Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sze-Looi Song | -- | 1888 | 2022-08-15 14:57:14 | | | |
| 2 | Rita Xu | Meta information modification | 1888 | 2022-08-16 03:00:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cheng, A.; Lim, W.Y.; Lim, P.; Amri, A.Y.; Poong, S.; Song, S.; Ilham, Z. Marine Autotroph-Herbivore Synergies. Encyclopedia. Available online: https://encyclopedia.pub/entry/26150 (accessed on 08 February 2026).
Cheng A, Lim WY, Lim P, Amri AY, Poong S, Song S, et al. Marine Autotroph-Herbivore Synergies. Encyclopedia. Available at: https://encyclopedia.pub/entry/26150. Accessed February 08, 2026.
Cheng, Acga, Wai Yin Lim, Phaik-Eem Lim, Affendi Yang Amri, Sze-Wan Poong, Sze-Looi Song, Zul Ilham. "Marine Autotroph-Herbivore Synergies" Encyclopedia, https://encyclopedia.pub/entry/26150 (accessed February 08, 2026).
Cheng, A., Lim, W.Y., Lim, P., Amri, A.Y., Poong, S., Song, S., & Ilham, Z. (2022, August 15). Marine Autotroph-Herbivore Synergies. In Encyclopedia. https://encyclopedia.pub/entry/26150
Cheng, Acga, et al. "Marine Autotroph-Herbivore Synergies." Encyclopedia. Web. 15 August, 2022.
Copy Citation
Species invasion is a leading threat to marine ecosystems worldwide, being deemed as one of the ultimate jeopardies for biodiversity along with climate change. Tackling the emerging biodiversity threat to maintain the ecological balance of the largest biome in the world has now become a pivotal part of the Sustainable Development Goals (SDGs). Marine herbivores are often considered as biological agents that control the spread of invasive species, and their effectiveness depends largely on factors that influence their feeding preferences, including the specific attributes of their food–the autotrophs.
autotroph-herbivore interactions
feeding behaviour
macroalgae
marine herbivores
1. Introduction
In recent decades, mounting evidence suggests that biological invasions by invasive (also called alien or non-native) species are a growing threat to global biodiversity, and is exacerbated by climate warming [1][2]. Globalization, the transformation of technological regimes and expansions of transportation networks which modify the marine habitats are other recognized drivers behind the rapid shifting of invasive species across a broad geographical range [3][4][5]. In a narrower sense, species invasions can adversely influence the dynamics of specific communities, particularly concerning the extirpation of native species [6][7] and the reduction of species richness [8]. Climate, recipient communities, and invaders are considered the prime determinants of invasion impacts, with the characteristics of recipient communities being the most critical determinant [9]. The mechanisms of invasion impact on the diversity of native species, however, are still not well understood and in fact, previous findings on invasion consequences for species richness have been contradictory; viz., either positive, negative, neutral, or multifarious impacts [9]. This invasion paradox has led to many controversial debates over the past two decades [10]. The diversity and impact of invasive species on marine ecosystems are extensively covered in a recent review by Salimi et al. (2021) [11].
Studies on aquatic ecosystems showed that the interactions between marine herbivores and various plants and/or algae (hereinafter referred to as the “autotrophs”) could reduce or even prevent the detrimental impacts of species invasions [12][13]. Lyons and Scheibling [14] reported that the establishment of the invasive green algae Codium fragile was enhanced by sea urchin food preference for kelps under increased water temperature and wave action, leading to increased herbivore pressure on local kelp stands. By and large, generalist marine herbivores such as most fishes and sea urchins that feed on autotrophs are common biological control agents that suppress the establishment and abundance of invasive species in the recipient communities [15][16]. It has been reported that the feeding (or grazing) preferences of the herbivores can determine the relationship between native or invasive autotrophs [17][18]. Recent findings also suggested that mechanisms underlying autotroph palatability could help resolve the inconsistent results of novelty [19][20].
Since the 1980s, efforts have been undertaken to understand the foraging behaviour of generalist marine herbivores [21][22][23][24]. Their selective foraging behaviour, which aims chiefly to regulate their nutritional needs for growth, fecundity, and performance [25][26], has been found to exert a profound impact on the biological structure of many marine ecosystems [27]. As such, theoretic insights on the nutritional relationships between herbivores and autotrophs will assist in the control and management of invasive species [28][29][30]. Generalist herbivores have also been found to make their food selection based on autotroph palatability, which depends primarily on their other unique attributes including, among others, secondary metabolites, morphology and physical stress [31][32][33][34][35]. Significant research has been devoted to examining the role and importance of some secondary metabolites in the survival and adaptation of autotrophs [36][37][38], but less attention has been paid to dissecting the value of their other attributes that may also influence the preferences of herbivores, i.e., whether to feed on native or invasive plants, or both [12]. It is worth noting that autotrophic characteristics may have the opposite effect on autotroph-herbivore interactions in controlled experimental studies where herbivores are restricted to a single autotroph species than in effects seen in field studies where herbivores are free to move around and cause natural autotroph damage. Future research examining the significance of autotroph features in interactions between autotrophs and herbivores must therefore carefully take into account the context in which the relationships have been observed [19].
2. Marine Algae and Their Unique Attributes
Algae are the ultimate source of nutrients and energy for other organisms living in aquatic ecosystems. Although not considered plants, algae are photosynthetic in nature and produce over 70% of the global oxygen content [39][40][41]. Algae are also effective at sequestering carbon by converting almost 50% of the atmospheric carbon dioxide into organic molecules that build essential cellular constituents and intensify their energy production [42][43][44][45][46]. Macroalgae, being the most important primary producers in the oceans, house a wide range of nutritional quality within and among groups which often influences their palatability to herbivores [25][47]. For the most part, the proteins in macroalgae contain important amino acids, particularly the ones that cannot be synthesized by the animal body [48][49]. Animal hosts can thus obtain all these essential amino acids through symbiosis with the algae [50]. A variety of macroalgae reproduce either exclusively sexually or asexually, whilst some species demonstrate an alternation of generations involving both reproductive strategies in succession [51][52][53]. The following subsections discuss the unique characteristics and ecological relationships of each major group of macroalgae, including red algae (Rhodophytes), brown algae (Phaeophytes), and green algae (Chlorophytes) [45][46]. Figure 1 depicts the three major groups of macroalgae and examples of their common species.
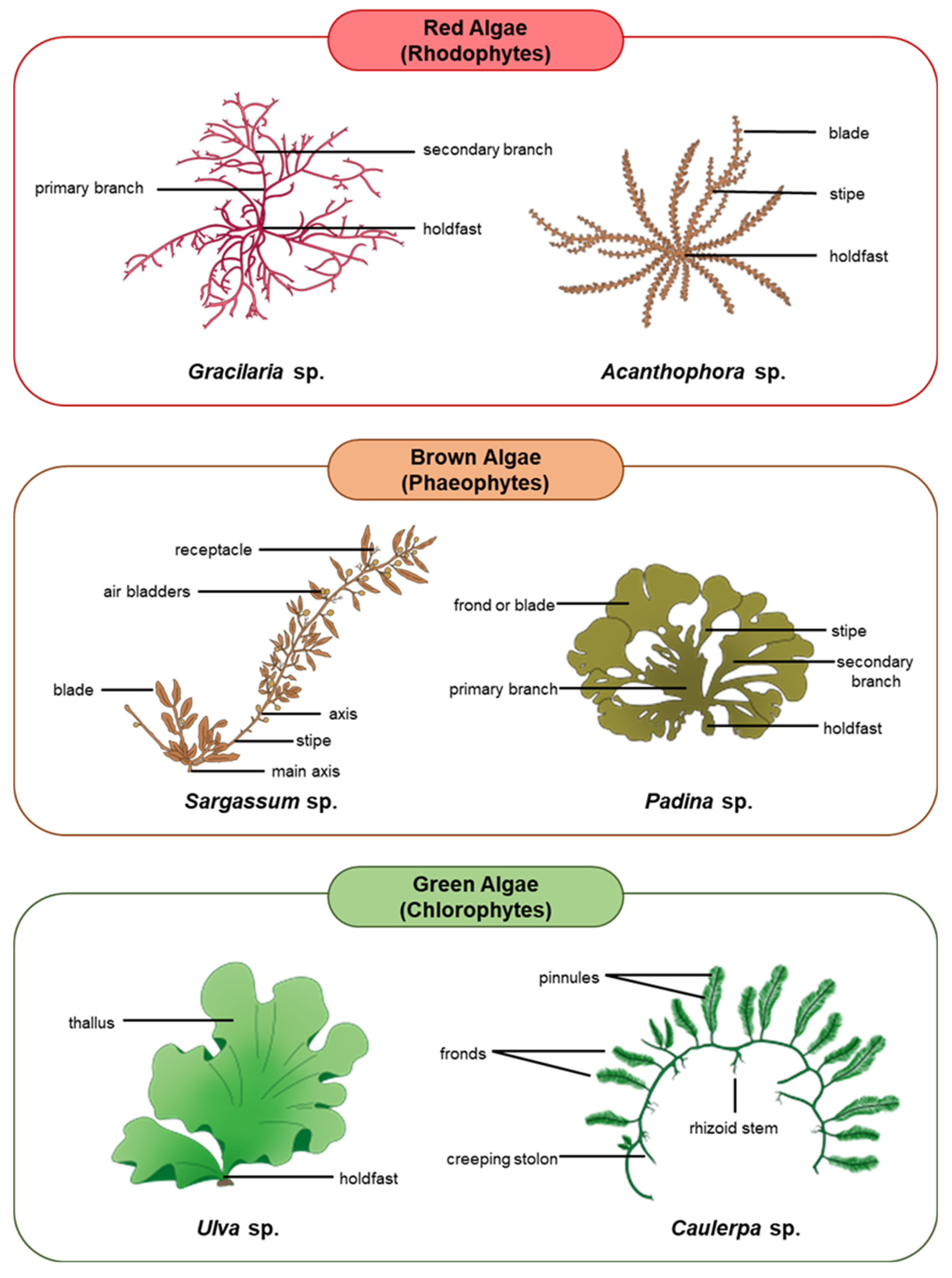
Figure 1. Major groups of macroalgae and examples of their common species.
2.1. Red Algae (Division Rhodophyta)
The first group is the eukaryotic red algae, or the Rhodophytes, comprising more than 6000 species of primarily marine algae ranging from microscopic to macroscopic in size [54][55]. These algae store their energy as a specialized polysaccharide, known as floridean starch, and their cell walls are made of unique cellulose and polysaccharides, such as agars and carrageenan galactans [56][57][58]. However, some other red algae may adopt sulfated mannans or neutral xylans as the main cell wall components rather than carrageenans [57]. Their photosynthetic pigments include chlorophylls a and d, while their accessory pigments are carotenoids, phycobilins, and xanthophyll [54][59][60] (Table 1). Some notable examples of red algae are, among others, filamentous species like Pleonosporum spp. and coralline algae like Porolithon spp., which contribute significantly to the building of tropical reefs and thalloid species. It is worth noting that the red algae have no flagellated cells or cells with any vestigial structure of flagellation [20]. Irish moss (Chondrus crispus Stackhouse), also known as the carrageen moss, is an example of an economically important red alga which has been used to bind proteins together to stabilize and add texture to various foods and beverages like ice cream, yogurt, and deli meats [61][62]. Another economically and nutritionally important species of red algae is nori (Porphyra umbilicalis Kützing); a high-protein and high-fibre algae which is commonly used in Japanese cuisine as an ingredient to wrap sushi [63]. Porphyra was proved to have the greatest protein content (ca. 35%) among the marine macroalgae, while some members of the brown algae in the order Laminariales have the lowest content (ca. 7%) [64][65].
Table 1. Major groups of macroalgae and their attributes.
| Major Groups | Pigments | Cell Wall | Storage Components |
|---|---|---|---|
| Red algae (Rhodophytes) |
Chlorophyll a (d in some Florideophyceae), R- and C- phycocyanin, allophycocyanin, R- and B-phycoerythrin, Alpha- and Beta-carotene, xanthophylls | Cellulose, xylans, galactan, alginate in corallinaceae | Floridean starch |
| Brown algae (Phaeophytes) |
Chlorophyll a, c, Beta-carotene, fucoxanthin, xanthophylls | Cellulose, alginic acid, fucoidan | Laminaran, mannitol |
| Green algae (Chlorophytes) |
Chlorophyll a, b, Alpha-, Beta- and Gamma- carotene, xantophylls | Cellulose, hydroxyproline glucosides, xylans, mannans, absent wall, calcified in some | Starch, oil |
2.2. Brown Algae (Division Chromophyta)
In contrast to other algal groups, brown algae or the Phaeophytes are mostly developed from a secondary endosymbiosis event which involved a non-photosynthetic eukaryote and a unicellular red alga. Resultantly, brown algae exhibit several morphological and metabolic features that make them the most complex macroalgae [66]. Phaeophytes are mostly macroscopic in size, inclusive of the giant kelp (Macrocystis pyrifera (Linnaeus) C.Agardh), which can grow up to 10 m in length [67]. Most of the approximately 1800 species of brown algae live in the marine environment, especially in cool temperate waters located in both the Northern and Southern Hemispheres [68][69]. Fucans and alginates are the specific polysaccharides compounds, which can be found in the cell wall of brown algae [66]. Generally, brown algae consist of three distinctly recognizable parts–the holdfast, stipe, and leaf-like blades [70]. The holdfast is a root-like structure at the bottom, which is often joined by a stipe to one or more leaf-like blades depending on the species. The blades serve as the primary surface for important processes including photosynthesis and nutrient exchange in the algae [71][72]. Although photosynthesis takes place predominantly in the blades, it is crucial that the stipe has the adequate length to place the blades sufficiently close to the light source. Alternatively, algae can absorb sufficient light by swelling the body (thallus) or increasing their growth rate [73]. The photosynthetic pigments in brown algae are chlorophylls a and c, and their accessory pigments include carotenoids and xanthophylls [74] (Table 1). Fucoxanthin contains brown-coloured pigment and the unique xanthophyll in brown algae which gives them their characteristic dark colour [75]. Unlike red algae, most of the brown algae have two flagella which help them achieve locomotion [76]. Some examples of brown algae include the rockweeds (Ascophyllum spp. and Fucus spp.) and the giant kelps (Macrocystis sp.). These algae usually contain laminarin and mannitol, storage sugars which can be fermented to make alcohol [77]. Some brown algae possess the ability to take up certain important substances from seawater. For instance, the iodine concentration in an edible kelp, kombu, can be thousands of times as great in the cells of the species as in its surrounding water [78].
2.3. Green Algae (Division Chlorophyta)
On the other hand, green algae or the Chlorophytes are generally more closely related to the higher plants in comparison to brown and red algae, in particular their chloroplast structure [79][80]. The cell walls of most species of green algae are built mainly by cellulose, with some incorporation of glycans (hemicelluloses) [81]. Their photosynthetic pigments in the chloroplast are chlorophylls a and b, while their accessory pigments are carotenoids and xanthophylls, found in embryophytes [81] (Table 1). Green algae comprise of 9000 to 12,000 species, with the majority of them occurring in freshwater rather than the marine environments [80][81]. Most green algae are microscopic, except for a small number of species in some specific genera such as those in Cladophora which are multicellular and macroscopic [81][82][83]. The unicellular genera Chlamydomonas and Chlorella are some common examples of green algae in both marine and freshwater ecosystems worldwide, which consist of species that disperse in a wide range of habitats [84]. An example of more complex green algae includes Volvox, which forms large hollow-spherical colonies that consist of thousands of cells [85]. The green algae Ulva spp., Caulerpa spp., Enteromorpha spp., and Codium spp. are commonly used as a food source for humans. The Ulva spp., known generally as sea lettuce, are extensively consumed in many Asian countries especially in Japan, China, and the Republic of Korea [80][86]. Access to nitrogen is one of the major limiting factors in the growth of green algae on the grounds that most of them thrive in shallow water [87]. Nevertheless, the increased runoff of fertilizer-related nitrogen into the oceans, mainly from agriculture has created favourable conditions for the growth of green algae and also other groups of algae in the past few decades [88]. According to Lee (2018), the majority of green algae form zoogametes, which are motile flagellated gametes [20]. The review by Moreira et al. (2021) details how macroalgae from various divisions differ in their flagellal construction, orientation, and life cycle in general [83].
References
- Thuiller, W.; Richardson, D.M.; Midgley, G.F. Will climate change promote alien plant invasions? In Biological Invasions; Springer: Berlin, Germany, 2008; pp. 197–211.
- Seebens, H.; Essl, F.; Dawson, W.; Fuentes, N.; Moser, D.; Pergl, J.; Pyšek, P.; van Kleunen, M.; Weber, E.; Winter, M.; et al. Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Chang. Biol. 2015, 21, 4128–4140.
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Kühn, I.; Wild, J.; Arianoutsou, M.; Bacher, S.; Chiron, F.; Didžiulis, V.; Essl, F.; et al. Disentangling the role of environmental and human pressures on biological invasions across Europe. Proc. Natl. Acad. Sci. USA 2010, 107, 12157–12162.
- Richardson, D.M.; Holmes, P.M.; Esler, K.J.; Galatowitsch, S.M.; Stromberg, J.C.; Kirkman, S.P.; Pyšek, P.; Hobbs, R.J. Riparian vegetation: Degradation, alien plant invasions, and restoration prospects. Divers. Distrib. 2007, 13, 126–139.
- Seebens, H.; Gastner, M.T.; Blasius, B. The risk of marine bioinvasion caused by global shipping. Ecol. Lett. 2013, 16, 782–790.
- Didham, R.K.; Tylianakis, J.M.; Hutchison, M.A.; Ewers, R.M.; Gemmell, N.J. Are invasive species the drivers of ecological change? Trends Ecol. Evol. 2005, 20, 470–474.
- Gilbert, B.; Levine, J.M. Plant invasions and extinction debts. Proc. Natl. Acad. Sci. USA 2013, 110, 1744–1749.
- Winter, M.; Schweiger, O.; Klotz, S.; Nentwig, W.; Andriopoulos, P.; Arianoutsou, M.; Basnou, C.; Delipetrou, P.; Didžiulis, V.; Hejda, M.; et al. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl. Acad. Sci. USA 2009, 106, 21721–21725.
- Dong, L.J.; Yu, H.W.; He, W.M. What determines positive, neutral, and negative impacts of Solidago canadensis invasion on native plant species richness? Sci. Rep. UK 2015, 5, 16804.
- Stohlgren, T.J.; Rejmánek, M. No universal scale-dependent impacts of invasive species on native plant species richness. Biol. Lett. 2014, 10, 20130939.
- Salimi, P.A.; Creed, J.C.; Esch, M.M.; Fenner, D.; Jaafar, Z.; Levesque, J.C.; Montgomery, A.D.; Salimi, M.A.; Edward, J.K.P.; Raj, K.D.; et al. A review of the diversity and impact of invasive non-native species in tropical marine ecosystems. Mar. Biodivers. Rec. 2021, 14, 11.
- Grutters, B.M.C.; Roijendijk, Y.O.A.; Verberk, W.; Bakker, E. Plant traits and plant biogeography control the biotic resistance provided by generalist herbivores. Funct. Ecol. 2017, 31, 1184–1192.
- Parker, J.D.; Burkepile, D.E.; Hay, M.E. Opposing Effects of Native and Exotic Herbivores on Plant Invasions. Science 2006, 311, 1459–1461.
- Lyons, D.; Scheibling, R. Context-dependant survival of the invasive seaweed Codium fragile ssp. tomentosoides in kelp bed and urchin barren habitats off Nova Scotia. Aquat. Biol. 2008, 2, 17–27.
- Cebrian, E.; Ballesteros, E.; Linares, C.; Tomas, F. Do native herbivores provide resistance to Mediterranean marine bioinvasions? A seaweed example. Biol. Invasions 2010, 13, 1397–1408.
- Seastedt, T.R. Biological control of invasive plant species: A reassessment for the Anthropocene. New Phytol. 2015, 205, 490–502.
- Joshi, J.; Vrieling, K. The enemy release and EICA hypothesis revisited: Incorporating the fundamental difference between specialist and generalist herbivores. Ecol. Lett. 2005, 8, 704–714.
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176.
- Münzbergová, Z.; Skuhrovec, J. Data on Herbivore Performance and Plant Herbivore Damage Identify the Same Plant Traits as the Key Drivers of Plant–Herbivore Interaction. Insects 2020, 11, 865.
- Lee, R.E. Phycology; Cambridge University Press: Cambridge, UK, 2018; pp. 510–546.
- Behmer, S.T.; Simpson, S.J.; Raubenheimer, D. Herbivore foraging in chemically heterogeneous environments: Nutrients and secondary metabolites. Ecology 2002, 83, 2489–2501.
- Martinez, A.S.; Byrne, M.; Coleman, R.A. What and when to eat? Investigating the feeding habits of an intertidal herbivorous starfish. Mar. Biol. 2016, 163, 166.
- Senft, R.L.; Coughenour, M.B.; Bailey, D.W.; Rittenhouse, L.R.; Sala, O.; Swift, D.M. Large Herbivore Foraging and Ecological Hierarchies. BioScience 1987, 37, 789–799.
- Wahl, M.; Hay, M. Associational resistance and shared doom: Effects of epibiosis on herbivory. Oecologia 1995, 102, 329–340.
- Duarte, C.; Navarro, J.; Acuña, K.; Gómez, I. Feeding preferences of the sandhopper Orchestoidea tuberculata: The importance of algal traits. Hydrobiologia 2010, 651, 291–303.
- Johnson, J.S.; Clements, K.D.; Raubenheimer, D. The nutritional basis of seasonal selective feeding by a marine herbivorous fish. Mar. Biol. 2017, 164, 201.
- Taylor, D.I.; Schiel, D.R. Algal populations controlled by fish herbivory across a wave exposure gradient on southern temperate shores. Ecology 2010, 91, 201–211.
- Sagerman, J.; Enge, S.; Pavia, H.; Wikström, S.A. Low feeding preference of native herbivores for the successful non-native seaweed Heterosiphonia japonica. Mar. Biol. 2015, 162, 2471–2479.
- Schwartz, N.; Rohde, S.; Hiromori, S.; Schupp, P.J. Understanding the invasion success of Sargassum muticum: Herbivore preferences for native and invasive Sargassum spp. Mar. Biol. 2016, 163, 181.
- Thomas, M.B.; Reid, A.M. Are exotic natural enemies an effective way of controlling invasive plants? Trends Ecol. Evol. 2007, 22, 447–453.
- Chavanich, S.; Harris, L.G. The influence of macroalgae on seasonal abundance and feeding preference of a subtidal snail, lacuna vincta (montagu) (littorinidae) in the gulf of maine. J. Molluscan Stud. 2002, 68, 73–78.
- Molis, M.; Scrosati, R.A.; El-Belely, E.; Lesniowski, T.J.; Wahl, M. Wave-induced changes in seaweed toughness entail plastic modifications in snail traits maintaining consumption efficacy. J. Ecol. 2015, 103, 851–859.
- Pennings, S.C.; Siska, E.L.; Bertness, M.D. Latitudinal differences in plant palatability in Atlantic coast salt marshes. Ecology 2001, 82, 1344–1359.
- Rodríguez, A.; Clemente, S.; Hernández, J.C.; Brito, A.; García, I.; Becerro, M.A. Nutritional, structural and chemical defenses of common algae species against juvenile sea urchins. Mar. Biol. 2017, 164, 127.
- Sudatti, D.B.; Fujii, M.; Rodrigues, S.V.; Turra, A.; Pereira, R.C. Prompt induction of chemical defenses in the red seaweed Laurencia dendroidea: The role of herbivory and epibiosis. J. Sea Res. 2018, 138, 48–55.
- Ianora, A.; Boersma, M.; Casotti, R.; Fontana, A.; Harder, J.; Hoffmann, F.; Pavia, H.; Potin, P.; Poulet, S.A.; Toth, G. New trends in marine chemical ecology. Estuaries Coasts 2006, 29, 531–551.
- Nylund, G.M.; Enge, S.; Pavia, H. Costs and Benefits of Chemical Defence in the Red Alga Bonnemaisonia hamifera. PLoS ONE 2013, 8, e61291.
- Maschek, J.A.; Baker, B.J. The Chemistry of Algal Secondary Metabolism. In Algal Chemical Ecology; Springer: Berlin, Germany, 2008; pp. 1–24.
- Gislason, S. Air and Breathing; Environmed Research Inc.: Vancouver, BC, Canada, 2018; Volume 5.
- Greenbaum, E.; Guillard, R.R.L.; Sunda, W.G. Hydrogen and Oxygen Photoproduction by Marine Algae. Photochem. Photobiol. 1983, 37, 649–655.
- Souvorov, A.V. Marine Ecologonomics: The Ecology and Economics of Marine Natural Resources Management, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 1999; Volume 6.
- Arrigo, K.R. Carbon cycle: Marine manipulations. Nature 2007, 450, 491.
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using marine macroalgae for carbon sequestration: A critical appraisal. J. Appl. Phycol. 2010, 23, 877–886.
- Moreira, D.; Pires, J.C. Atmospheric CO2 capture by algae: Negative carbon dioxide emission path. Bioresour. Technol. 2016, 215, 371–379.
- Bocanegra, A.; Bastida, S.; Benedi, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and Nutritional and Cardiovascular-Health Properties of Seaweeds. J. Med. Food 2009, 12, 236–258.
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17.
- Rothäusler, E.; Macaya, E.; Molis, M.; Wahl, M.; Thiel, M. Laboratory experiments examining inducible defense show variable responses of temperate brown and red macroalgae. Rev. Chil. Hist. Nat. 2005, 78, 603–614.
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899.
- Wang, J.T.; Douglas, A.E. Essential amino acid synthesis and nitrogen recycling in an alga-invertebrate symbiosis. Mar. Biol. 1999, 135, 219–222.
- Douglas, A.E. Host benefit and the evolution of specialization in symbiosis. Heredity 1998, 81, 599.
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014.
- Frenkel, J.; Vyverman, W.; Pohnert, G. Pheromone signaling during sexual reproduction in algae. Plant J. 2014, 79, 632–644.
- Clifton, K.E.; Clifton, L.M. The Phenology of Sexual Reproduction by Green Algae (Bryopsidales) on Caribbean Coral Reefs. J. Phycol. 1999, 35, 24–34.
- Gantt, E.; Grabowski, B.; Cunningham, F.X. Antenna Systems of Red Algae: Phycobilisomes with Photosystem ll and Chlorophyll Complexes with Photosystem I. In Light-Harvesting Antennas in Photosynthesis; Springer: Dordrecht, The Netherlands, 2003; pp. 307–322.
- Masarin, F.; Cedeno, F.R.P.; Chavez, E.G.S.; De Oliveira, L.E.; Gelli, V.C.; Monti, R. Chemical analysis and biorefinery of red algae Kappaphycus alvarezii for efficient production of glucose from residue of carrageenan extraction process. Biotechnol. Biofuels 2016, 9, 122.
- Michel, G.; Helbert, W.; Kahn, R.; Dideberg, O.; Kloareg, B. The structural bases of the processive degradation of ι-carrageenan, a main cell wall polysaccharide of red algae. J. Mol. Biol. 2003, 334, 421–433.
- Usov, A.I. Polysaccharides of the red algae. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2011; Volume 65, pp. 115–217.
- Vreeland, V.; Kloareg, B. Cell wall biology in red algae: Divide and conquer. J. Phycol. 2000, 36, 793–797.
- Schubert, N.; García-Mendoza, E.; Pacheco-Ruiz, I. Carotrnoid composition of marine red algae. J. Phycol. 2006, 42, 1208–1216.
- Squires, A.H.; Moerner, W.E. Direct single-molecule measurements of phycocyanobilin photophysics in monomeric C-phycocyanin. Proc. Natl. Acad. Sci. USA 2017, 114, 9779–9784.
- Rioux, L.-E.; Beaulieu, L.; Turgeon, S.L. Seaweeds: A traditional ingredients for new gastronomic sensation. Food Hydrocoll. 2017, 68, 255–265.
- Trius, A.; Sebranek, J.G.; Lanier, T. Carrageenans and their use in meat products. Crit. Rev. Food Sci. Nutr. 1996, 36, 69–85.
- Blouin, N.A.; Brodie, J.A.; Grossman, A.C.; Xu, P.; Brawley, S.H. Porphyra: A marine crop shaped by stress. Trends Plant Sci. 2011, 16, 29–37.
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28.
- Fleurence, J.; Morançais, M.; Dumay, J. Seaweed proteins. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 245–262.
- Cock, J.M.; Sterck, L.; Rouzé, P.; Scornet, D.; Allen, A.; Amoutzias, G.; Anthouard, V.; Artiguenave, F.; Aury, J.-M.; Badger, J.H.; et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 2010, 465, 617–621.
- Westermeier, R.; Patiño, D.J.; Müller, H.; Müller, D.G. Towards domestication of giant kelp (Macrocystis pyrifera) in Chile: Selection of haploid parent genotypes, outbreeding, and heterosis. J. Appl. Phycol. 2009, 22, 357–361.
- Aven, J.A.R.; Johnston, A.M.; Kübler, J.E.; Orb, R.E.K.; Cinroy, S.G.M.; Andley, L.I.L.H.; Crimgeour, C.H.M.S.; Alker, D.I.W.; Beardall, J.; Layton, M.N.C.; et al. Seaweeds in Cold Seas: Evolution and Carbon Acquisition. Ann. Bot. 2002, 90, 525–536.
- Wei, N.; Quarterman, J.; Jin, Y.-S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77.
- Wernberg, T.; Thomsen, M.S. The effect of wave exposure on the morphology of Ecklonia radiata. Aquat. Bot. 2005, 83, 61–70.
- Koehl, M.A.R.; Silk, W.K.; Liang, H.; Mahadevan, L. How kelp produce blade shapes suited to different flow regimes: A new wrinkle. Integr. Comp. Biol. 2008, 48, 834–851.
- Stewart, H.L.; Carpenter, R.C. The Effects of Morphology and Water Flow on Photosynthesis of Marine Macroalgae. Ecology 2003, 84, 2999–3012.
- Toohey, B.; Kendrick, G.A.; Wernberg, T.; Phillips, J.C.; Malkin, S.; Prince, J. The effects of light and thallus scour from Ecklonia radiata canopy on an associated foliose algal assemblage: The importance of photoacclimation. Mar. Biol. 2004, 144, 1019–1027.
- Bidigare, R.R. Photosynthetic pigment composition of the brown tide alga: Unique chlorophyll and carotenoid derivatives. In Novel Phytoplankton Blooms; Springer: Berlin/Heidelberg, Germany, 1989; pp. 57–75.
- Maria, A.G.; Graziano, R.; Nicolantonio, D.O. Anti-Obesity Activity of the Marine Carotenoid Fucoxanthin. Mar. Drugs 2015, 13, 2196–2214.
- Fu, G.; Nagasato, C.; Oka, S.; Cock, J.M.; Motomura, T. Proteomics Analysis of Heterogeneous Flagella in Brown Algae (Stramenopiles). Protist 2014, 165, 662–675.
- Horn, S.J.; Aasen, I.M.; Østgaard, K. Production of ethanol from mannitol by Zymobacter palmae. J. Ind. Microbiol. Biotechnol. 2000, 24, 51–57.
- Amachi, S. Microbial Contribution to Global Iodine Cycling: Volatilization, Accumulation, Reduction, Oxidation, and Sorption of Iodine. Microbes Environ. 2008, 23, 269–276.
- Patron, N.J.; Keeling, P.J. Common evolutionary origin of starch biosynthetic enzymes in green and red algae1. J. Phycol. 2005, 41, 1131–1141.
- Kılınç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for Food and Industrial Applications; IntechOpen: London, UK, 2013.
- Lewis, L.A.; McCourt, R.M. Green algae and the origin of land plants. Am. J. Bot. 2004, 91, 1535–1556.
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648.
- Moreira, A.; Cruz, S.; Marques, R.; Cartaxana, P. The underexplored potential of green macroalgae in aquaculture. Rev. Aquac. 2021, 14, 5–26.
- Pinheiro, C.; Azevedo, J.; Campos, A.; Loureiro, S.; Vasconcelos, V. Absence of negative allelopathic effects of cylindrospermopsin and microcystin-LR on selected marine and freshwater phytoplankton species. Hydrobiologia 2012, 705, 27–42.
- Nozaki, H.; Mahakham, W.; Heman, W.; Matsuzaki, R.; Kawachi, M. A new preferentially outcrossing monoicous species of Volvox sect. Volvox (Chlorophyta) from Thailand. PLoS ONE 2020, 15, e0235622.
- García-Casal, M.N.; Ramirez, J.; Leets, I.; Pereira, A.C.; Quiroga, M.F. Antioxidant capacity, polyphenol content and iron bioavailability from algae (Ulva sp., Sargassum sp. and Porphyra sp.) in human subjects. Br. J. Nutr. 2008, 101, 79–85.
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636.
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloğlu, I.; et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452.
More
Information
Subjects:
Marine & Freshwater Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
16 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




