Environmental pollution deriving from anthropogenic activities is nowadays a serious problem that afflicts our planet and that cannot be neglected. Nanotechnologies and new performing nanomaterials, thanks to their unique features, such as high surface area (surface/volume ratio), catalytic capacity, reactivity and easy functionalization to chemically modulate their properties, represent potential for the development of sustainable, advanced and innovative products/techniques for environmental (bio)remediation. Metal nanoparticles (MNPs; related to metals or noble metals such as M = Pt, Pd, Ni, Ru, Al, Ag, Au, Cu) are nanomaterials with physical and chemical properties that differ from bulk materials due to their small size and high surface-to-volume ratio.
1. Properties of Metal Nanoparticles (NPs)
Metal nanoparticles (MNPs; related to metals or noble metals such as M = Pt, Pd, Ni, Ru, Al, Ag, Au, Cu) are nanomaterials with physical and chemical properties that differ from bulk materials due to their small size and high surface-to-volume ratio. One of the most important characteristics of MNPs is their antibacterial properties and bacterial killing capacity, that occur through different mechanisms such as production of reactive oxygen species, ATP depletion, damage to biomolecules, cation release, and membrane interaction
[1]. MNPs have also important optical and electrical properties (extinction, absorption, Rayleigh scattering, Raman scattering, Plasmon Resonances)
[2][3] and catalytic properties with a reactivity influenced by the particle size, geometry, composition, oxidation state, and chemical/physical environment
[4]. These characteristics allow these nanomaterials to find applications in various sectors such as nanocatalysis, sensing and biosensing, smart textiles
[5], biomedicine (molecular diagnostics, imaging, drug delivery and therapeutics)
[6][7][8][9], and also in the environmental remediation sector
[10]. Different techniques to synthesize MNPs have been developed, such as chemical (e.g., chemical reduction, chemical vapor deposition, photochemical reduction, co-precipitation, thermal decomposition, hydrolysis,) and physical methods (e.g., mechanical milling, laser ablation, vapor deposition, ion sputtering, grinding, flame pyrolysis). These methods can be categorized as bottom-up or top-down methods
[11].
Green processes for the synthesis of MNPs have also been reported
[12], together with their use in the sector of bioremediation and production of biosurfactants. Bioremediation processes, based on the use of biosurfactants that facilitate the solubility of hydrophobic contaminants and remediate contaminated marine sites with a green approach, can be improved by the use of nanoparticles. FeNPs, for example, are of significant importance for stimulation of the production (increased to 80%) of biosurfactants by bacteria such as the marine
Actinobacterium nocardiopsis MSA13A [13].
2. MNP Composites and Hybrids Applications
MNPs featuring very interesting and useful properties may be used in combination with polymeric matrices for the formation of functional nanocomposites or nanohybrids. to produce the physical properties of the final materials.
In this regard, they can allow the formation of different nanocomposites such as polymer-matrix composites, which consist of isolated nanoparticles finely dispersed in a polymer, composite nanoparticles, such as core/shell nanoparticles or surface modified nanoparticles, and microsphere composite nanoparticles that are larger nanocomposite spheres
[14].
There are reported examples of the use of these materials in the field of environmental remediation and pollutant detection, as showed in Table 1.
Table 1. Some MNP-based systems for remediation and bioremediation approaches.
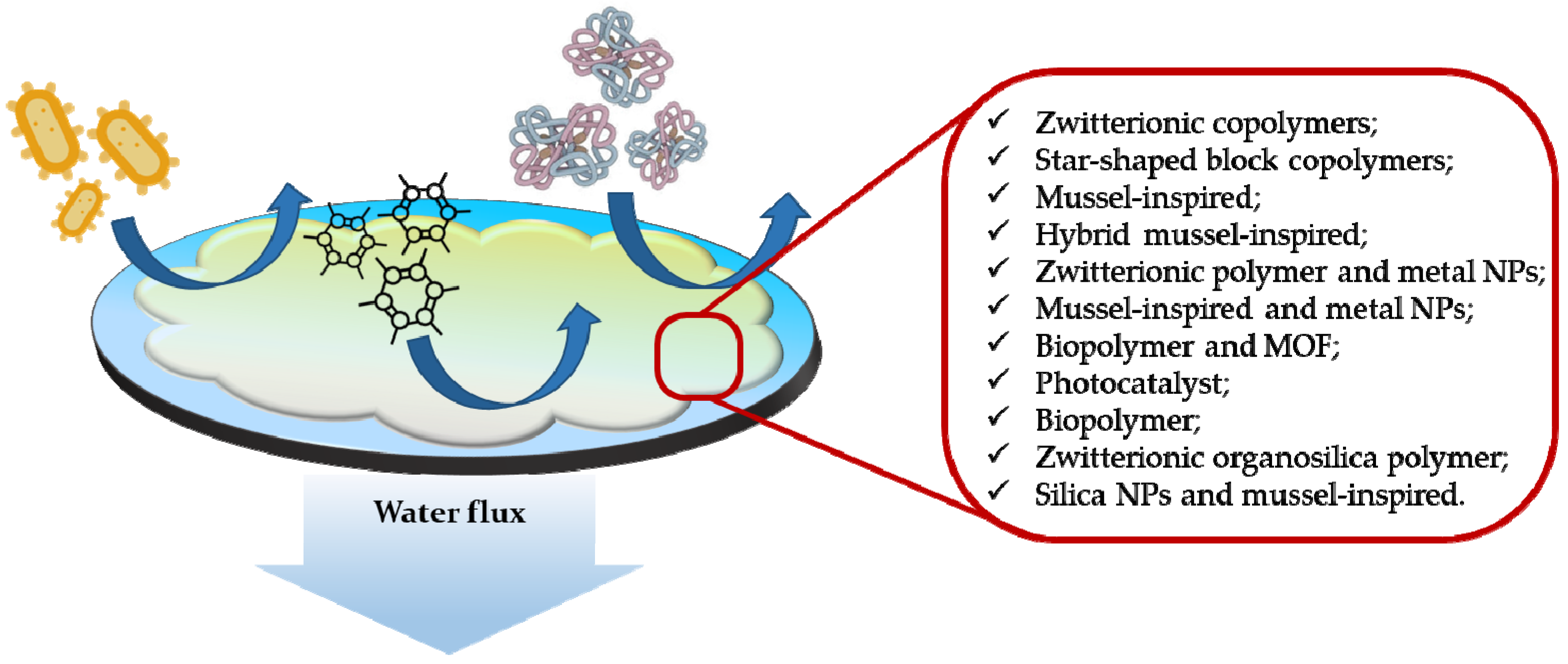
These functional materials can be combined with already employed remediation techniques using different approaches, such as grafting in polymeric membranes for nanofiltration and ultrafiltration processes to confer on them antifouling properties (
Figure 1)
[29]. Fouling represents one of the biggest problems of membrane technology, as it compromises filtration efficiency of membranes and reduces their lifespan. It is caused by agents present in wastewater such as inorganic compounds, proteins, bacteria and other organic organisms
[30][31].
Figure 1. Representation of a general antifouling coated membrane for common water foulants (bacteria, proteins, other organic compounds) and some antifouling functional agents
[31].
A poly(vinylidene fluoride) (PVDF) membrane grafted with silver nanoparticles-poly(carboxybetaine methacrylate) (AgNPs-PCBMA) nanocomposites, via a physiosorbed free-radical grafting technique, showed anti-protein and anti-bacterial fouling properties, especially against
E. coli. In addition, water flux recovery ratio (FRR) and bovine serum albumin (BSA) rejection ratio improved by 40% and 60%, respectively, due to better hydrophilicity performances. These properties were conferred by a synergistic effect due to the presence of AgNPs and the zwitterionic polymer brushes of PCBMA
[15].
The catalytic properties of MNPs can also be exploited in the environmental remediation field. For this purpose, a polypropylene hollow fiber membrane functionalized with osmium NPs obtained in situ from a solution of osmium tetroxide in
tert-butyl alcohol by reduction with molecular hydrogen, was fabricated and employed for a redox process in a membrane contactor. Due to the catalytic properties of OsNPs, the reduction of p-nitrophenol or the oxidation of 10-undecylenic acid was performed with conversions of about 90% and 80%, respectively, in
t-butanol. The obtained composite membranes have advantages such as large contact reactive area, the ability to operate at relatively low temperatures, to perform in the same module oxidation and reduction processes, no contamination of the working environment, and they represent a useful approach to recover the reaction mass through ultrafiltration
[16].
Another example is represented by the use of MNPs for UV/visible-light driven selective oxidation and reduction of some pollutants. Some nanocomposites of Au/Bi
2WO
6, fabricated via a hydrothermal approach combined with a rapid reduction–deposition method employing different weight ratios of Au, can be used as visible-light selective photocatalysts in water. In particular, this hybrid nanostructure shows a high capacity for benzylic alcohols oxidation and Cr(VI) reduction in water under visible-light radiation and aerobic conditions. The optimal catalysts for this oxidation/reduction process are 2.0 wt% and 1.0 wt% Au/Bi
2WO
6, respectively. The first target of this work was to find a catalyst for selective redox processes in water under the framework of green chemistry, but it also represents a possibility for environmental remediation
[17].
Moreover, the oxidation of polluting organic compounds, such as methylene blue, is possible by the employing olfactive photocatalysts in water. For example, a heterostructure based on a plasmonic bimetallic photocatalyst made of Pd-AgNPs/macro porous silicon (macroPSi) can be used to improve the activity of the methylene blue degradation in water under the ultraviolet light illumination. This heterostructure is obtained via a simple immersion process of macroporous silicon for the deposition of monometallic and bimetallic NPs of Ag and Pd. Monometallic photocatalysts AgNPs/macroPSi and PdNPs/macroPSi can also be prepared, but the bimetallic ones showed better performances with a higher efficiency (98.8%) and methylene blue degradation rate (0.033 min
−1), due to their highest specific surface area and plasmonic effect
[18].
Other types of aggregates and composite structures that involve MNPs and exploit their catalytic capacities are represented by gels, micelles and vesicles. Microgels based on poly(methacrylic acid-co-acrylonitrile) are obtained by inverse suspension polymerization, and subsequently the nitrile groups are converted into amidoxime groups to obtain a more hydrophilic amidoximated microgel. MNPs based on Cu and Co are synthetized in situ by loading the amid-microgels with the aqueous metal salt solutions of Cu(II) and Co(II) ions, and by subsequent treating with sodium borohydride (NaBH
4). The obtained microgel composites (amid-p(Mac-co-AN)-M, M: Cu, Co) showed high catalytic effectiveness for the simultaneous degradation of nitrophenoles and cationic and anionic organic dyes (eosin Y, methylene blue and methyl orange), that may exist in contaminated aquatic environments. The ability of such systems to be reused for more catalytic cycles is also observed. In particular, amid-p(Mac-co-AN)-Cu composites can be used up to four times as sacrificial catalyst systems and amid-p(Mac-co-AN)-Co composites do not show any loss in catalytic activity for up to seven cycles, so they appear to be more stable compared to Cu composites in similar aquatic environments. These experimental findings result from the strong coordinating interaction of Co nanoparticles with amidoxime groups
[19].
The reduction reaction of 4-nitrophenol can also be facilitated by a pH-responsive multifunctional homopolymer vesicle based on poly[2-hydroxy-3-(naphthalen- 1-ylamino) propyl methacrylate] (PHNA), in which AuNPs are supported. This system showed a synergistic effect between the AuNPs and the supporter (PHNA vesicle). The π-π interaction between the naphthalene pendants in PHNA vesicle occurs with polycyclic aromatic hydrocarbons (less than 0.876 ppb within 1 h) and makes those homopolymer vesicles useful as powerful adsorbents of these contaminants in polluted aquatic environments. This pH-responsive absorbent system (PHNA vesicle) decorated with AuNPs is also recyclable, as it acts as a nanoreactor for the reduction of 4-nitrophenol in water by adding NaBH
4 [20].
It is also possible to find examples of nanotechnologies based on nanocomposites that combine the antibacterial and catalytic properties of some MNPs and the mechanical properties of natural polymers such as cellulose. A composite material based on cellulose and AgNPs is simple to prepare. By a simple impregnation of cellulose, obtained from citrus waste, with AgNPs, a composite nanomaterial is developed that has antibacterial, antioxidant and photodegradation properties. In particular, discs made of the composite material, cellulose-AgNPs, display more than 90% reduction of
Staphylococcus aureus culture within 150 min, a moderate total antioxidant potential, minor 2,2-diphenyl 1-picryl-hydrazyl (DPPH) radical scavenging activity, and a moderate photodegradation capacity under sunlight of methylene blue dye of up to 63.16% (time of 60 min)
[21].
The use of MNPs composites and hybrids is not limited to e environmental remediation and extraction/degradation of pollutants, but is also of high importance for the production of selective sensors for some substances. An example of a detector based on MNPs is a polyurethane micelle/Ag nanoparticle (AgNPs) cluster fabricated as a surface-enhanced Raman scattering (SERS) substrate for in situ extraction and measurement of pesticide residues such as thiabendazole, phosmet, and acetamiprid. Due to their amphiphilic properties, polyurethane micelles have a two-fold function in capturing target molecules and stabilizing the nanoparticle cluster. This method consists of simply dropping the polyurethane micelle/AgNPs substrate on the sample surface (it was tested on surface of apple, orange, and spinach) to extract and detect the target molecules without any previous sample treatment
[25].
Another example is represented by a reduced graphene oxide detector decorated with AuNPs (rGO@Au NPs), produced to detect heavy metal contaminants in water bodies. In particular, this sensor is able to detect heavy metals such as Cd
2+, Pb
2+, Cu
2+ and Hg
2+, with a sensitivity of 19.05, 47.76, 22.10 and 29.28 µA µM
−1cm
−2, respectively. The rGO@AuNPs are synthetized by a green method utilizing
Abelmoschus esculentus vegetable extract as a reducing agent, and contaminated water is remediated by the bacteria
Pseudomonas aeruginosa,
Rhizobium gallicum,
Staphylococcus aureus, and
Bacillus subtilis, that have impressive absorption properties for heavy metal contaminants when acting as scavengers. The cell walls of these bacteria have the tendency to adsorb various inorganic and toxic heavy metal ions, which makes it possible for them to be used successfully for bioremediation. This represents a new green innovative method of detection and bioremediation that can be applied in contaminated aqueous sites and could encourage the development of further eco-friendly technologies for water remediation
[26].