1. Taurine-Conjugated BAs
Taurine-conjugated bile acids (BAs) are generally less toxic than unconjugated BAs and exhibit some beneficial properties. Particularly,
tauroursodeoxycholic acid (TUDCA) has broad therapeutic applications. It is more efficient in treating liver cirrhosis than its deconjugated counterpart ursodeoxycholic acid (UDCA)
[1][2]. Its administration in mice attenuates HFD-induced hepatic steatosis, inflammatory responses, insulin resistance, and obesity. Moreover, TUDCA improves intestinal barrier function, reduces inflammatory cytokine levels and intestine histopathology scores. Finally, the gut microbiota composition of the HFD-fed and TUDCA-treated mice differs from that in HFD-fed mice, but is similar to that in chow diet-fed animals
[3].
As a supplement, TUDCA is orally bioavailable, and due to its capacity to cross the blood–brain barrier, it can penetrate the central nervous system
[4]. Similar to free taurine
[5][6][7], TUDCA has proven neuroprotective properties which were researched in the models of Alzheimer’s disease (AD)
[8][9][10][11][12][13], amyotrophic lateral sclerosis (ALS)
[14], Huntington’s disease (HD)
[4][15], and stroke
[16][17]. In the mouse model of AD, TUDCA prevents cognitive impairments
[9], interferes with amyloid-β production
[8] as well as suppresses amyloid β-induced synaptic toxicity inhibiting organelle-driven apoptosis
[13], and interferes with upstream molecular targets of p53 pathways
[10][11][12]. In the case of ALS patients, compared with placebo treatment, TUDCA slows the progression of disability
[14]. Supplementation of TUDCA in a genetic mouse model of HD reduces striatal atrophy and decreases striatal apoptosis, resulting in fewer and smaller sized ubiquitinated neuronal intranuclear huntingtin inclusions, as well as improving locomotor and sensorimotor deficits
[4]. Administration of TUDCA before or up to 6 h after induction of intracerebral hemorrhage reduces apoptosis and inhibits caspase activity by 50% in the area immediately surrounding the hematoma, as well as improves neurobehavioral deficits
[16].
Given that taurine supplementation increases the pool of taurine-conjugated BAs
[18][19], many of the benefits of taurine mentioned in the section “Taurine” overlap with and are due to BAs activity. Furthermore, TGR5, as a BAs receptor, likely plays an essential role in these processes.
2. Taurine and Microbiota in the Gastrointestinal (GI) Tract
Upon release from conjugated BAs, taurine creates multiple secondary conjugates of unknown roles
[20] or may be metabolized by bacteria (
Figure 1). Taurine remains largely stable when cultured with human fecal samples in the absence of oxygen, although under aerobic conditions, the majority of the taurine is degraded
[21]. However, another report showed that when using cat feces as the inoculum, taurine is also degraded in anaerobic cultures
[22].
E.
coli is an example of a bacterium that imports and utilizes taurine when cultured under aerobic conditions
[23]. Therefore, the availability of taurine and its impact on the intestinal epithelium depends on whether conditions favor taurine metabolism.
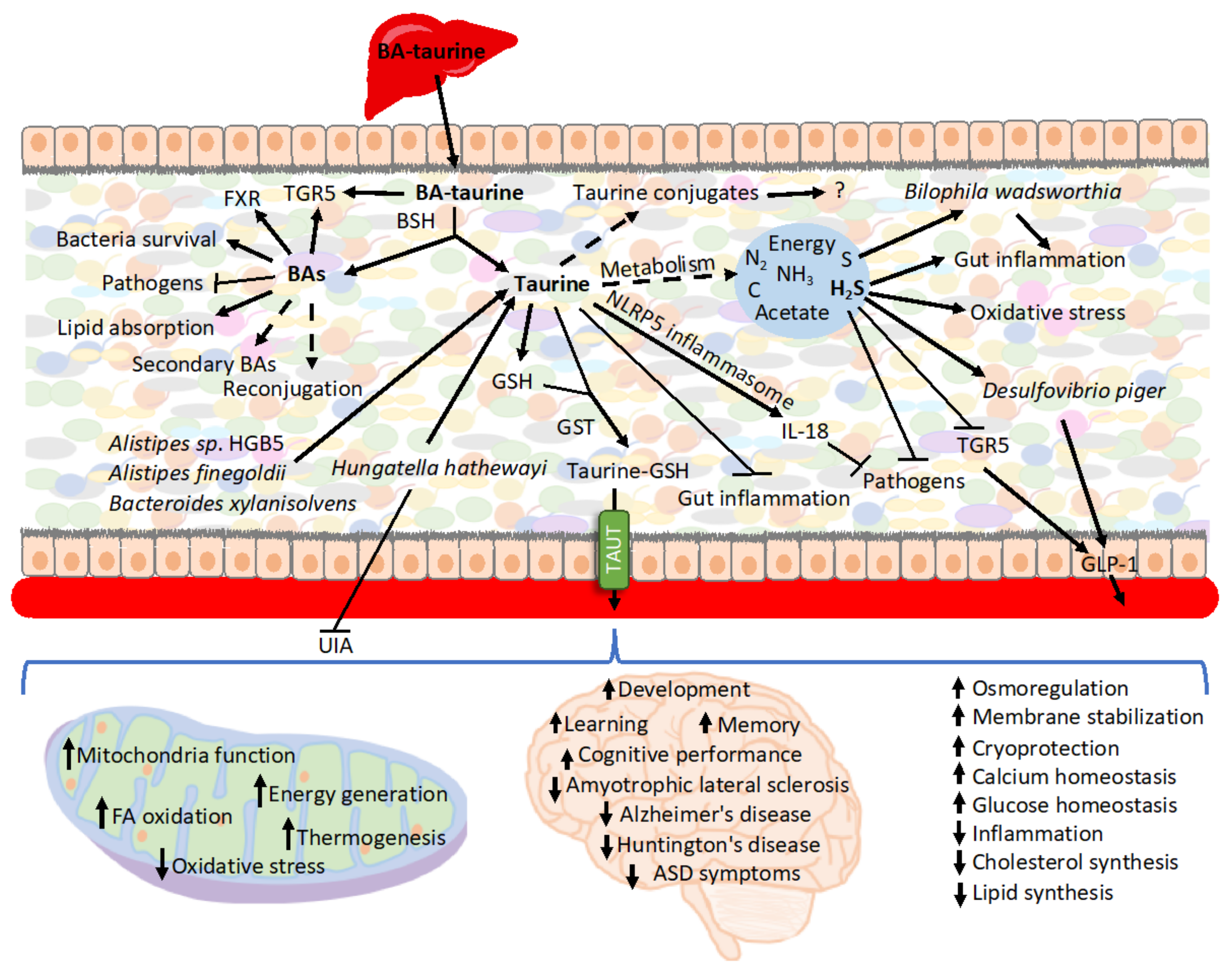
Figure 1. Summary of the roles of BAs-derived taurine. Various bacterial strains have the capacity to deconjugate BAs. The released unconjugated BAs modulate gut microbiota composition, signal various functions through its receptors, and impact nutrient uptake. BAs are also submitted modifications by microbiota, including reconjugation and generation of secondary BAs. The faith of taurine released from conjugated BAs in the intestine can follow various paths. It is metabolized for energy and generates secondary metabolites, which, such as H2S, may play a role in interacting with bacteria, inflammation, and oxidative stress. Taurine also signals within the intestine to extinguish inflammation and prevent pathogens colonization. Additionally, various compounds can conjugate taurine, and, e.g., conjugation to GSH enhances taurine uptake during caloric restriction. The exported taurine plays various roles in other organs, particularly in the nerve system as well as in mitochondria all over the body.
Caloric restriction, which is accompanied by decreased expression of inflammatory and antibacterial genes
[24], is also characterized by increased levels of taurine in the intestinal epithelium
[20]. As a positive inflammasome modulator, taurine is responsible for enhanced NLRP6 inflammasome-induced IL-18 secretion upon intestinal microbial colonization, and, therefore, it modulates bacteria composition
[25][26]. Consequently, taurine has been associated with inhibited growth of harmful bacteria, including
Proteobacteria and especially
Helicobacter, and also increasing the production of SCFA in mouse feces
[26] as well as the metabolism of taurine by microbiota
[27]. Controversially, some reports show that taurine does not trigger significant change in the microbiota’s diversity or composition as well as the composition of SCFA produced when cultured with human feces
[21]. Reciprocally, the presence of taurine in the intestine depends on microbiota
[25][28]. One of the mechanisms involves microbiota-mediated BAs deconjugation and release of free taurine
[20]. Thus, microbiota transplant from calorie-restricted mice characterized by increased levels of BAs and taurine in the intestinal mucosa, raises levels of intestinal free taurine and various secondary taurine conjugates
[28].
Taurine has been shown to have a substantial impact on the GI tract due to its anti-inflammatory properties and, therefore, impacting the environment of resident gut bacteria. When supplemented in the model of an immunosuppressive mouse, taurine improves immune cell numbers in Payer’s patches
[29]. It also attenuates dextran sulfate sodium (DSS)-induced colitis reducing the severity of diarrhea, rectal bleeding, colon shortening, histological score, myeloperoxidase (MPO) activity elevation, abnormal macrophage inflammatory protein (MIP-2) gene expression, and infiltration of neutrophils
[30][31]. Consequently, it retards DSS-induced weight loss and lowers mortality upon DSS-induced treatment
[25][30]. In the model of trinitrobenzene sulfonic acid (TNBS)-induced inflammatory bowel disease, taurine reduces the inflammatory parameters in rat colon by increasing capacity to defend against oxidative damage
[32]. Taurine also inhibits the TNF-α-induced secretion of IL-8 from human intestinal epithelial Caco-2 cells
[30]. Particularly noteworthy is the role of taurine in T lymphocytes, where it accounts for approximately 44% of the total free amino acid pool
[33] and is critical for cell survival, T cell-mediated immune reactions, and memory development
[34]. However, recently an extraordinary mechanism preventing infection has been described in which BA-derived taurine mediates long-term metaorganism colonization resistance (
Table 1). Mechanistically, the host, triggered by transient intestinal infection, alters BAs metabolism and deploys taurine leading to the expansion of taurine-utilizing taxa. Taurine nourishes and trains the selected microbiota. Following infection,
Deltaproteobacteria, typically a minor class, expands up to 100-fold. Importantly,
Deltaproteobacteria encompasses sulfate-reducing bacteria possessing the capacity to convert taurine to ammonia, acetate, and sulfide. Subsequently, sulfide serves as an inhibitor of cellular respiration, which is key to host invasion by numerous pathogens. The conversion of taurine occurs via taurine–pyruvate aminotransferase encoded by the tpa gene, which is more prevalent in infection-trained microbiota metagenomes. Following oral gavage with
Klebsiella pneumoniae, the subsequent fecal transfer of
Klebsiella pneumoniae infection-trained microbiota enhanced resistance to colonization.
Klebsiella pneumoniae grows on 1,2-propanediol by scavenging oxygen via cytochrome oxidase bd-II. Sulfide at concentration >250 mM potently inhibited
Klebsiella pneumoniae respiration and ability to grow on 1,2- propanediol with oxygen. Notably, supplying exogenous taurine alone was sufficient to induce alteration in microbiota function and enhance resistance. However, taurine treatment of GF mice did not enhance resistance to
Klebsiella pneumoniae [35]. Previously, TCA has also been implied to stimulate intestinal bacteria capable of converting taurine to hydrogen sulfide
[27]. Sadly, bismuth subsalicylate, a common over-the-counter drug for diarrhea and upset stomach, neutralizes infection protection as it inhibits hydrogen sulfide production
[35].
In addition to being toxic to bacteria, sulfide can also be toxic to the host. Sulfide increases proliferation in the intestinal crypt, epithelial cells, and the upper colonic crypts, accompanied by induction of inflammatory pathways
[36][37][38]. Furthermore, sulfide, as a genotoxic compound, triggers oxidative stress leading to cell-cycle arrest and DNA damage in the human colon
[37][38][39][40]. Interestingly, in the adenocarcinoma cell line HCT116, sulfide was implicated in preventing apoptosis induced by β-phenylethyl isothiocyanate, a phytochemical found in cruciferous vegetables
[41].
Endogenous concentrations of sulfide range between 0.2–3.4 mmol/L in the GI tract of mice and humans
[42][43]. Sulfide-detoxifying enzymes are upregulated during differentiation in the human colon
[44], and rat colonocytes express protective enzymes on the mucosal surface that oxidize sulfide
[45]. However, these enzymes are decreased in the colon of patients suffering from cancer and active ulcerative colitis
[44]. Accordingly, fecal sulfide is significantly elevated in ulcerative colitis patients experiencing active disease
[36][46][47]. Controversially, some reports have shown no significant increase in sulfide in ulcerative colitis patients
[48].
Concerning microbiota, sulfidogenic bacterium (
Fusobacterium spp.) is associated with the tumor surface in a subset of colorectal cancer
[49][50], while sulfite-reducing opportunistic pathogen
Bilophila wadsworthia bacteria, which is difficult to detect in healthy individuals, emerges under pathological conditions such as appendicitis
[51], ulcerative colitis
[52][53], and colorectal cancer
[54].
B. wadsworthia thrives in the presence of taurine-conjugated BAs since it utilizes taurine-derived organic sulfur as the terminal electron acceptor of the electron transport chain resulting in the generation of sulfide as a byproduct
[55][56]. Increased taurine conjugation of hepatic BAs, e.g., by consuming a diet high in saturated (milk-derived) fat, breeds
B. wadsworthia. What is relevant in this context, milk fat promotes the onset and incidence of colitis in IL-10
−/− mice, driving it from a spontaneous rate of 25–30% to over 60% in a 6-month period. Correspondingly,
B. wadsworthia occurrence, as well as TCA supplementation, are also associated with colitis development in IL-10
−/− mice. The mechanisms involve
B.
wadsworthia-driven activation of dendritic cells in a way that selectively induces the production of interferon-γ (IFNγ) and Th1- mediated colitis
[57]. A diet high in meat has been shown to significantly increase both the levels of taurine conjugation to BAs
[18][19] and the production of hydrogen sulfide in the colon
[42]. The low occurrence rate of cancer in African populations consuming small amounts of meat is associated with colonic bacteria fermentation
[58]. However, native black Africans also have decreased ratios of taurine to glycine conjugation (1:9) and low hydrogen sulfide production compared with populations consuming a “Western diet”. Therefore, consumption of a diet low in taurine may contribute to the reduced frequency of cancer. Opportunely, supplementation with ω-3 fish oil inhibits the bloom of
B.
wadsworthia, most likely because of alterations in the BA composition
[59].
In addition to its toxic properties, sulfide and sulfate prebiotics also stimulate GLP-1 secretion and its downstream metabolic actions
[60]. Furthermore, supplementation of prebiotic chondroitin sulfate to mice increases the proportion of
Desulfovibrio piger, a sulfate-reducing bacterium, and accentuates GLP-1 levels, leading to an improved glucose tolerance
[60]. In contrast, another cell-based study revealed that sulfide has potent inhibitory effects on TGR5-mediated GLP-1 and PYY release
[61]. Thus, the effect of hydrogen sulfide on GLP-1 release remains controversial and calls for further investigation.
3. Taurine, Microbiota, and Cardiovascular Diseases
Gut microbiota is a critical risk factor in cardiovascular diseases as it impacts host metabolism and immune homeostasis. Depletion of the gut microbiota by antibiotics has been shown to reduce the incidence of intracranial aneurysms in mice
[62]. Similarly, taurine plays a protective role in acute ischemic stroke
[63], subarachnoid hemorrhage
[64], and aortic aneurysm formation
[65].
The microbiome of atherosclerotic cardiovascular disease is characterized by depletion of the taurine transport system
[66]. Importantly, taurine depletion is a key factor in the pathogenesis of unruptured intracranial aneurysms (UIA)
[67]. Microbiota transplantation from UIA patients’ donors is sufficient to induce UIAs and decrease the serum taurine levels of mice, indicating that UIA microbiota mediates the low level of taurine in mice. Specifically, the abundance of
Hungatella hathewayi is strikingly reduced in UIA and correlates positively with the circulating taurine concentration in humans and mice. Consequently, gavage with
H. hathewayi normalizes the taurine serum levels and protects mice against the formation and rupture of intracranial aneurysms
[67].
H. hathewayi also reduces the release of cytokines and lowers NF-κB activation in dendritic cells
[68], whereas taurine supplementation reverses the progression of intracranial aneurysms
[67].
4. Taurine and GSH
Taurine and GSH are linked on several levels. Both require cysteine for their synthesis, therefore, the responsible enzymes may compete for the substrate
[69]. Both act as antioxidants and play a vital function in mitochondria. GSH serves as a mitochondrial redox buffer to stabilize the electrical gradient, whereas taurine is applied as a pH buffer, but simultaneously establishes the equilibrium between the NADH/NAD+ redox pair and the redox buffer pair GSH/GSSG
[70]. Taurine enhances the activity of antioxidant enzymes, including SOD, CAT, GSH peroxidase (GPx), and GSH reductase (GR), thus preserving redox levels and GSH stores
[71][72][73][74]. Upon one-time taurine supplementation in rats, GPx activity shows an increase in liver, heart, stomach, and plasma; GR activity increased in the kidney and decreased in liver and plasma, whereas GSH levels increased in the liver and stomach and decreased in the kidney
[72]. Therefore, in several instances, taurine has been found to prevent or repair oxidative damage by acting on GSH. Following nicotine administration, taurine protects against oxidative stress by normalizing GSH stores in rats
[75]. It reduces oxidative stress in iron-overloaded mice and protects the levels of reduced GSH
[76]. Further, taurine treatment alleviates adverse effects of mitochondrial oxidative stress found in induced pluripotent stem cells (iPSCs) from a patient with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) by normalizing stores and the ratio of the reduced to oxidized GSH (GSSG)
[77]. Finally, taurine administration improves both DNA damage and oxidative indices triggered by acetaminophen. In this case, taurine was shown to act by reducing MDA formation, increasing the activity of antioxidant enzymes, and regulating synthesis, utilization, and reduction in GSH
[78].
Gut microbiota influences both host taurine
[79] and GSH metabolism
[80]. Recently, the researchers have shown that upon deconjugation from BAs, taurine creates various conjugates, among others, with GSH. The occurrence of the conjugate in the intestinal epithelium and activity of GSH S-transferases (GST) catalyzing the reaction is modulated by microbiota. This takes place, likely, by bacterial BSH activity regulating taurine availability. The conjugation of taurine with GSH increases intestinal taurine uptake during caloric restriction, playing an important role in taurine circulation and reuse. However, the potential roles of other taurine conjugates remain unknown
[28][81].
5. Taurine and Microbiota in Autism
Autism is associated with frequent dietary issues and GI symptoms, including abdominal pain, constipation, diarrhea, gastroesophageal reflux, bloody stools, vomiting, and gaseousness
[82][83][84]. The symptoms correlate with the severity of core autism-related behavioral abnormalities on measures of irritability, anxiety, and social withdrawal
[85]. Increased intestinal permeability, which is connected with the potential for translocation of intestinal metabolites or bacteria and consequent immune activation, is linked to autism
[82][86][87]. Dysregulated GI motility and secretion in autistic individuals are connected with an altered composition of intestinal microbiota, dysbiosis. The changes in the GI tract influence higher-order behavioral and brain function via the gut–brain axis, vagus nerve, indirect immune and metabolic signals
[88][89][90][91].
In one study, taurine serum concentrations in children with autism spectrum disorder (ASD) were not significantly different from their parents or siblings; however, 21 out of 66 children with ASD had low taurine concentrations, which may have consequences on their mitochondria function. Accordingly, lowered taurine levels were proposed as a biomarker of autism
[92]. Transplanting gut microbiota from human donors with ASD into GF mice induces hallmark autistic behaviors. The brains of mice colonized with ASD microbiota display alternative splicing of ASD-relevant genes
[93]. The circulating concentration of taurine is ~50% reduced in GF mice receiving fecal transplantation from individuals with an ASD. Furthermore, the metabolism of various amino acids, specifically that of proline, taurine, glutamate, and glutamine, are differentially represented in the metagenomes of mice receiving ASD-microbiota. Bioinformatical predictions imply that taurine concentrations might result from differential synthesis potential by three species:
Alistipes sp. HGB5,
Alistipes finegoldii, and
Bacteroides xylanisolvens, whereas taurine supplementation improves repetitive and social behaviors and reduces anxiety in mice by acting locally in the gut
[93].
6. Taurine and Microbiota in Non-GI Tissues
Importantly, due to its role in preventing infections, taurine interacts with bacteria also in tissues other than the intestine. The infection of mammary epithelial cells with
Streptococcus uberis is connected with the internalization of the pathogen, thus leading to avoiding the elimination of bacteria by medication and host responses. Taurine attenuates the infection via phosphoinositides/Ca
2+ signaling, inhibition of over-activation of the NF-κB pathway, and stimulation of Treg cells
[94][95][96]. It also activates autophagy via phosphatase and tensin homolog (PTEN) and Akt/mTOR, which accelerates the degradation of intracellular
S. uberis, reduces intracellular bacterial load, and alleviates the inflammation and damage caused by the infection
[97].
Both taurine and microbiota play multiple roles in organs outside of the GI tract as well as in response to various diseases. Due to its anti-inflammatory and anti-oxidative properties, taurine alleviates liver injury and its consequent events, including a rise in plasma and brain ammonia and brain oedema
[98][99]. It also prevents liver steatosis by reducing oxidative damage, inhibiting lipogenesis, and promoting energy expenditure
[99]. Importantly, liver diseases are tightly connected with dysbiosis, and taurine has been suggested to prevent hepatic inflammation by inhibiting TLR4/MyD88
[100][101][102]. TLR4 recognizes the pathogen-associated molecular pattern and allows the host to identify microorganisms, ultimately transmitting bacterial signals the play a pivotal role in the gut-liver axis
[102]. Thus, taurine may influence the bacteria signaling to extragastrointestinal tissues.
In the renal system, taurine is particularly important for osmoregulation. However, it also reduces the injurious effect of several kidney diseases, including diabetic nephropathy, glomerulonephritis, chronic renal failure, and acute kidney injury
[103][104][105][106]. Similarly, intestinal flora has been reported to prevent the development and progression of several renal diseases, such as lupus nephritis, chronic kidney disease, diabetic nephropathy, and renal ischemia–reperfusion injury
[107]. Similarly, both taurine and gut bacteria play a role in cardiovascular diseases
[108][109], neurological
[5][6][7][110][111][112][113][114][115][116][117][118][119] and multiple other disorders. Their activity is very likely coordinated; however, so far, it lacks evidence.