Lipids, in addition to providing energy, texture, and mouthfeel, play an important role in the odor and flavor development of food. This could be due to the ability of lipids to generate odors and flavors, act as precursors of odor and flavor compounds, or modify the odor and flavor of other components
[1]. Lipids are responsible for both the undesirable and desirable flavors of food; the oxidation of lipids mainly results in the development of off-flavor and lipoxygenase-derived lipid-based volatiles that are responsible for flavor generation. Food flavor is the manifestation of interactions between aroma, taste, and oral sensations, where the aroma is linked with mostly volatile compounds, and taste is associated with non-volatile high-molecular-weight components
[2]. Generally, raw foods, mainly meat, provide little aroma and have a mild taste, but this can change to a strong aroma and taste upon processing. For example, odorants of raw and cooked sheep meat have been reported to be due to
cis-1,5-octadien-3-one (geranium-like), 4-ethyloctanoic acid (mutton-like),
trans-4,5-epoxy-2-decenal (metallic), and
trans-2,4-decadienal (deep-fried). Moreover, flavor dilution (FD) factors of aroma compounds such as 2-acetyl-1-pyrroline, 4-hydroxy-2,5-dimethyl-3(2
H)-furanone, and 2-aminoacetophenone were clearly increased upon cooking
[3]. However, aquatic foods are slightly different as they have a comparatively strong flavor in their raw state. This is because of the presence of amines, lipoxygenase-derived lipid-based volatile components, and the development of secondary oxidation products in the raw stage
[4][5]. On the other hand, lipids with low and high volatility are almost odorless and tasteless, respectively, partly because of their non-polar nature.
Due to the lipid oxidation in food, an off-flavor is developed, and some bioactive compounds and fat-soluble vitamins are lost. The oxidation of unsaturated fatty acids is a complex process that can occur in the presence of oxygen or via non-enzymatic (autoxidation and photooxidation) and enzymatic (lipoxygenase) pathways. Lipid oxidation and the development of off-flavor compounds are catalyzed by light, heat, oxygen, photosensitizers, and transition metal ions (e.g., Fe
2+ and Cu
2+). Autoxidation occurs in the presence of triplet oxygen (
3O
2), while photooxidation happens in the presence of singlet oxygen (
1O
2)
[6]. In autoxidation, primary oxidation products (e.g., hydroperoxides) are formed, and their subsequent break down into volatile secondary lipid oxidation products such as ketones, alcohols, and aldehydes, among others, are believed to play the main role in the development of an off-flavor. Due to the multiple methylene-interrupted
cis-double bonds, polyunsaturated fatty acids (PUFAs) are highly prone to oxidation. For example, edible oils and fish lipids are rich in omega-3 fatty acids, where propanal and acrolein act as good indicators for assessing the degree of oxidation. Similarly, meat and meat products are rich in omega-6 fatty acids, and hexanal serves as a reliable indicator for flavor deterioration
[7][8]. Moreover, heterocyclic compounds (e.g., pyrazines, oxazoles, pyridines, and thiazole) are formed mainly via non-enzymatic browning reactions, which generally play a key and desirable role in flavor generation
[9]. On the other hand, upon thermal processing and heating experienced during frying, cooking, and grilling, hundreds of compounds can possibly be generated from lipid degradation, Maillard reactions, or Strecker degradation, which contribute to the overall desirable aroma upon interacting with each other. This could occur via forming a wide range of new volatile products upon interaction or entirely or partially blocking the presence of volatiles from others
[10]. For instance, at both the initial and later stages of thermal processing, aldehydes that are produced upon the thermal degradation of lipids could join in the Maillard reactions
[11]. In addition, the thermal generation of the oxidation products of lipids may be accompanied by the production of polar lipids and polymers that have no real effect on flavor
[12].
2. Role of Lipids Oxidation in Flavor or Off-Flavor Development
Lipids are susceptible to oxidation, which is believed to play the main non-microbial role in the quality degradation of food and food products including meat, fish, and oil-based products. Usually, off-flavors are linked very closely to lipids compared to proteins and carbohydrates. Lipid oxidation not only lessens the nutritional benefits of products due to the changes in essential fatty acids and vitamins but also negatively affects the sensory qualities such as flavor, texture, and color, which impacts on the overall consumer acceptance
[6][13]. However, in some cases, lipid oxidation promotes the development of pleasant aromas, mainly during the ripening or dry-cured stages of meat products, which is one of the quality parameters of these products
[14]. Lipid oxidation is a very complex phenomenon that undergoes a variety of reactions and produces a wide range of compounds. In a nutshell, fatty acids, mainly PUFAs, react with molecular oxygen and produce primary oxidation products (hydroperoxides) via free radical mechanisms. These compounds are highly unstable due to a weak oxygen–oxygen bond and are believed to make no contribution to odor and aroma development, but they break down rapidly and produce a wide variety of secondary constituents, which are responsible for the formation of off-flavors
[15]. However, not all of these constituents similarly contribute to the flavor profiles, since the overall aroma perception is mainly dependent on the olfactory threshold, concentration, and type of products. Among these compounds, aldehydes seem to be the major compounds contributing to flavor development due to their low odor threshold and higher concentration. The most common aldehydes generated from lipid oxidation are
n-alkanals, 4-hydroxy-2-alkenals, 2-alkenals, and malondialdehyde (MDA)
[14]. Hence, lipid oxidation can occur in various pathways including autoxidation, photooxidation, thermal oxidation, and enzyme-catalyzed oxidation. Autoxidation is a very common lipid oxidation pathway, occurring through a continuous free-radical chain reaction. However, hydroperoxides are formed during the initiation stage of autoxidation, which is the main difference between the mechanisms of photo and enzymatic oxidation
[12].
2.1. Formation of Oxidation Products via Autoxidation
Autoxidation is a combination of three different phases, namely, initiation, propagation, and termination. These steps are mainly responsible for producing radicals, followed by the multiplication of reactive compounds, finally degrading or reacting with each other in order to produce non-reactive products
[16]. Autoxidation is the spontaneous oxidation of lipids in the presence of oxygen, mainly
3O
2, which can interact with radical species due to its diradical nature. However, the interaction between unsaturated fatty acids (singlet electronic state) and oxygens (triplet electronic state) is not possible because of their chemical natures. Moreover, the triplet oxygen (
3O
2) cannot change its state to singlet states (
1O
2) without external support. Therefore, an initiator is necessary to convert
3O
2 into
1O
2, or reactive oxygen species (ROS) such as hydroxyl radicals, hydrogen peroxide (H
2O
2), superoxide anions, or to generate a radical by removing an electron from the unsaturated lipids
[6][14]. The activation of oxygen mainly occurs in the presence of light, temperature, or metal ions such as Fe
2+ and Cu
2+. At the end of the propagation stage, primary oxidation products such as lipid hydroperoxides and conjugated dienes/trienes are produced and these further break down into a series of secondary oxidation products such as aldehydes, ketones, alcohols, hydrocarbons, epoxy compounds, and volatile organic acids. Among them, some compounds are responsible for the off-flavor at very low threshold values
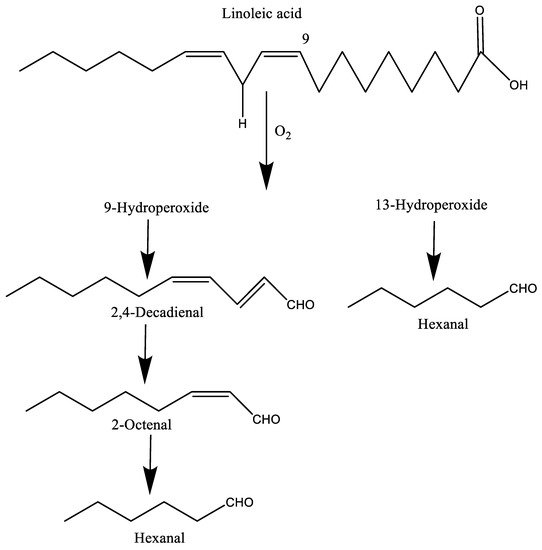
[12]. For example, linoleic acid produces 9- and 13-hydroperoxides in the presence of oxygen, and finally generates various volatiles including 2,4-decadienal, 2-octenal, and hexanal, among others, due to the cleavage of the C–O bond by alkoxy radicals derived from these hydroperoxides (
Figure 1). Similarly, oleic acid forms 8-, 9-, 10-, and 11-hydroperoxides, which leads to many secondary compounds such as pentanal, octanal, hexanal, and heptanal, among others
[9][17].
Figure 1. The formation of some volatiles from the autoxidation of linoleic acid.
2.2. Formation of Oxidation Products via Photooxidation
A possible route for lipid-derived flavor generation is lipolysis, which raises the level of free fatty acids (FFAs). The FFAs may then be oxidized via various oxidation mechanisms and produces hydroperoxides, and further decompose to a series of volatile flavor compounds
[18]. Photooxidation is much faster than autoxidation, occurring in the presence of singlet oxygen (
1O
2), which can be generated by ultraviolet (UV) light, and/or photosensitizers including riboflavin, chlorophyll, and myoglobin. Singlet oxygen is more reactive than triplet oxygen due to its higher electrophilicity. For instance, the interaction between
1O
2 and linoleic acid is about 1500 times faster than that with
3O
2 [19]. The photooxidation also produces hydroperoxides in many ways, where excited triplet sensitizers (e.g., myoglobin and hemoglobin) react with molecular oxygen (
3O
2) and produce
1O
2. Subsequently, hydroperoxides are formed upon interactions between
1O
2 and double bonds of unsaturated fatty acids without forming alkyl radical
[20]. Moreover, ROS such as superoxide radical anion can be formed when the sensitizer interacts with
3O
2, resulting in lipid oxidation due to the abstraction of hydrogen atoms from unsaturated fatty acids. In addition, hydroxyl radicals and
1O
2 produced from the interaction between superoxide radical anions and H
2O
2 can initiate lipid oxidation in the presence of metal ions. Furthermore, alkyl radicals produced from the reaction of fatty acids and sensitizers can react with molecular oxygen and produce peroxyl radicals, initiating oxidation via a free radical chain mechanism
[14]. Finally, primary oxidation products such as various hydroperoxides including 9- and 10-hydroperoxides for oleic acid, 9-, 10-, 12-, 13-, 15-, and 16-hydroperoxides for linolenic acid, and 9-, 10-, 12-, and 13-hydroperoxides for linoleic acid are formed, which lead to a series of volatile compounds
[6]. The formation of these hydroperoxides mainly depends on the nature of the fatty acids involved in the oxidation reaction.
2.3. Formation of Oxidation Products via Enzymatic Oxidation
Lipoxygenase, the main enzyme involved in enzymatic oxidation, is found abundantly in various species of plants, animals, and fish. It is a globular protein comprising a single polypeptide chain with a molecular mass of 75–80 kDa in animals and 94–104 kDa in plants. Lipoxygenase plays an important role in the degradation of unsaturated fatty acids, resulting in flavor generation. Lipoxygenase-mediated reactions produce the aromas of freshly harvested fish through the production of both volatile alcohols and carbonyl compounds
[21]. For instance, lipoxygenase in fish can react with fatty acids and produces
cis-4-heptenal and 2,4,7-decatrienal isomers, which are responsible for a fishy aroma
[15]. The rate of enzyme-catalyzed lipid oxidation mainly depends on the concentration of enzymes, which is proportional to the oxidation
[14]. Lipoxygenase produces conjugated hydroperoxides through oxidation when the active site of the enzyme abstracts a hydrogen atom from the methylene group of fatty acids. In order to exhibit activity, the active site of the enzyme containing iron needs to be in the ferrous form
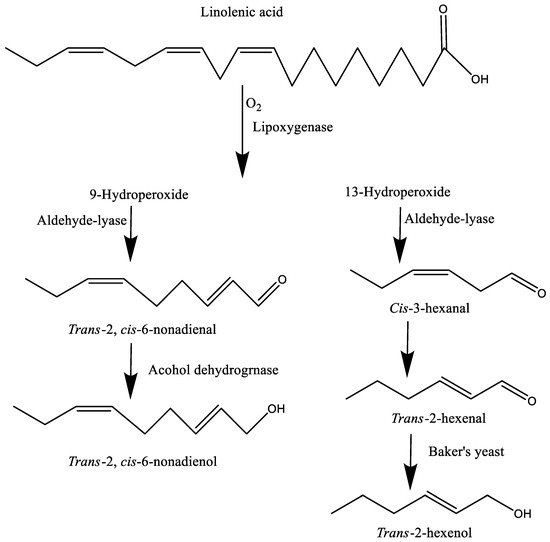
[22]. In short, lipase catalyzes the first step of the lipid by hydrolysis and produces free fatty acids, followed by the production of conjugated hydroperoxy fatty acids through lipoxygenase and finally, the formation of volatiles such as carbonyl compounds. In plants, 9- and 13-hydroperoxy-octadecadienoates are transformed into reactive intermediates by hydroperoxide dehydrases, hydroperoxide lyases, epoxide hydrolases, and hydroperoxide epoxygenase
[21][23]. For example, the oxidation of linolenic acid by a lipoxygenase and a subsequent lyase cleavage reaction produces
trans-2,
cis-6-nonadienal in cucumbers, and
trans-2-hexenal in fresh tomatoes. Moreover, these carbonyl compounds may further degrade into alcohol (e.g.,
trans-2,
cis-6-nonadienol and
trans-2-hexenol), which provides a stronger aroma than the precursor of carbonyls
[15] (
Figure 2). Apart from lipoxygenase, alcohol dehydrogenase, hydroperoxide lyase, and 3Z, 2E (3
cis, 2
trans) enal isomerase are also involved in the biosynthetic pathway. For instance, hydroperoxy fatty acids further break down into C6 or C9 volatiles such as 3-hexenal and 3,6-nonadienal, respectively, in the presence of hydroperoxide lyase
[6].
Figure 2. The formation of carbonyls and alcohols from linolenic acid.
In addition to the enzymatic and non-enzymatic pathways, there is a heat-mediated approach that also initiates lipid oxidation. The thermal oxidation of frying oil is the effect of the development of a complex form of the decomposition of fatty acids. The formation of cyclic and dimeric compounds leads to the thermolytic reaction in unsaturated fatty acids. Even saturated fatty acids become oxidized when heated at a high temperature (~150 °C) and produce
n-alkanols,
n-alkanes, lactones, 2-alkanones, 1-alkenes, and carboxylic acid
[24].
Several analytical techniques have been used to identify and quantify lipid oxidation products. Generally, techniques that are used to measure the changes in primary oxidation products are changes in the fatty acid contents, peroxide value (PV) (e.g., iodometric titration and ferric-xylenol orange), and conjugated dienes/trienes, while the thiobarbituric acid reactive substances (TBARS) assay, p-anisidine value, TOTOX value (2 PV + p-anisidine), and volatile measurements using GC-MS are used to determine secondary oxidation products. Both primary and secondary oxidation products are recommended to be measured for better reliability regarding the state of oxidation.
3. Flavor Chemistry: Lipid Participation in Maillard Interaction
The Maillard reaction involves a series of complex chemical reactions between the carbonyls of reducing sugars and proteins, mainly primary or secondary amines, which is responsible for the browning of food and its distinctive flavor
[25]. The Maillard reaction develops a wide range of chemical constituents belonging to oxazole, thiophene, furan, thiazole, pyrrole, pyrazine, and pyridine, among others. Generally, compounds such as peptides, amino acids, thiamine, carbohydrates, nucleotides, and lipids in food promote the development of flavor. Hence, the generation of aroma volatiles is basically related to the break down and oxidation of lipids, the Maillard reaction, and the degradation of vitamins, mainly thiamine
[11]. For example, the Maillard reaction between ribose and cysteine produces sulfur-containing compounds including thiophan-3-thiol and furan-3-thiol, which are responsible for the desirable meaty aroma of cooked meat
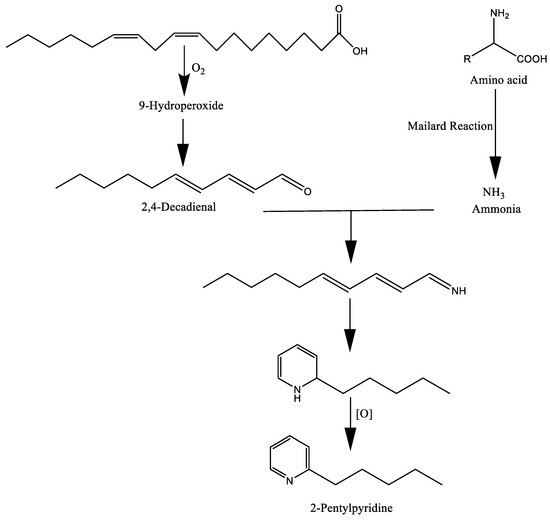
[9]. In particular, the products of these reactions can act as precursors and interact with other degradation constituents of food and produce a number of long-chain heterocyclic compounds during cooking. For instance, Henderson and Nawar
[26] reported the formation of 2-pentylpyridine through the interaction of lipid (linoleic acid) and Maillard reaction by-products (
Figure 3). Amadori products are produced during the first stages of the reaction via glycosylamine because of the interaction between the carbonyls of reducing sugars and primary amines of amino acids, peptides, or other components. The formation of Strecker aldehydes through the degradation of amino acids and lipid oxidation is another example of developing aroma compounds
[27]. These compounds react with Maillard reaction-derived carbonyls and form intermediates, which further break down into flavor compounds. Apart from amino acids, phenolic compounds may promote the generation of Strecker aldehydes. For example, amino acids and quinones originating from phenolics could produce flavor precursor volatile aldehydes through Strecker degradation
[28]. The same study also reported that amino acids (e.g., phenylalanine and methionine) were capable of interacting with phenolic compounds (e.g., chlorogenic acid, caffeic acid, epicatechin, and catechin) and developing Strecker aldehydes (e.g., phenylacetaldehyde and methional) in a ferricyanide-based model system.
Figure 3. The formation of 2-pentylpyridine by lipid–Maillard interaction.
Numerous sugar dehydration and degradation compounds such as dicarbonyl compounds, furanone and furfural derivatives, and hydroxy ketones are developed by the dehydration and rearrangement of the subsequent compounds
[6][29]. However, the formation of Maillard reaction products depends on the moisture content, cooking temperature and time, pH, and the nature of the reactants involved. The reaction rate increases markedly with the temperature at low moisture levels, and the production of flavor compounds is linked with areas of the food dehydrated by heat. Moreover, the formation of Maillard reaction products is affected by the presence of a discrete non-polar environment and the reactants’ location. For example, the interaction between lipid oxidation and Maillard reaction products was examined in a model system of oil-in-water emulsion containing canola oil, water, glucose, phenylalanine, and a surfactant (Tween 20)
[30]. It was found that the development of Maillard reaction products was 16 times greater in the emulsion system than in the aqueous solution.
Maillard reaction produces a myriad of components that are involved in flavor generation alone or with the lipid oxidation products. The interaction of these products produces new volatile compounds in some cases, or partially/wholly blocks other compounds
[10]. For instance, aldehydes generated from lipid oxidation could participate in the initial and later stages of the Maillard reaction during cooking to form volatiles including pyrazines, thiophenes, pyridines, oxazoles, and thiazoles with alkyl side chains
[11][31]. Several thiazoles and thiophenes such as 2-alkyl-3-thiazolines, 2-alkylthiazoles, and 2-alkylthiophenes have been reported in roasted meat. Particularly, carbonyls and sulfur compounds derived from ribose and cysteine are the major precursors to the flavor of meat. It has been reported that phospholipid oxidation produced 2,4-decadienal, which participated in the Maillard reaction and developed 2-alkyl heterocyclic products
[9]. Usually, volatiles obtained from the combination lipid–Maillard reaction exhibit a higher odor threshold than those developed from its parent reaction, resulting in a weak odor intensity from the lipid–Maillard by-products. However, it may modify the aroma profiles obtained from this complex system and provide an indirect impact on the aroma compounds
[10][11].