Harwalkar, M.; Bhambere, D.; Nirgude, P.S. Lyophilization/Freeze Drying—A Review. World J. Pharm. Res. 2015, 4, 29.
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.J.; Oliveira, A.; Pintado, M.E.; Teixeira, P. Comparison of Spray Drying, Freeze Drying and Convective Hot Air Drying for the Production of a Probiotic Orange Powder. J. Funct. Foods 2015, 17, 340–351.
- Jiang, H.; Zhang, M.; Mujumdar, A.S.; Lim, R.-X. Comparison of Drying Characteristic and Uniformity of Banana Cubes Dried by Pulse-Spouted Microwave Vacuum Drying, Freeze Drying and Microwave Freeze Drying: Comparison of Banana Cubes Dried by Different Drying Method. J. Sci. Food Agric. 2014, 94, 1827–1834.
- Liu, W.; Zhang, M.; Bhandari, B.; Yu, D. A Novel Combination of LF-NMR and NIR to Intelligent Control in Pulse-Spouted Microwave Freeze Drying of Blueberry. LWT 2021, 137, 110455.
- Morgan, C.A.; Herman, N.; White, P.A.; Vesey, G. Preservation of Micro-Organisms by Drying; A Review. J. Microbiol. Methods 2006, 66, 183–193.
- Fu, N.; Chen, X.D. Towards a Maximal Cell Survival in Convective Thermal Drying Processes. Food Res. Int. 2011, 44, 1127–1149.
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Alternative Drying Processes for the Industrial Preservation of Lactic Acid Starter Cultures. Biotechnol. Prog. 2007, 23, 302–315.
- Sánchez-Portilla, Z.; Melgoza-Contreras, L.M.; Reynoso-Camacho, R.; Pérez-Carreón, J.I.; Gutiérrez-Nava, A. Incorporation of Bifidobacterium Sp. into Powder Products through a Fluidized Bed Process for Enteric Targeted Release. J. Dairy Sci. 2020, 103, 11129–11137.
- Strasser, S.; Neureiter, M.; Geppl, M.; Braun, R.; Danner, H. Influence of Lyophilization, Fluidized Bed Drying, Addition of Protectants, and Storage on the Viability of Lactic Acid Bacteria. J. Appl. Microbiol. 2009, 107, 167–177.
- Stummer, S.; Toegel, S.; Rabenreither, M.-C.; Unger, F.M.; Wirth, M.; Viernstein, H.; Salar-Behzadi, S. Fluidized-Bed Drying as a Feasible Method for Dehydration of Enterococcus Faecium M74. J. Food Eng. 2012, 111, 156–165.
- Jacobsen, N.M.Y.; Caglayan, I.; Caglayan, A.; Bar-Shalom, D.; Müllertz, A. Achieving Delayed Release of Freeze-Dried Probiotic Strains by Extrusion, Spheronization and Fluid Bed Coating—Evaluated Using a Three-Step in Vitro Model. Int. J. Pharm. 2020, 591, 120022.
- Wirunpan, M.; Savedboworn, W.; Wanchaitanawong, P. Survival and Shelf Life of Lactobacillus Lactis 1464 in Shrimp Feed Pellet after Fluidized Bed Drying. Agric. Nat. Resour. 2016, 50, 1–7.
- Škrlec, K.; Zupančič, Š.; Prpar Mihevc, S.; Kocbek, P.; Kristl, J.; Berlec, A. Development of Electrospun Nanofibers That Enable High Loading and Long-Term Viability of Probiotics. Eur. J. Pharm. Biopharm. 2019, 136, 108–119.
- Yoha, K.S.; Anukiruthika, T.; Anila, W.; Moses, J.A.; Anandharamakrishnan, C. 3D Printing of Encapsulated Probiotics: Effect of Different Post-Processing Methods on the Stability of Lactiplantibacillus Plantarum (NCIM 2083) under Static in Vitro Digestion Conditions and during Storage. LWT 2021, 146, 111461.
- Anukiruthika, T.; Moses, J.A.; Anandharamakrishnan, C. 3D Printing of Egg Yolk and White with Rice Flour Blends. J. Food Eng. 2020, 265, 109691.
- Galvão, A.M.M.T.; Rodrigues, S.; Fernandes, F.A.N. Probiotic Dried Apple Snacks: Development of Probiotic Coating and Shelf-life Studies. J. Food Process. Preserv. 2020, 44, e14974.
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Inactivation Mechanisms of Lactic Acid Starter Cultures Preserved by Drying Processes. J. Appl. Microbiol. 2008, 105, 1–13.
- Pispan, S.; Hewitt, C.J.; Stapley, A.G.F. Comparison of Cell Survival Rates of E. Coli K12 and L. Acidophilus Undergoing Spray Drying. Food Bioprod. Process. 2013, 91, 362–369.
- Arepally, D.; Goswami, T.K. Effect of Inlet Air Temperature and Gum Arabic Concentration on Encapsulation of Probiotics by Spray Drying. LWT 2019, 99, 583–593.
- Perdana, J.; Fox, M.B.; Siwei, C.; Boom, R.M.; Schutyser, M.A.I. Interactions between Formulation and Spray Drying Conditions Related to Survival of Lactobacillus Plantarum WCFS1. Food Res. Int. 2014, 56, 9–17.
- Perdana, J.; Fox, M.B.; Schutyser, M.A.I.; Boom, R.M. Mimicking Spray Drying by Drying of Single Droplets Deposited on a Flat Surface. Food Bioprocess Technol. 2013, 6, 964–977.
- Ghandi, A.; Powell, I.B.; Chen, X.D.; Adhikari, B. The Effect of Dryer Inlet and Outlet Air Temperatures and Protectant Solids on the Survival of Lactococcus Lactis during Spray Drying. Dry. Technol. 2012, 30, 1649–1657.
- Bauer, S.A.W.; Kulozik, U.; Foerst, P. Drying Kinetics and Survival of Bacteria Suspensions of L. paracasei F19 in Low-Temperature Vacuum Drying. Dry. Technol. 2013, 31, 1497–1503.
- Hernández, A.; Larsson, C.U.; Sawicki, R.; van Niel, E.W.J.; Roos, S.; Håkansson, S. Impact of the Fermentation Parameters PH and Temperature on Stress Resilience of Lactobacillus Reuteri DSM 17938. AMB Express 2019, 9, 66.
- Nag, A.; Das, S. Improving Ambient Temperature Stability of Probiotics with Stress Adaptation and Fluidized Bed Drying. J. Funct. Foods 2013, 5, 170–177.
- Ananta, E.; Volkert, M.; Knorr, D. Cellular Injuries and Storage Stability of Spray-Dried Lactobacillus Rhamnosus GG. Int. Dairy J. 2005, 15, 399–409.
- Avila-Reyes, S.V.; Garcia-Suarez, F.J.; Jiménez, M.T.; San Martín-Gonzalez, M.F.; Bello-Perez, L.A. Protection of L. Rhamnosus by Spray-Drying Using Two Prebiotics Colloids to Enhance the Viability. Carbohydr. Polym. 2014, 102, 423–430.
- Zhao, G.; Zhang, G. Effect of Protective Agents, Freezing Temperature, Rehydration Media on Viability of Malolactic Bacteria Subjected to Freeze-Drying. J. Appl. Microbiol. 2005, 99, 333–338.
- Tymczyszyn, E.E.; Díaz, R.; Pataro, A.; Sandonato, N.; Gómez-Zavaglia, A.; Disalvo, E.A. Critical Water Activity for the Preservation of Lactobacillus Bulgaricus by Vacuum Drying. Int. J. Food Microbiol. 2008, 128, 342–347.
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Effect of Carbohydrates on the Survival of Lactobacillus helveticus during Vacuum Drying. Lett. Appl. Microbiol. 2006, 42, 271–276.
- Santivarangkna, C.; Naumann, D.; Kulozik, U.; Foerst, P. Protective Effects of Sorbitol during the Vacuum Drying of Lactobacillus Helveticus: An FT-IR Study. Ann. Microbiol. 2010, 60, 235–242.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
6.9K
Revisions:
3 times
(View History)
Update Date:
18 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





