
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Bonelli | -- | 2643 | 2022-08-13 17:12:22 | | | |
| 2 | Peter Tang | Meta information modification | 2643 | 2022-08-16 05:49:25 | | |
Video Upload Options
The association between aortic stenosis (AS) and cardiac amyloidosis (CA) is more frequent than expected. Albeit rare, CA, particularly the transthyretin (ATTR) form, is commonly found in elderly people. ATTR-CA is also the most prevalent form in patients with AS. These conditions share pathophysiological, clinical and imaging findings, making the diagnostic process very challenging.
1. Introduction
|
Fibril Protein |
Precursor Protein |
Target Organs |
|---|---|---|
|
ATTR |
Transthyretin (wild-type or variant) |
Wild type: heart, carpal tunnel syndrome (bilateral), ligaments, lumbar spinal stenosis Variant (variable): heart, PNS, ANS, ligaments, lumbar spinal stenosis, leptomeningeal, eye, gastrointestinal tract |
|
AL |
Monoclonal immunoglobulin light chain |
Heart, PNS (no CNS), ANS, liver, lung, gastrointestinal tract, soft tissues (tongue), kidney, myopathy |
Legend: ANS: autonomic nervous system; CNS: central nervous system; PNS: peripheral nervous system.
2. General Features (Epidemiology, Pathophysiology)
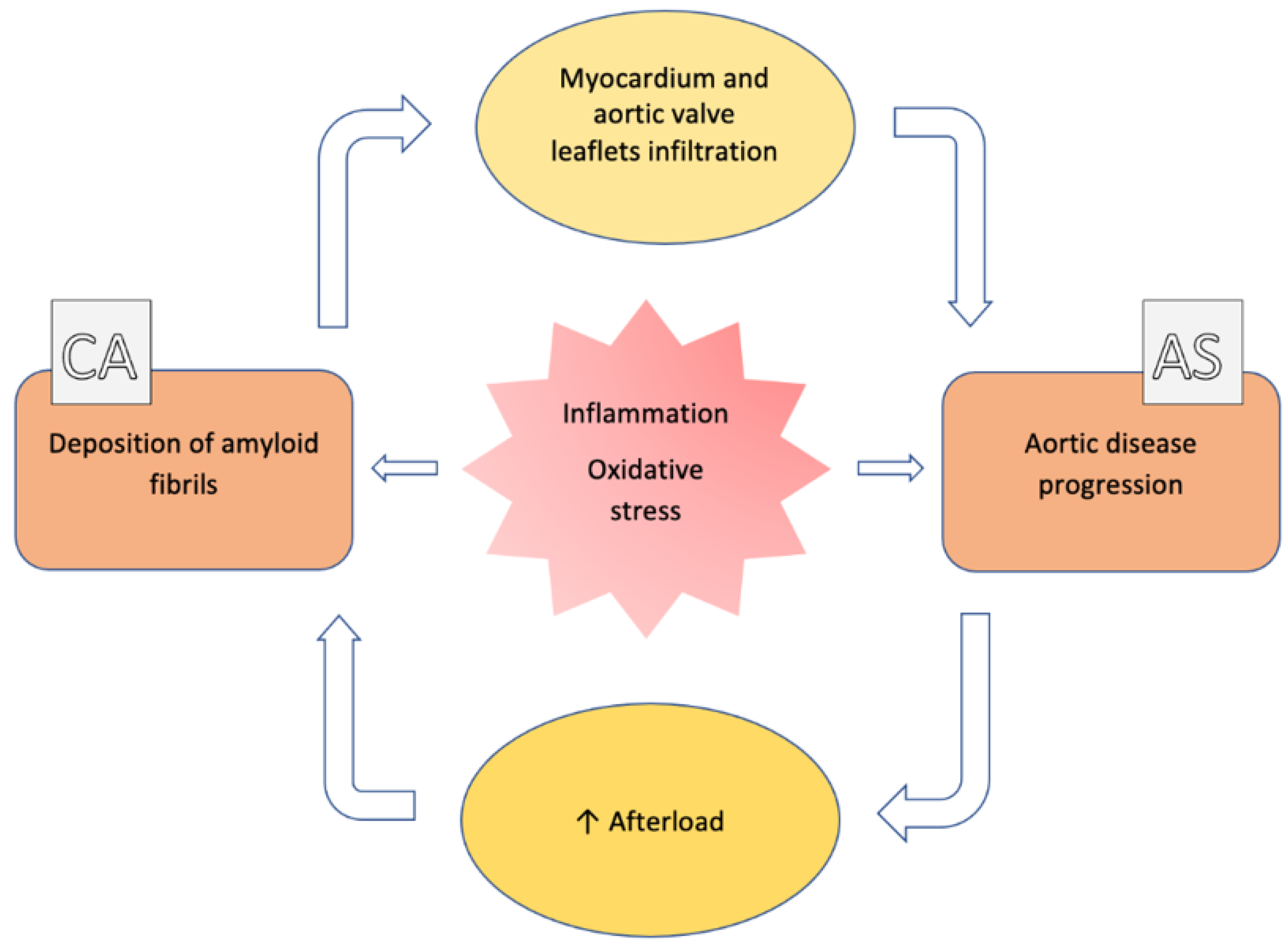
3. Clinical and Imaging Assessment
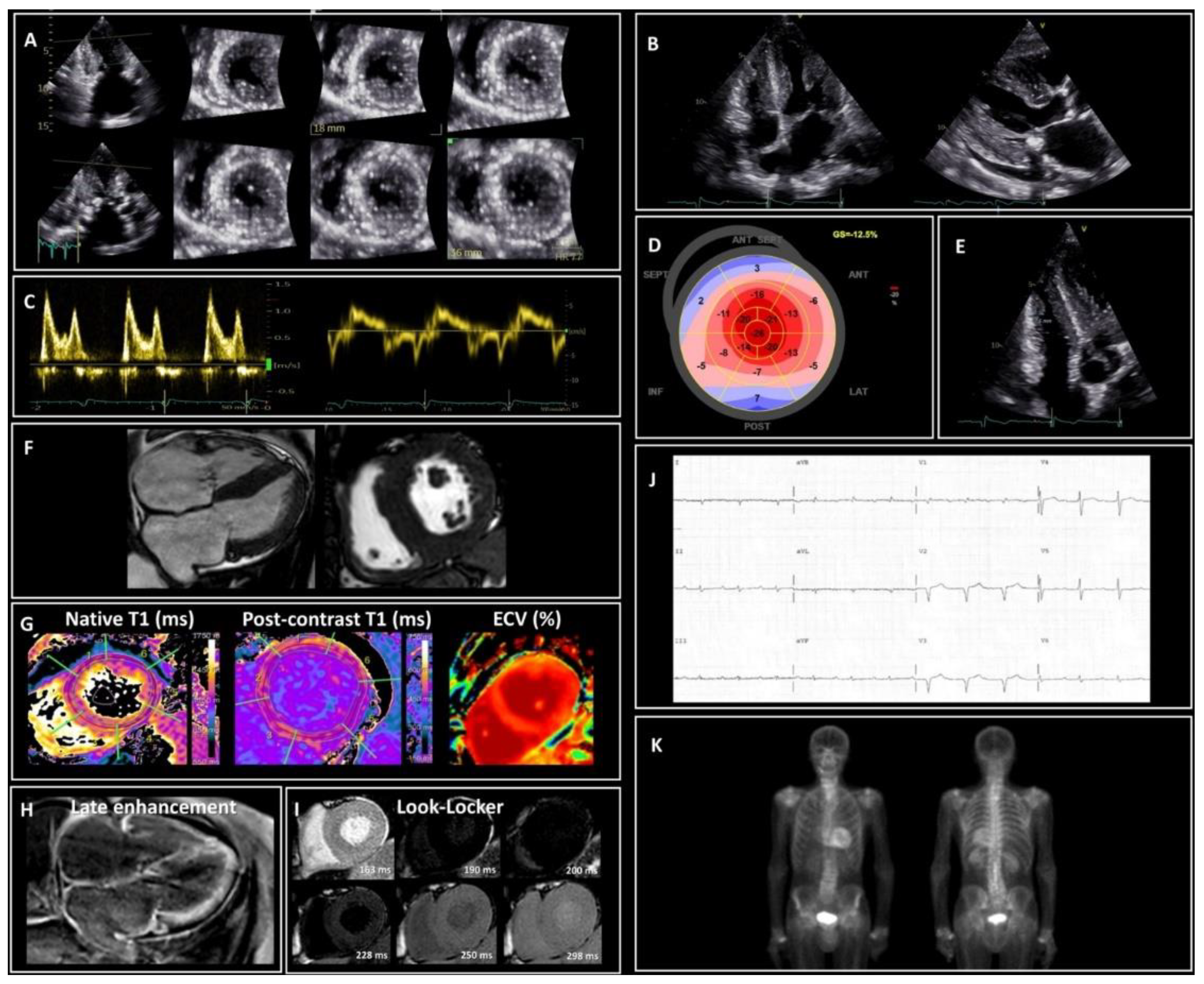
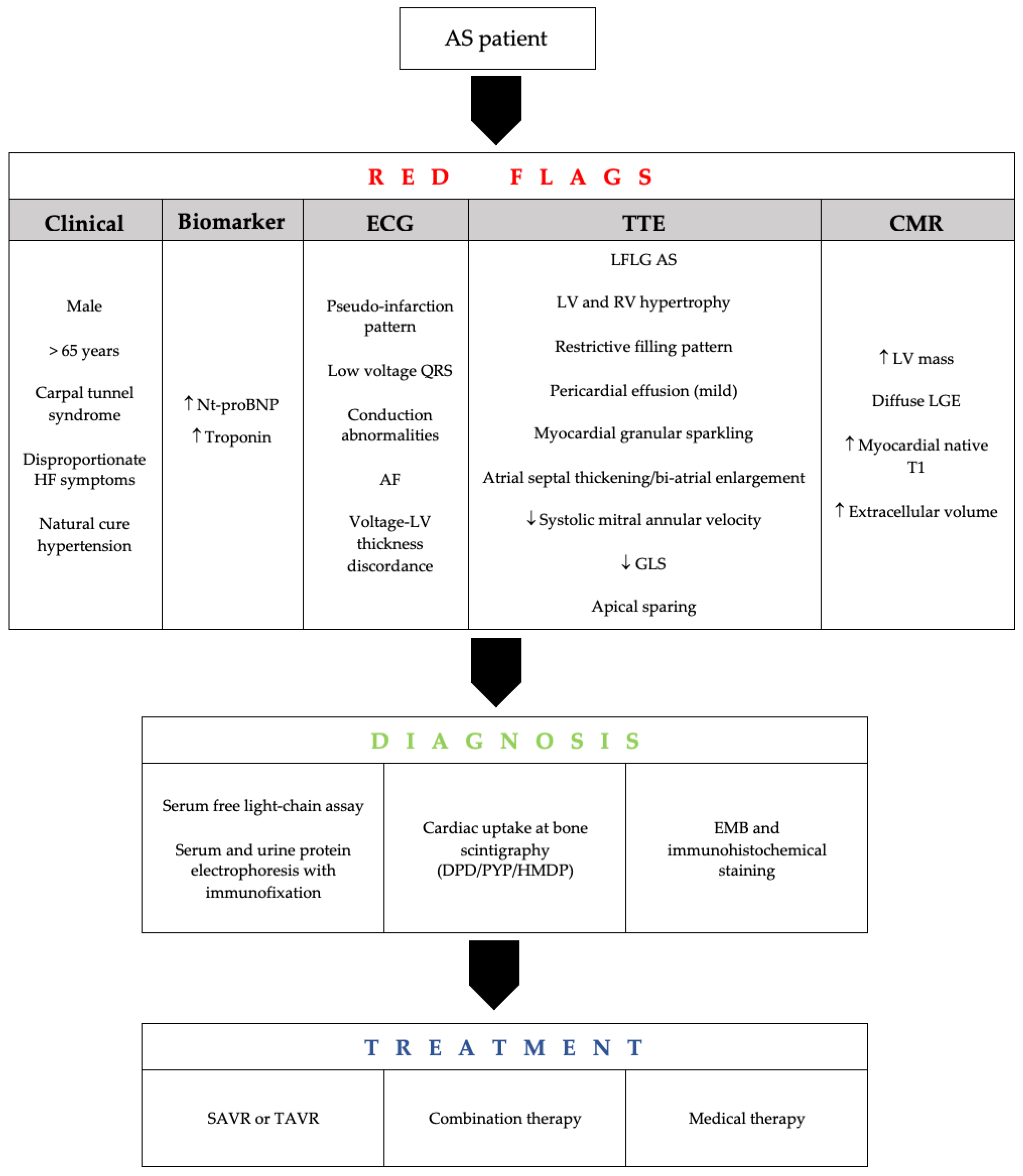
4. Screening and Predictors
5. Prognosis and Management
6. Conclusion
The combination of AS and CA, especially ATTR, is an important clinical problem. Its already high prevalence is destined to grow because of the aging population. However, current literature shows that CA is often underdiagnosed in old adults, resulting in underestimation of the AS-CA combination. Based on the available uncertainty of the clinical outcome of combined AS and CA, prospective multicenter studies in large cohorts are necessary to suggest an optimum road map for managing such patients.
References
- Coffey, S.; Cox, B.; Williams, M.J.A. The prevalence, incidence, progression, and risks of aortic valve sclerosis: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2014, 63, 2852–2861.
- Cornwell, G.G.; Murdoch, W.L.; Kyle, R.A.; Westermark, P.; Pitkänen, P. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am. J. Med. 1983, 75, 618–623.
- Merlini, G.; Bellotti, V. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 2003, 349, 583–596.
- Gertz, M.A.; Dispenzieri, A.; Sher, T. Pathophysiology and treatment of cardiac amyloidosis. Nat. Rev. Cardiol. 2015, 12, 91–102.
- Balciunaite, G.; Rimkus, A.; Zurauskas, E.; Zaremba, T.; Palionis, D.; Valeviciene, N.; Aidietis, A.; Serpytis, P.; Rucinskas, K.; Sogaard, P.; et al. Transthyretin cardiac amyloidosis in aortic stenosis: Prevalence, diagnostic challenges, and clinical implications. Hell J. Cardiol. HJC Hell Kardiol. Ep. 2020, 61, 92–98.
- Nitsche, C.; Scully, P.R.; Patel, K.P.; Kammerlander, A.A.; Koschutnik, M.; Dona, C.; Wollenweber, T.; Ahmed, N.; Thornton, G.D.; Kelion, A.D.; et al. Prevalence and Outcomes of Concomitant Aortic Stenosis and Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2021, 77, 128–139.
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L. American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e7–e22.
- Ternacle, J.; Krapf, L.; Mohty, D.; Magne, J.; Nguyen, A.; Galat, A.; Gallet, R.; Teiger, E.; Côté, N.; Clavel, M.A.; et al. Aortic Stenosis and Cardiac Amyloidosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2638–2651.
- Gargiulo, P.; Perrone-Filardi, P. Dangerous relationships: Aortic stenosis and transthyretin cardiac amyloidosis. Eur. Heart, J. 2017, 38, 2888–2889.
- Damy, T.; Judge, D.P.; Kristen, A.V.; Berthet, K.; Li, H.; Aarts, J. Cardiac findings and events observed in an open-label clinical trial of tafamidis in patients with non-Val30Met and non-Val122Ile hereditary transthyretin amyloidosis. J. Cardiovasc. Transl. Res. 2015, 8, 117–127.
- Falk, R.H.; Alexander, K.M.; Liao, R.; Dorbala, S. AL (Light-Chain) Cardiac Amyloidosis: A Review of Diagnosis and Therapy. J. Am. Coll. Cardiol. 2016, 68, 1323–1341.
- Dweck, M.R.; Boon, N.A.; Newby, D.E. Calcific aortic stenosis: A disease of the valve and the myocardium. J. Am. Coll. Cardiol. 2012, 60, 1854–1863.
- Joseph, J.; Naqvi, S.Y.; Giri, J.; Goldberg, S. Aortic Stenosis: Pathophysiology, Diagnosis, and Therapy. Am. J. Med. 2017, 130, 253–263.
- Zhao, L.; Buxbaum, J.N.; Reixach, N. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry 2013, 52, 1913–1926.
- Henderson, B.C.; Tyagi, N.; Ovechkin, A.; Kartha, G.K.; Moshal, K.S.; Tyagi, S.C. Oxidative remodeling in pressure overload induced chronic heart failure. Eur. J. Heart Fail. 2007, 9, 450–457.
- Kristen, A.V.; Schnabel, P.A.; Winter, B.; Helmke, B.M.; Longerich, T.; Hardt, S.; Koch, A.; Sack, F.U.; Katus, H.A.; Linke, R.P.; et al. High prevalence of amyloid in 150 surgically removed heart valves--a comparison of histological and clinical data reveals a correlation to atheroinflammatory conditions. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2010, 19, 228–235.
- Yingchoncharoen, T.; Agarwal, S.; Popović, Z.B.; Marwick, T.H. Normal ranges of left ventricular strain: A meta-analysis. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2013, 26, 185–191.
- Porciani, M.C.; Lilli, A.; Perfetto, F.; Cappelli, F.; Rao, C.M.; Del Pace, S.; Ciaccheri, M.; Castelli, G.; Tarquini, R.; Romagnani, L.; et al. Tissue Doppler and strain imaging: A new tool for early detection of cardiac amyloidosis. Amyloid. Int. J. Exp. Clin. Investig. Off. J. Int. Soc. Amyloidosis 2009, 16, 63–70.
- Koyama, J.; Ray-Sequin, P.A.; Falk, R.H. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation 2003, 107, 2446–2452.
- Treibel, T.A.; Fontana, M.; Gilbertson, J.A.; Castelletti, S.; White, S.K.; Scully, P.R.; Roberts, N.; Hutt, D.F.; Rowczenio, D.M.; Whelan, C.J.; et al. Occult Transthyretin Cardiac Amyloid in Severe Calcific Aortic Stenosis: Prevalence and Prognosis in Patients Undergoing Surgical Aortic Valve Replacement. Circ. Cardiovasc. Imaging 2016, 9, e005066.
- Galat, A.; Guellich, A.; Bodez, D.; Slama, M.; Dijos, M.; Zeitoun, D.M.; Milleron, O.; Attias, D.; Dubois-Randé, J.L.; Mohty, D.; et al. Aortic stenosis and transthyretin cardiac amyloidosis: The chicken or the egg? Eur. Heart J. 2016, 37, 3525–3531.
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2872–2891.
- Castaño, A.; Narotsky, D.L.; Hamid, N.; Khalique, O.K.; Morgenstern, R.; DeLuca, A.; Rubin, J.; Chiuzan, C.; Nazif, T.; Vahl, T.; et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. 2017, 38, 2879–2887.
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412.
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Reggiani, M.L.B.; et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084.
- Hutt, D.F.; Quigley, A.-M.; Page, J.; Hall, M.L.; Burniston, M.; Gopaul, D.; Lane, T.; Whelan, C.J.; Lachmann, H.; Gillmore, J.D.; et al. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur. Heart J. Cardiovasc. Imaging. 2014, 15, 1289–1298.
- Dorbala, S.; Cuddy, S.; Falk, R.H. How to Image Cardiac Amyloidosis: A Practical Approach. JACC Cardiovasc. Imaging 2020, 13, 1368–1383.
- Nitsche, C.; Aschauer, S.; Kammerlander, A.A.; Schneider, M.; Poschner, T.; Duca, F.; Binder, C.; Koschutnik, M.; Stiftinger, J.; Goliasch, G.; et al. Light-chain and transthyretin cardiac amyloidosis in severe aortic stenosis: Prevalence, screening possibilities, and outcome. Eur. J. Heart Fail. 2020, 22, 1852–1862.
- Scully, P.R.; Patel, K.P.; A Treibel, T.; Thornton, G.D.; Hughes, R.K.; Chadalavada, S.; Katsoulis, M.; Hartman, N.; Fontana, M.; Pugliese, F.; et al. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. Eur. Heart J. 2020, 41, 2759–2767.
- Carroll, J.D.; Gaasch, W.H.; McAdam, K.P. Amyloid cardiomyopathy: Characterization by a distinctive voltage/mass relation. Am. J. Cardiol. 1982, 49, 9–13.
- Longhi, S.; Lorenzini, M.; Gagliardi, C.; Milandri, A.; Marzocchi, A.; Marrozzini, C.; Saia, F.; Ortolani, P.; Biagini, E.; Guidalotti, P.L.; et al. Coexistence of Degenerative Aortic Stenosis and Wild-Type Transthyretin-Related Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2016, 9, 325–327.
- Cavalcante, J.L.; Rijal, S.; Abdelkarim, I.; Althouse, A.D.; Sharbaugh, M.S.; Fridman, Y.; Soman, P.; Forman, D.E.; Schindler, J.T.; Gleason, T.G.; et al. Cardiac amyloidosis is prevalent in older patients with aortic stenosis and carries worse prognosis. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2017, 19, 98.
- Salinger, T.; Hu, K.; Liu, D.; Herrmann, S.; Lorenz, K.; Ertl, G.; Nordbeck, P. Cardiac amyloidosis mimicking severe aortic valve stenosis—A case report demonstrating diagnostic pitfalls and role of dobutamine stress echocardiography. BMC Cardiovasc. Disord. 2017, 17, 86.
- Salinger, T.; Hu, K.; Liu, D.; Herrmann, S.; Lorenz, K.; Ertl, G.; Nordbeck, P. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: Results of an international registry study. J. Am. Coll. Cardiol. 2014, 64, 1202–1213.
- Sperry, B.W.; Jones, B.M.; Vranian, M.N.; Hanna, M.; Jaber, W.A. Recognizing Transthyretin Cardiac Amyloidosis in Patients with Aortic Stenosis: Impact on Prognosis. JACC Cardiovasc. Imaging 2016, 9, 904–906.
- Java, A.P.; Greason, K.L.; Dispenzieri, A.; Grogan, M.; King, K.S.; Maleszewski, J.J.; Daly, R.C.; Eleid, M.F.; Pochettino, A.; Schaff, H.V. Aortic valve replacement in patients with amyloidosis. J. Thorac. Cardiovasc. Surg. 2018, 156, 98–103.




