
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David Smith | -- | 3333 | 2022-08-16 10:17:55 | | | |
| 2 | Vivi Li | -6 word(s) | 3327 | 2022-08-17 04:53:23 | | |
Video Upload Options
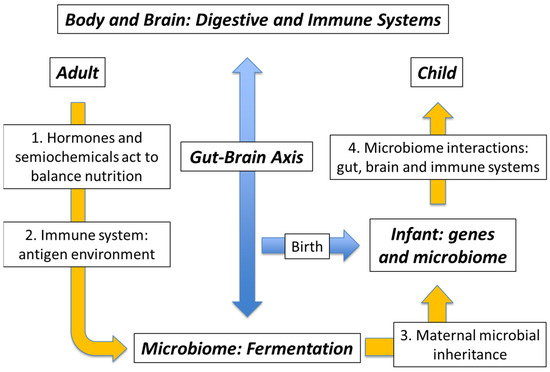
The expression microbiota-gut-brain axis is well recognised nowadays (alongside the related terms microbiome-gut-brain axis and, occasionally, brain-gut-microbiota axis), however it is also clear that the action of the microbiome includes an immune system component. In turn, any concerning reaction from this system will necessarily involve the brain in fashioning a coordinated response, such as is seen in the case of traveller’s diarrhoea, for example. The interaction of the microbiota with the body is therefore bidirectional: with the gut wall itself and with the immune system, both of which link through to the brain. Any chemical communication with the microbiome is actually semiochemical, in the sense of the transfer of signalling information between the Kingdoms of Life, i.e. prokaryote to eukaryote and vice versa. Accordingly, the two terms “microbiota-gut-brain axis” and “immune/semiochemical system” should be considered alongside one another.
1. Introduction: The Unstoppable Rise of Obesity
2. Health versus Pollution: The Degraded Microbiome
3. Genes versus Environment: Maternal Microbial Inheritance
4. The Mutualistic Microbiome: External Microbes and Their Antigens
5. The Mutualistic Microbiome: The Immune/Semiochemical Complex
6. Breaking the Contract: Dysbiosis as Microbiome Failure
References
- Garner, D.M.; Wooley, S.C. Confronting the failure of behavioral and dietary treatments for obesity. Clin. Psychol. Rev. 1991, 11, 729–780.
- Prentice, A.M.; Jebb, S.A. Obesity in Britain: Gluttony or sloth? BMJ 1995, 311, 437–439.
- Westerterp, K.R.; Speakman, J.R. Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int. J. Obes. 2008, 32, 1256–1263.
- Jou, C. The biology and genetics of obesity—A century of inquiries. N. Engl. J. Med. 2014, 370, 1874–1877.
- Casazza, K.; Brown, A.; Astrup, A.; Bertz, F.; Baum, C.; Brown, B.B.; Dawson, J.; Durant, N.; Dutton, G.; Fields, D.A.; et al. Weighing the evidence of common beliefs in obesity research. Crit. Rev. Food Sci. Nutr. 2015, 55, 2014–2053.
- Hruby, A.; Hu, F.B. The epidemiology of obesity: A big picture. Pharmacoeconomics 2015, 33, 673–689.
- Reilly, J.J.; El-Hamdouchi, A.; Diouf, A.; Monyeki, A.; Somda, S.A. Determining the world-wide prevalence of obesity. Lancet 2018, 39, 1773–1774.
- Sandercock, G.R.H.; Cohen, D.D. Temporal trends in muscular fitness of English 10-year-olds 1998–2014: An allometric approach. J. Sci. Med. Sport 2019, 22, 201–205.
- Ðuric, S.; Sember, V.; Starc, G.; Soric, M.; Kovac, M.; Jurak, G. Secular trends in muscular fitness from 1983 to 2014 among Slovenian children and adolescents. Scand. J. Med. Sci. Sports 2021, 31, 1853–1861.
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031.
- Berthoud, H.-R.; Morrison, C.D.; Münzberg, H. The obesity epidemic in the face of homeostatic body weight regulation: What went wrong and how can it be fixed? Physiol. Behav. 2020, 222, 112959.
- Torres-Fuentes, C.; Schellenkens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756.
- Burkitt, D.P. Some diseases characteristic of modern western civilization. BMJ 1973, 1, 274–278.
- O’Keefe, S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996.
- Smith, D.; Palacios-Pérez, M.; Jheeta, S. The enclosed intestinal microbiome: Semiochemical signals from the Precambrian and their disruption by heavy metal pollution. Life 2022, 12, 287.
- Smith, D.; Jheeta, S. Microbiome-gut dissociation: Investigating the origins of obesity. Gastrointest. Disord. 2021, 3, 156–172.
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359.
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179.
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715.
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214.
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A Gut-Brain Neural Circuit for Nutrient Sensory Transduction. Science 2018, 361, eaat5236.
- Hidalgo, G.; Marini, E.; Sanchez, W.; Contreras, M.; Estrada, I.; Comandini, O.; Buffa, R.; Magris, M.; Dominguez-Bello, M.G. The nutrition transition in the Venezuelan Amazonia: Increased overweight and obesity with transculturation. Am. J. Hum. Biol. 2014, 26, 710–712.
- Clemente, J.C.; Pehrsson, E.C.; Blaser, M.J.; Sandhu, K.; Gao, Z.; Wang, B.; Magris, M.; Hidalgo, G.; Contreras, M.; Noya-Alarcón, Ó.; et al. The Microbiome of Uncontacted Amerindians. Sci. Adv. 2015, 1, e1500183.
- Kaplan, H.; Thompson, R.C.; Trumble, B.C.; Wann, L.S.; Allam, A.H.; Beheim, B.; Frohlich, B.; Sutherland, M.L.; Sutherland, J.D.; Stieglitz, J.; et al. Coronary atherosclerosis in indigenous South American Tsimane: A cross sectional cohort study. Lancet 2017, 389, 1730–1739.
- Irimia, A.; Chaudhari, N.N.; Robles, D.J.; Rostowsky, K.A.; Maher, A.S.; Chowdhury, N.F.; Calvillo, N.F.; Ngo, V.; Gatz, M.; Mack, W.J.; et al. The indigenous South American Tsimane exhibit relatively modest decrease in brain volume with age despite high systemic inflammation. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2147–2155.
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654.
- Mizuno, S.; Masaoka, T.; Naganuma, M.; Kishimoto, T.; Kitazawa, M.; Kurokawa, S.; Nakashima, M.; Takeshita, K.; Suda, W.; Mimura, M.; et al. Bifidobacterium-rich fecal donor may be a positive predictor for successful fecal microbiota transplantation in patients with irritable bowel syndrome. Digestion 2017, 96, 29–38.
- Pratt, C.; Campbell, M.D. The effect of bifidobacterium on reducing symptomatic pain in patients with irritable bowel syndrome: A systematic review. Probiotics Antimicrob. Proteins 2020, 12, 834–839.
- Smith, D.; Jheeta, S. The epidemiology of the dysfunctional microbiome in animals and in humans: The propensity for the development of non-communicable disease. EC Gastroenterol. Dig. Syst. 2020, 7, 83–93.
- Bouchard, C.; Tremblay, A.; Després, J.-P.; Nadeau, A.; Lupien, P.J.; Thériault, G.; Dussault, J.; Moorjani, S.; Pinault, S.; Fournier, G. The response to long-term overfeeding in identical twins. N. Engl. J. Med. 1990, 322, 1477–1482.
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111.
- Eriksson, J.G. The fetal origins hypothesis—10 years on. BMJ 2005, 330, 1096–1097.
- Almond, D.; Currie, J. Killing me softly: The fetal origins hypothesis. J. Econ. Perspect. 2011, 25, 153–172.
- Waddington, C.H. Toward a Theoretical Biology; Edinburgh University Press: Edinburgh, UK, 1968; pp. 1–32.
- Trerotola, M.; Relli, V.; Simeone, P.; Alberti, S. Epigenetic inheritance and the missing heritability. Hum. Genom. 2015, 9, 17.
- Horsthemke, B. A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 2018, 9, 2973.
- Qin, Y.; Wade, P.A. Crosstalk between the microbiome and the epigenome: Messages from bugs. J. Biochem. 2018, 163, 105–112.
- Yuan, C.; Gaskins, A.J.; Blaine, A.I.; Zhang, C.; Gillman, M.W.; Missmer, S.A.; Field, A.E.; Chavarro, J.E. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. 2016, 170, e162385.
- Zhao, Q.; Elson, C.O. Adaptive Immune Education by Gut Microbiota Antigens. Immunology 2018, 154, 28–37.
- Strachan, D.P. Hay fever, hygiene and household size. BMJ 1989, 299, 1259–1260.
- Rook, G.A.W.; Lowry, C.A.; Raison, C.L. Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol. Med. Public Health 2013, 1, 46–64.
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811.
- Jheeta, S.; Smith, D. Seeing the wood for the trees: A new way to view the human intestinal microbiome and its connection with non-communicable disease. Med. Hypotheses 2019, 125, 70–74.
- Tuladhar, E.; Hazeleger, W.C.; Koopmans, M.; Zwietering, M.H.; Beumer, R.R.; Duizer, E. Residual viral and bacterial contamination of surfaces after cleaning and disinfection. Appl. Environ. Microbiol. 2012, 78, 7769–7775.
- Tuladhar, E.; Bouwknegt, M.; Zwietering, M.H.; Koopmans, M.; Duizer, E. Thermal stability of structurally different viruses with proven or potential relevance to food safety. J. Appl. Microbiol. 2012, 112, 1050–1057.
- Giddings, S.L.; Stevens, A.M.; Leung, D.T. Traveler’s diarrhea. Med. Clin. N. Am. 2016, 100, 317–330.
- Underhill, D.M.; Iliev, I.D. The Mycobiota: Interactions between Commensal Fungi and the Host Immune System. Nat. Rev. Immunol. 2014, 14, 405–416.
- Laforest-Lapointe, I.; Arrieta, M.-C. Microbial eukaryotes: A missing link in gut microbiome studies. mSystems 2018, 3, e00201-17.
- Ward, T.L.; Dominguez-Bello, M.G.; Heisel, T.; Al-Ghalith, G.; Knights, D.; Gale, C.A. Development of the human mycobiome over the first month of life and across body sites. mSystems 2018, 3, e00140.
- Berdoy, M.; Webster, J.P.; Macdonald, D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. Royal Soc. B 2000, 267, 1591–1594.
- Scanlan, P.D.; Stensvold, C.R.; Rajilic-Stojanovic, M.; Heilig, H.G.H.J.; De Vos, W.M.; O’Toole, P.W.; Cotter, P.D. The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol. Ecol. 2014, 90, 326–330.
- Noel, C.; Dufemez, F.; Gerbod, G.; Egcomb, V.P.; Delgado-Viscogliosi, P.; Ho, L.-C.; Singh, M.; Wintjens, R.; Soglin, M.L.; Capron, M.; et al. Molecular phylogenies of Blastocystis isolates from different hosts: Implications for genetic diversity, identification of species, and zoonosis. J. Clin. Microbiol. 2005, 43, 348–355.
- Bostock, J. Case of a periodical affection of the eyes and chest. Med. Chir. Trans. 1819, 10, 161–165.
- Walker, S.; Khan-Wasti, S.; Fletcher, M.; Sheikh, A. Prevalence of hayfever symptoms and diagnosis in UK teenagers. Prim. Care Respir. J. 2005, 14, 270.
- Loh, W.; Tang, M.L.K. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 2018, 15, 2043.
- Hill, D.A.; Spergel, J.M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 2018, 120, 131–137.
- Bostock, J. Of the catarrhus aestivus or summer catarrh. Med. Chir. Trans. 1828, 14, 437–446.
- Corson, R. Fashions in Makeup: From Ancient to Modern Times; Peter Owen Ltd.: London, UK, 1972.
- Tansley, A.G. Sigmund Freud, 1856–1939. Obit. Not. Fellows R. Soc. 1941, 3, 246–275.
- Yan, F.; Polk, D.B. Commensal Bacteria in the Gut: Learning Who Our Friends Are. Curr. Opin. Gastroenterol. 2004, 20, 565–571.
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275.
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tarantino, L.M.; Bulik, C.M.; Carroll, I.M. The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. Psychosom. Med. 2015, 77, 969–981.
- Vandeputte, D.; Falony, G.; Veira-Silva, S.; Tito, R.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62.
- Sudo, N. Biogenic amines: Signals between commensal microbiota and gut physiology. Front. Endocrinol. 2019, 10, 504.
- Xue, R.; Zhang, H.; Pan, J.; Du, Z.; Zhou, W.; Zhang, Z.; Tian, Z.; Zhou, R.; Bai, L. Peripheral dopamine controlled by gut microbes inhibits invariant natural killer T cell-mediated hepatitis. Front. Immunol. 2018, 9, 2398.
- Ter Horst, K.W.; Lammers, N.M.; Trinko, R.; Opland, D.M.; Figee, M.; Ackermans, M.T.; Booij, J.; van den Munckhof, P.; Schuurman, P.R.; Fliers, E.; et al. Striatal dopamine regulates systemic glucose metabolism in humans and mice. Sci. Transl. Med. 2018, 10, eaar3752.
- LeBlanc, J.G.; Chain, F.; Martin, R.; Bermùndez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories 2017, 16, 79.
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403.
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573.
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546.
- Iyer, L.M.; Aravind, L.; Coon, S.L.; Klein, D.C.; Koonin, E.V. Evolution of cell-cell signaling in animals: Did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004, 20, 292–299.
- Hooks, K.B.; O’Malley, M.A. Dysbiosis and its discontents. mBio 2017, 8, e01492-17.
- Brüssow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2019, 13, 423–434.
- Woese, C. On the evolution of cells. Proc. Natl. Acad. Sci. USA 2002, 99, 8742–8747.
- Smith, D.; Palacios-Pérez, M.; Jheeta, S. Microbiome–Gut Dissociation in the Neonate: Obesity and Coeliac Disease as Examples of Microbiome Function Deficiency Disorder. Gastrointest. Disord. 2022, 4, 108–128.
- Margulis, L. Symbiogenesis and symbionticism. In Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis; Margulis, L., Fester, R., Eds.; MIT Press: Cambridge, MA, USA, 1991; pp. 49–92.
- Guerrero, R.; Margulis, L.; Berlanga, M. Symbiogenesis: The holobiont as a unit of evolution. Int. Microbiol. 2013, 16, 133–143.
- Simon, J.-C.; Marchesi, J.R.; Mougel, C.; Selosse, M.-A. Host-microbiota interactions: From holobiont theory to analysis. Microbiome 2019, 7, 5.
- Mesa, D.M.; Loureiro, B.; Iglesia, I.; Gonzalez, S.F.; Olivé, E.L.; Algar, O.G.; Solana, M.J.; Cabero, M.J.; Sainz, T.; Martinez, L.; et al. The evolving microbiome from pregnancy to early infancy: A comprehensive review. Nutrients 2020, 12, 133.




