Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gary Hin-Fai Yam | -- | 2235 | 2022-08-10 19:19:46 | | | |
| 2 | Conner Chen | + 31 word(s) | 2266 | 2022-08-11 02:24:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Santra, M.; Liu, Y.; Jhanji, V.; Yam, G. Corneal Regenerative Approach. Encyclopedia. Available online: https://encyclopedia.pub/entry/26053 (accessed on 04 March 2026).
Santra M, Liu Y, Jhanji V, Yam G. Corneal Regenerative Approach. Encyclopedia. Available at: https://encyclopedia.pub/entry/26053. Accessed March 04, 2026.
Santra, Mithun, Yu-Chi Liu, Vishal Jhanji, Gary Yam. "Corneal Regenerative Approach" Encyclopedia, https://encyclopedia.pub/entry/26053 (accessed March 04, 2026).
Santra, M., Liu, Y., Jhanji, V., & Yam, G. (2022, August 10). Corneal Regenerative Approach. In Encyclopedia. https://encyclopedia.pub/entry/26053
Santra, Mithun, et al. "Corneal Regenerative Approach." Encyclopedia. Web. 10 August, 2022.
Copy Citation
A transparent cornea is paramount for vision. Corneal opacity is one of the leading causes of blindness. Although conventional corneal transplantation has been successful in recovering patients’ vision, the outcomes are challenged by a global lack of donor tissue availability. Bioengineered corneal tissues are gaining momentum as a new source for corneal wound healing and scar management. Extracellular matrix (ECM)-scaffold-based engineering offers a new perspective on corneal regenerative medicine.
corneal regeneration
cornea
tissue engineering
1. Introduction
1.1. Human Cornea Anatomy, Composition and Cell Types
A healthy cornea with high transparency and optimal refractivity is paramount for vision. As the first layer of the eye, the cornea refracts light onto the lens, which then reaches the retina. It also provides mechanical support and protection to the intraocular tissues and defense against pathogens. The adult cornea measures 11 to 12.5 mm in diameter, with a mean anterior corneal curvature radius of around 8 mm. The corneal thickness is 500 to 600 μm (an average of 540 μm in the center and 700 μm in the periphery) with a refractive index of 1.38 [1][2]. The human cornea comprises five layers: the epithelium, Bowman’s layer, stroma, Descemet’s membrane, and innermost corneal endothelium (Figure 1).
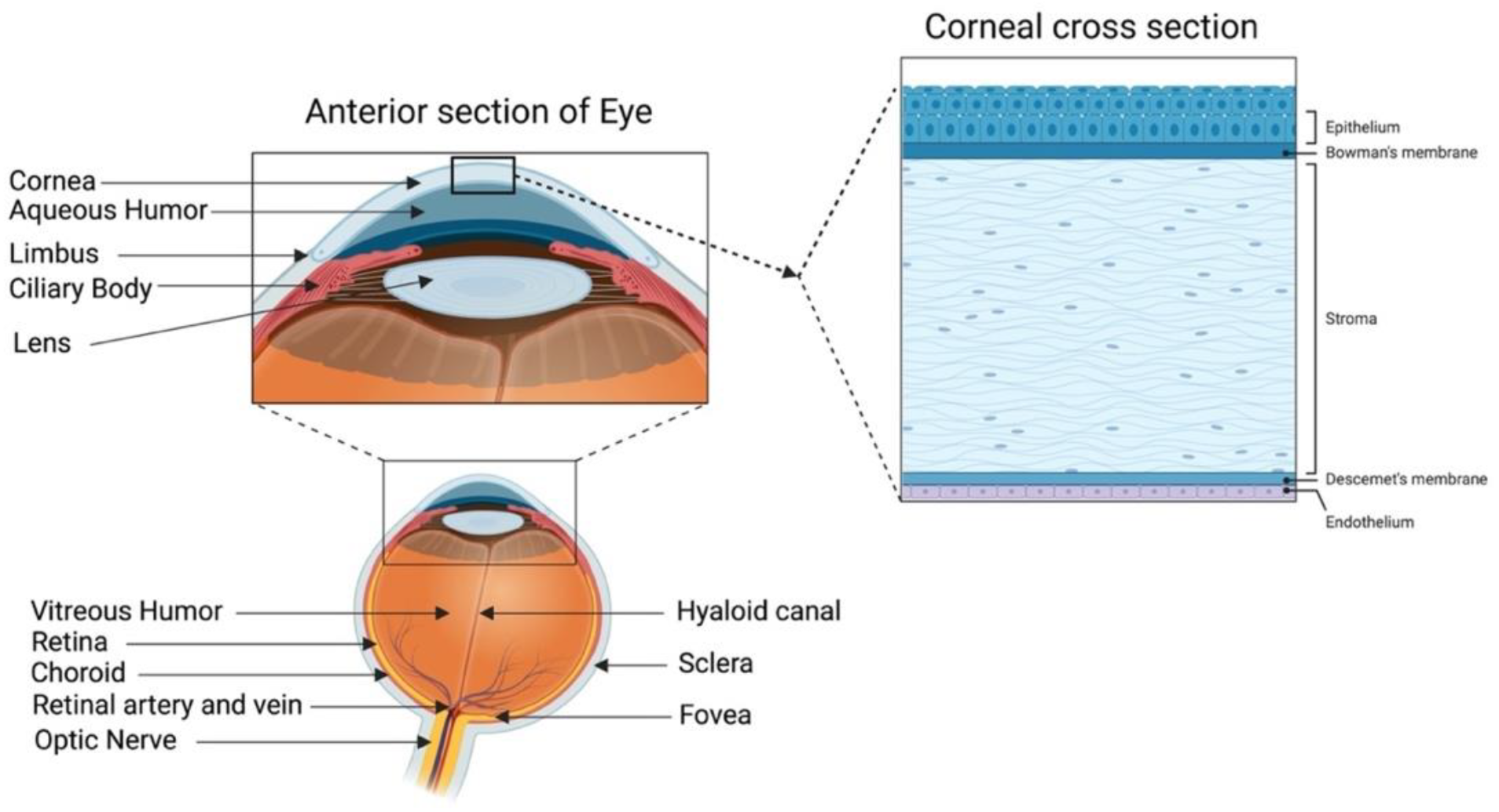
Figure 1. Human cornea: anatomy and structure. In the anterior segment, the cornea is highlighted in relation to the rest of the eye. A schematic representation of the structure and composition of the cornea is presented in the corneal cross-section. It consists of 3 cellular layers (epithelium, stroma, and endothelium) and 2 basement membranes (Bowman’s layer and Descemet’s membrane) (figure created using BioRender.com, BioRender, Toronto, ON, Canada).
The corneal epithelium is a non-keratinized stratified squamous epithelium. It functions as a physical barrier against external hazards and protects the eye from chemicals and microbes, which helps in maintaining visual acuity [3]. It is the only corneal tissue that undergoes both maintenance and injury-triggered regeneration due to the presence of the epithelial stem cell population in the peripheral limbus. The Bowman’s layer is a collagen-rich basement membrane of the corneal epithelium [4]. Corneal stroma, the thickest layer of the cornea, accounts for 80–90% of the overall corneal volume [5]. It provides mechanical strength to the cornea, comprising highly structured collagen fibers and a stromal matrix. A syncytial network of corneal stromal keratocytes populates the inside of the stroma. These cells are quiescent and are indispensable for stromal homeostasis, as they primarily produce the collagens and proteoglycans that comprise the stromal extracellular matrix (ECM) [6]. Parallel layers of collagen fibrils (predominantly type I and V) form an organized matrix architecture with an orthogonally aligned pattern for undisturbed light passage without scattering. Furthermore, small leucine-rich keratan sulfate proteoglycans (SLRPs) (such as lumican, keratocan, mimecan, and decorin) regulate the stromal collagen fibrillar spacing and play a significant role in maintaining the structural integrity of the stromal matrix [7]. Descemet’s membrane is the basement membrane of the innermost corneal endothelium. It is composed of collagen types IV and VIII [8][9]. The corneal endothelium is lined with a monolayer of tightly packed hexagonal-shaped corneal endothelial cells. Its continuous pump/leak activity regulates stromal hydration preventing corneal edema and loss of transparency [10].
1.2. Corneal Blindness and Conventional Treatments with Tissue Grafting
The cornea is susceptible to abrasive insults, including mechanical, chemical, and thermal injury and infections. Globally, corneal blindness ranks fifth after refractive errors, cataracts, age-related macular degeneration, and glaucoma. In a recent report issued by the World Health Organization (WHO), around 2.2 billion people have vision impairment, including 4.2 million with unaddressed corneal opacities (WHO World report on vision, October 2019; https://www.who.int/publications/i/item/9789241516570, accessed on 13 February 2022). Corneal transplantation (keratoplasty) is the primary and most successful treatment modality for severe corneal opacities (Figure 2). However, the scarcity of transplantable donor tissues has limited the treatment outcomes. A recent report projected that 12.7 million people worldwide are waiting for corneal transplantations [11][12]. The highest corneal transplantation rate is in the USA (199 cases per million), followed by Lebanon (122 per million) and Canada (117 per million). However, the transplantation rate for the other 116 countries is only 19 per million. Approximately 53% of the world’s population has no access to corneal transplantation, and only 1 out of 70 patients with corneal blindness can access a transplantable donor cornea [11].
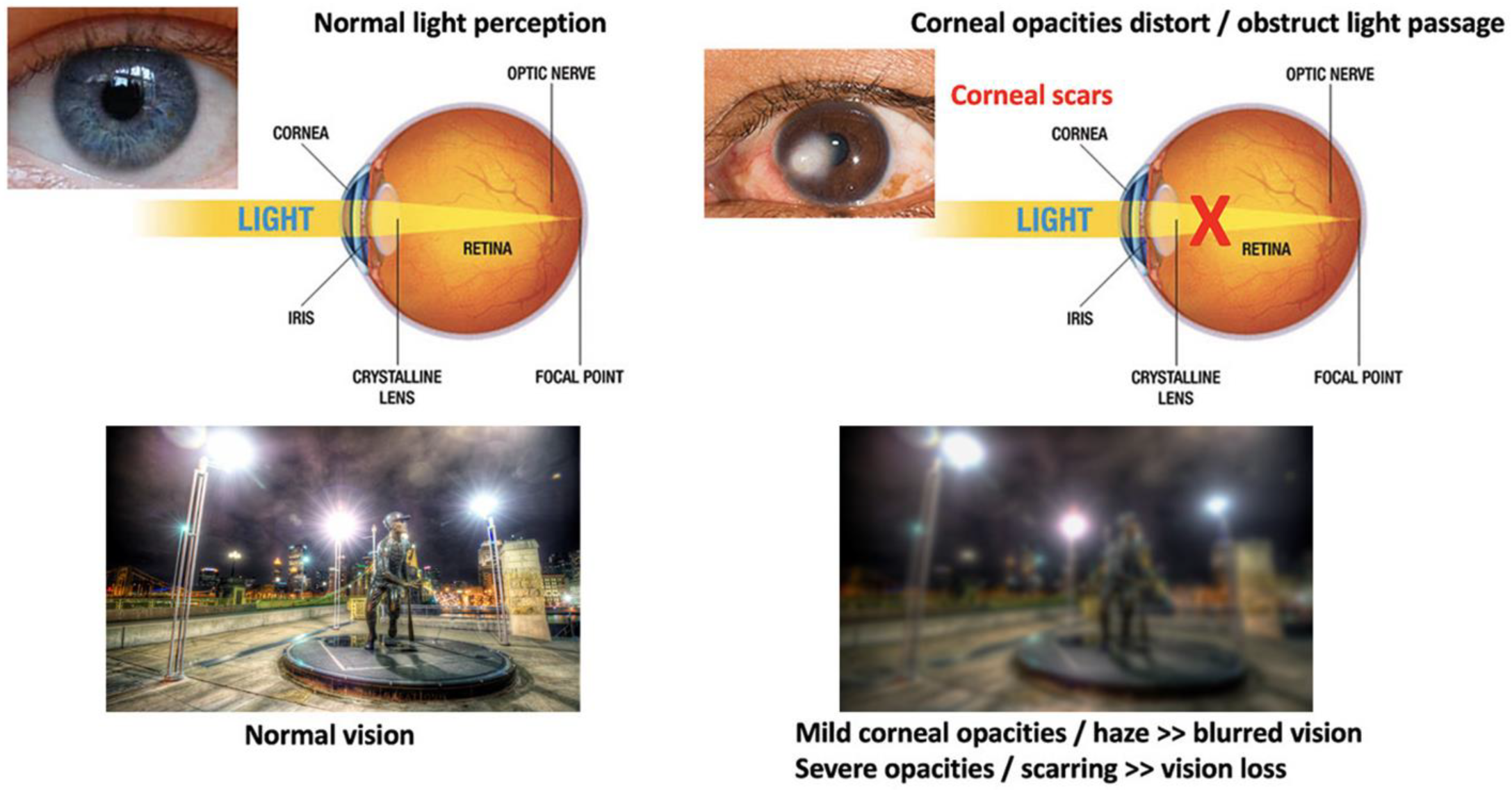
Figure 2. Comparison of vision between clear and opaque corneas and conventional corneal grafting to replace scarred corneas. Normal vision with unblocked light passage through a healthy and clear cornea, leading to clear and sharp visual acuity. The presence of corneal scarring/opacities blocks light passages, resulting in vision loss (corneal blindness).
Although corneal transplantation is the most frequent and successful type of organ transplant worldwide, various postoperative complications have been reported, such as astigmatism, infection, wound dehiscence, and graft rejection [13][14]. Astigmatism affects almost 15–31% of patients (greater than five diopters) undergoing penetrating keratoplasty [15].
1.3. Corneal Wound Healing and Scar Development
Corneal opacity or scarring results from injury, infection, or hereditary or inflammatory corneal diseases. After injury, the corneal epithelium, stroma, and endothelium heal with different but interlinked mechanisms [16]. Corneal epithelial cells and stromal keratocytes produce and release cytokines to initiate epithelial–stromal interaction for coordinated wound healing. IL-1α and platelet-derived growth factor (PDGF) secreted by the injured epithelium induce stromal keratocytes to be activated and undergo fibrosis (more details in the following paragraph) [4][16][17]. Moreover, keratocyte-secreted Growth Factors (GF), such as keratinocyte growth factor (KGF) and hepatocyte growth factor (HGF), aid epithelial wound healing by influencing cell behaviors, including cell migration and proliferation [16][18]. Adult limbal epithelial stem cells are also activated and generate transit-amplifying cells that migrate and regenerate the corneal epithelium [19][20]. On the other hand, the corneal endothelium has a specific pump and leak action to keep the corneal stroma in a partially dehydrated status for corneal clarity. Endothelial wounds mostly heal through cell shape enlargement and the sliding of adjacent endothelial cells rather than mitosis, as adult corneal endothelial cells are post-mitotic quiescent and lack regenerative capacity. Hence, endothelial cell loss, if severe, causes the cell density to drop below the threshold needed to maintain an efficient pump/leak action, resulting in corneal edema and opacities [21][22].
Inside the corneal stroma, the dominant population of stromal keratocytes regulates stromal homeostasis by producing and depositing stromal-specific collagens and matrix proteoglycans, and this maintains the structural integrity with minimal light scattering. One recent review detailed the role of stromal keratocytes in corneal health and visual functions [17]. Mature keratocytes are quiescent and show minimal mitosis in adult life. There is an estimated 0.45% cell loss per annum. Keratocyte-secreted maspin inhibits cell migration and stimulates cell adhesion to ECM [23]. Keratocytes also have phagocytic functions [17][24]. Upon injury, keratocytes at the wound site undergo apoptosis due to IL-1α and PDGF produced by the corneal epithelium. Cells in the peripheral regions are activated to re-enter the cell cycle. This event generates proliferative and motile stromal fibroblasts (SFs) with an accompanying actin cytoskeleton-mediated morphological change from a dendritic to a spindle shape. The activated SFs have a loss of keratocyte phenotypes, including the expression of keratan sulfate proteoglycans and stromal crystallins. On the other hand, there is an increased production of ECM proteins, including collagen I, fibronectin, and biglycan, an initiation of fibronectin receptor and integrin (α5β1) interaction, and an expression of IL-1α-controlled metalloproteinases (MMPs). These features are related to stromal tissue remodeling that alters the stromal matrix architecture, resulting in corneal haze development [25]. Further generation of contractile myofibroblasts due to the presence of pro-fibrotic transforming growth factor (TGF)-β isoforms exaggerates these fibrotic events with disorganized ECM and excessive matrix contraction, forming scars and opacities. SFs also produce growth factors (GFs) to trigger neovascularization into the avascular stroma and the expression of MMP to degrade stromal collagens to assist new blood vessel formation and penetration [26].
2. Corneal Regenerative Approach: Cell-Based vs. Scaffold-Based Strategies
2.1. Cell-Based Therapies
Conservative treatments (topical anti-inflammatory medications or steroid eye drops and minor surgeries) and donor tissue grafting are the major options for managing corneal opacities and scarring. The limited supply of transplantable donor tissue and the risk of allogenic graft rejections have urged researchers to look for alternative options to restore corneal functions. The regenerative ability of corneal cells has led to extensive research into regenerating and reconstructing various layers of the cornea, starting from the corneal epithelium [27][28][29][30] to current developments in the corneal stroma [31][32][33] and endothelium [21][34][35][36][37]. In the regeneration of corneal layers, cell and tissue engineering have emerged as essential strategies for developing novel substitutes [38]. Specific corneal stem cells have immense potential to induce respective tissue regeneration through multiple modes of action, including: (1) differentiation of limbal epithelial stem cells to corneal epithelial cells and corneal stromal stem cells (CSSCs) into keratocytes to replenish the lost/damaged cells; (2) activation of resident cells to assist in tissue repair; (3) secretion of regenerative molecules to reduce tissue inflammation, promote tissue remodeling, and activate the signaling pathways associated with tissue healing and regeneration [31]. Different studies have reported the differentiation of CSSCs, extraocular mesenchymal stem cells (MSCs), and induced pluripotent stem cells (iPSCs) to survive and differentiate in vivo along the keratocyte lineage and synthesize new collagens in the recipient stroma [31][38]. MSC treatment also reduces pre-existing scars and stromal defects, resulting in improved corneal clarity [38]. In addition, the immunomodulatory properties of MSC make them ideal for corneal regeneration in syngeneic, allogeneic, and even xenogeneic scenarios [39][40].
Despite the potential of cell-based therapies, there are limitations regarding the direct delivery of cells. The therapeutic efficacy depends on the precise location and retention of viable cells at the target site and their functions in the new niche [41]. However, a number of attempts have not been met with critical success due to post-transplantation complications, such as poor cell localization, short-term survival, and side effects (including the transition to other cell types and tumorigenesis) [41]. Without appropriate data from corneal research, it can be found from other studies that less than 5% of transplanted cells reach the target site after intravenous administration, and their survival rate can be as low as 1% [42][43][44][45].
2.2. Scaffold-Based Cell Delivery Strategies
Scaffold-based cell delivery has the potential to overcome some limitations associated with cell-based therapy, such as poor cell localization, as mentioned above. Bioscaffolding can assist in efficient cell delivery; at the same time, cells introduced inside the scaffold can create 3D tissue analogs. It also provides a suitable matrix environment for cell adhesion and growth [46] and maintains cellular functions, such as cytoskeleton reorganization, integrin activation, gene expression, and ECM organization [47]. Compared with cell suspensions, cell-laden scaffolds show higher cell viability and better integration at the host site [48]. Furthermore, in tissues where cell orientations are necessary, a good alignment of cells and ECM is feasible. Such cell guidance inside the scaffold can assist host tissue regeneration and functional recovery [47]. In tissues and organs, such as corneal stroma, tendons, bones, and skeletal muscles, specific cell–ECM alignment is crucial for organ function [49]. In the corneal stroma, keratocytes are sparsely located between the parallel running collagen lamellae, and this is essential to minimize incident light scattering for perfect light transmission [17][50]. In tendons, the presence of a unique cell–matrix alignment provides substantial resistance and exceptional mechanical properties to the tissue in that axis [51][52]. Other similar examples can be observed in cartilage [53], dental enamel [54], and basal epithelium [55]. Reproducing those cell–matrix alignment patterns within the tissue-engineered substitutes can generate a more native physiological representation of biological tissues, leading to a better recovery of tissue functions.
Engineering cells in a scaffold also warrant mechanical support and protection for the cells [37]. Last but not least, a bioscaffold used for corneal tissue engineering should have excellent optical characteristics, biocompatibility, and stability [56].
The ECM is a non-cellular component present in every tissue and organ in our body, providing the physical scaffolding support and essential biochemical, biophysical, and biomechanical signals necessary for tissue morphogenesis, differentiation, and homeostasis. Therefore, an ECM-derived scaffold is an ideal biocompatible material for cell incorporation compared with other biosynthetic platforms. In corneas, stromal matrix scaffolds have shown the potential to be used for such a purpose due to the good preservation of the native ECM structure [57].
Decellularization of ECM Tissues for Scaffold-Based Engineering
Decellularization aims to eliminate cellular and antigenic molecules, including genetic materials while preserving the structural, biochemical, and biomechanical properties of the matrix scaffold [46][58][59]. In the context of tissue engineering, a decellularized scaffold provides a native-like ECM environment with high bioactivity and compatibility for cell–ECM interaction, and this promotes subsequent cell adhesion, proliferation, and survival [60][61]. In addition, it offers the advantage of a remarkable similarity with the tissue to be replaced. After delivery to the target site, the decellularized ECM can be repopulated with the recipient cells to produce an integrated cell–tissue composite. Various decellularization methods have been developed to efficiently remove immunogenic cellular materials, hence maintaining low immunogenicity [62]. A good decellularization protocol would produce non-immunogenic ECM with the original structural integrity and a preserved protein content, including collagen, fibronectin, and glycosaminoglycans (GAGs). As a criterion to be justified as non-immunogenic, decellularized ECM should contain less than 50 ng of double-stranded DNA per mg ECM dry weight, and the residual DNA fragments must be less than 200 bp in length [58][63].
In recent years, different protocols have been developed to decellularize human corneal tissue [64][65][66][67][68][69][70]. The different approaches, methodology details, reagent requirements, and efficiency, as well as limitations, have been broadly reviewed recently [63][71]. Ideally, decellularization techniques aim to completely remove cellular and antigenic materials. However, most protocols inevitably cause tissue disruption and a loss of intrinsic biological cues. Similarly, residues of decellularization reagents might be retained in the resulting matrix, which could have a negative influence on further engineering events and/or tissue transplantation. From a manufacturing point of view, simple protocols with fewer steps and minimal reagent use are desirable. Stringent checks on decellularized tissue architecture, cellular material, and protein content, as well as any residual reagents, are necessary.
References
- Patel, S.; Alió, J.L.; Pérez-Santonja, J.J. Refractive Index Change in Bovine and Human Corneal Stroma before and after LASIK: A Study of Untreated and Re-treated Corneas Implicating Stromal Hydration. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3523–3530.
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23.
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194.
- Wilson, S.E. Bowman’s layer in the cornea—Structure and function and regeneration. Exp. Eye Res. 2020, 195, 108033.
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598.
- Zhang, L.; Anderson, M.C.; Liu, C.Y. The role of corneal stroma: A potential nutritional source for the cornea. J. Nat. Sci. 2017, 3, e428.
- Chen, S.; Mienaltowski, M.J.; Birk, D.E. Regulation of corneal stroma extracellular matrix assembly. Exp. Eye Res. 2015, 133, 69–80.
- Kabosova, A.; Azar, D.T.; Bannikov, G.A.; Campbell, K.P.; Durbeej, M.; Ghohestani, R.F.; Jones, J.C.R.; Kenney, M.C.; Koch, M.; Ninomiya, Y.; et al. Compositional Differences between Infant and Adult Human Corneal Basement Membranes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4989–4999.
- Jacobsen, I.E.; Jensen, O.A.; Prause, J.U. Structure and composition of Bowman’s membrane. Study by frozen resin cracking. Acta Ophthalmol. Cph. 1984, 62, 39–53.
- Bonanno, J.A. Identity and regulation of ion transport mechanisms in the corneal endothelium. Prog. Retin. Eye Res. 2003, 22, 69–94.
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173.
- Guérin, L.-P.; Le-Bel, G.; Desjardins, P.; Couture, C.; Gillard, E.; Boisselier, É.; Bazin, R.; Germain, L.; Guérin, S.L. The Human Tissue-Engineered Cornea (hTEC): Recent Progress. Int. J. Mol. Sci. 2021, 22, 1291.
- Price, M.O.; Feng, M.T.; Price, F.W.J. Endothelial Keratoplasty Update 2020. Cornea 2021, 40, 541–547.
- Price, M.O.; Mehta, J.S.; Jurkunas, U.V.; Price, F.W. Corneal endothelial dysfunction: Evolving understanding and treatment options. Prog. Retin. Eye Res. 2021, 82, 100904.
- Feizi, S.; Zare, M. Current approaches for management of postpenetrating keratoplasty astigmatism. J. Ophthalmol. 2011, 2011, 708736.
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45.
- Yam, G.H.F.; Riau, A.K.; Funderburgh, M.L.; Mehta, J.S.; Jhanji, V. Keratocyte biology. Exp. Eye Res. 2020, 196, 108062.
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Ambrósio, R.; Hong, J.; Lee, J. The Corneal Wound Healing Response: Cytokine-mediated Interaction of the Epithelium, Stroma, and Inflammatory Cells. Prog. Retin. Eye Res. 2001, 20, 625–637.
- Ruan, Y.; Jiang, S.; Musayeva, A.; Pfeiffer, N.; Gericke, A. Corneal Epithelial Stem Cells—Physiology, Pathophysiology and Therapeutic Options. Cells 2021, 10, 2302.
- Saghizadeh, M.; Kramerov, A.A.; Svendsen, C.N.; Ljubimov, A.V. Concise review: Stem cells for corneal wound healing. Stem Cells 2017, 35, 2105–2114.
- Català, P.; Thuret, G.; Skottman, H.; Mehta, J.S.; Parekh, M.; Dhubhghaill, S.N.; Collin, R.W.J.; Nuijts, R.M.M.A.; Ferrari, S.; LaPointe, V.L.S. Approaches for corneal endothelium regenerative medicine. Prog. Retin. Eye Res. 2022, 87, 100987.
- Vercammen, H.; Miron, A.; Oellerich, S.; Melles, G.R.; Dhubhghaill, S.N.; Koppen, C.; van den Bogerd, B. Corneal endothelial wound healing: Understanding the regenerative capacity of the innermost layer of the cornea. Transl. Res. 2022.
- Warejcka, D.J.; Narayan, M.; Twining, S.S. Maspin increases extracellular plasminogen activator activity associated with corneal fibroblasts and myofibroblasts. Exp. Eye Res. 2011, 93, 618–627.
- Nishida, T.; Ueda, A.; Otori, T.; Fujita, H. Long-term storage of endocytosed latex beads in keratocytes in vivo. Cornea 1991, 10, 532–535.
- Funderburgh, J.L.; Mann, M.M.; Funderburgh, M.L. Keratocyte Phenotype Mediates Proteoglycan Structure: A role for fibroblasts in corneal fibrosis. J. Biol. Chem. 2003, 278, 45629–45637.
- Ma, J.; Zhou, D.; Fan, M.; Wang, H.; Huang, C.; Zhang, Z.; Wu, Y.; Li, W.; Chen, Y.; Liu, Z. Keratocytes Create Stromal Spaces to Promote Corneal Neovascularization Via MMP13 Expression. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6691–6703.
- Nosrati, H.; Alizadeh, Z.; Nosrati, A.; Ashrafi-Dehkordi, K.; Banitalebi-Dehkordi, M.; Sanami, S.; Khodaei, M. Stem cell-based therapeutic strategies for corneal epithelium regeneration. Tissue Cell 2021, 68, 101470.
- Kobayashi, T.; Kan, K.; Nishida, K.; Yamato, M.; Okano, T. Corneal regeneration by transplantation of corneal epithelial cell sheets fabricated with automated cell culture system in rabbit model. Biomaterials 2013, 34, 9010–9017.
- Nosrati, H.; Abpeikar, Z.; Mahmoudian, Z.G.; Zafari, M.; Majidi, J.; Alizadeh, A.; Moradi, L.; Asadpour, S. Corneal epithelium tissue engineering: Recent advances in regeneration and replacement of corneal surface. Regen. Med. 2020, 15, 2029–2044.
- Bandeira, F.; Goh, T.W.; Setiawan, M.; Yam, G.H.; Mehta, J.S. Cellular therapy of corneal epithelial defect by adipose mesenchymal stem cell-derived epithelial progenitors. Stem Cell Res. Therapy 2020, 11, 14.
- Kumar, A.; Yun, H.; Funderburgh, M.L.; Du, Y. Regenerative therapy for the Cornea. Prog. Retin. Eye Res. 2021, 87, 101011.
- Basu, S.; Hertsenberg, A.J.; Funderburgh, M.L.; Burrow, M.K.; Mann, M.M.; Du, Y.; Lathrop, K.L.; Syed-Picard, F.N.; Adams, S.M.; Birk, D.E. Human limbal biopsy–derived stromal stem cells prevent corneal scarring. Sci. Transl. Med. 2014, 6, ra172–ra266.
- Jhanji, V.; Santra, M.; Riau, A.K.; Geary, M.L.; Yang, T.; Rubin, E.; Yusoff, N.Z.B.M.; Dhaliwal, D.K.; Mehta, J.S.; Yam, G.H.-F. Combined therapy using human corneal stromal stem cells and quiescent keratocytes to prevent corneal scarring after injury. Int. J. Mol. Sci. 2022, 23, 6980.
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N. Engl. J. Med. 2018, 378, 995–1003.
- Ying, L.Y.; Qiu, W.Y.; Wang, B.H.; Zhou, P.; Zhang, B.; Yao, Y.F. Corneal endothelial regeneration in human eyes using endothelium-free grafts. BMC Ophthalmol. 2022, 22, 32.
- Okumura, N.; Koizumi, N. Regeneration of the corneal endothelium. Curr. Eye Res. 2020, 45, 303–312.
- Mahdavi, S.S.; Abdekhodaie, M.J.; Mashayekhan, S.; Baradaran-Rafii, A.; Djalilian, A.R. Bioengineering Approaches for Corneal Regenerative Medicine. Tissue Eng. Regen. Med. 2020, 17, 567–593.
- El Zarif, M.; Alió, J.L.; Alió del Barrio, J.L.; De Miguel, M.P.; Abdul Jawad, K.; Makdissy, N. Corneal Stromal Regeneration: A Review of Human Clinical Studies in Keratoconus Treatment. Front. Med. 2021, 8, 650724.
- De Miguel, M.P.; Fuentes-Julián, S.; Blázquez-Martínez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr. Mol. Med. 2012, 12, 574–591.
- Kao, W.W.Y.; Coulson-Thomas, V.J. Cell Therapy of Corneal Diseases. Cornea 2016, 35 (Suppl. 1), S9–S19.
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. Regen. Med. 2021, 6, 18.
- Rossetti, T.; Nicholls, F.; Modo, M. Intracerebral Cell Implantation: Preparation and Characterization of Cell Suspensions. Cell Transplant. 2016, 25, 645–664.
- Amer, M.H.; Rose, F.R.A.J.; Shakesheff, K.M.; Modo, M.; White, L.J. Translational considerations in injectable cell-based therapeutics for neurological applications: Concepts, progress and challenges. Regen. Med. 2017, 2, 23.
- De Becker, A.; Riet, I.V. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J. Stem Cells 2016, 8, 73–87.
- Kurtz, A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cells 2008, 1, 1–7.
- García-Gareta, E.; Abduldaiem, Y.; Sawadkar, P.; Kyriakidis, C.; Lali, F.; Greco, K.V. Decellularised scaffolds: Just a framework? Current knowledge and future directions. J. Tissue Eng. 2020, 11, 2041731420942903.
- Li, Y.; Xiao, Y.; Liu, C. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem. Rev. 2017, 117, 4376–4421.
- Gu, C.; Feng, J.; Waqas, A.; Deng, Y.; Zhang, Y.; Chen, W.; Long, J.; Huang, S.; Chen, L. Technological Advances of 3D Scaffold-Based Stem Cell/Exosome Therapy in Tissues and Organs. Front. Cell Dev. Biol. 2021, 9, 709204.
- Bourget, J.M.; Guillemette, M.; Veres, T.; Auger, F.A.; Germain, L. Alignment of cells and extracellular matrix within tissue-engineered substitutes. In Advances in Biomaterials Science and Biomedical Applications; IntechOpen: London, UK, 2013; pp. 365–390.
- Jester, J.V.; Moller-Pedersen, T.; Huang, J.; Sax, C.M.; Kays, W.T.; Cavangh, H.D.; Petroll, W.M.; Piatigorsky, J. The cellular basis of corneal transparency: Evidence for ‘corneal crystallins’. J. Cell Sci. 1999, 112, 613–622.
- Lewis, G.; Shaw, K.M. Modeling the tensile behavior of human Achilles tendon. Bio-Med. Mater. Eng. 1997, 7, 231–244.
- Lynch, H.A.; Johannessen, W.; Wu, J.P.; Jawa, A.; Elliott, D.M. Effect of fiber orientation and strain rate on the nonlinear uniaxial tensile material properties of tendon. J. Biomech. Eng. 2003, 125, 726–731.
- Jeffery, A.; Blunn, G.; Archer, C.; Bentley, G. Three-dimensional collagen architecture in bovine articular cartilage. J. Bone Jt. Surg. Br. Vol. 1991, 73, 795–801.
- Paine, M.L.; Snead, M.L. Protein interactions during assembly of the enamel organic extracellular matrix. J. Bone Miner. Res. 1997, 12, 221–227.
- Evans, M.J.; Cox, R.A.; Shami, S.G.; Wilson, B.; Plopper, C.G. The role of basal cells in attachment of columnar cells to the basal lamina of the trachea. Am. J. Respir. Cell Mol. Biol. 1989, 1, 463–469.
- Lin, L.; Jin, X. The development of tissue engineering corneal scaffold: Which one the history will choose? Ann. Eye Sci. 2018, 3, 6.
- Mobaraki, M.; Abbasi, R.; Vandchali, S.O.; Ghaffari, M.; Moztarzadeh, F.; Mozafari, M. Corneal Repair and Regeneration: Current Concepts and Future Directions. Front. Bioeng. Biotechnol. 2019, 7, 135.
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243.
- Wilson, S.L.; Sidney, L.E.; Dunphy, S.E.; Rose, J.B.; Hopkinson, A. Keeping an eye on decellularized corneas: A review of methods, characterization and applications. J. Funct. Biomater. 2013, 4, 114–161.
- Liao, J.; Xu, B.; Zhang, R.; Fan, Y.; Xie, H.; Li, X. Applications of decellularized materials in tissue engineering: Advantages, drawbacks and current improvements, and future perspectives. J. Mater. Chem. B 2020, 8, 10023–10049.
- Unnikrishnan, K.; Thomas, L.V.; Kumar, R.M.R. Advancement of Scaffold-Based 3D Cellular Models in Cancer Tissue Engineering: An Update. Front. Oncol. 2021, 11, 733652.
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed. Res. Int. 2017, 2017, 9831534.
- Riau, A.K.; Liu, Y.C.; Yam, G.H.; Mehta, J.S. Stromal keratophakia: Corneal inlay implantation. Prog. Retin. Eye Res. 2020, 75, 100780.
- Shafiq, M.A.; Gemeinhart, R.A.; Yue, B.Y.; Djalilian, A.R. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue Eng. Part C Method. 2012, 18, 340–348.
- Shafiq, M.A.; Milani, B.Y.; Djalilian, A.R. In vivo evaluation of a decellularized limbal graft for limbal reconstruction. Int. J. Tissue Eng. 2014, 2014, 754245.
- Choi, J.S.; Williams, J.K.; Greven, M.; Walter, K.A.; Laber, P.W.; Khang, G.; Soker, S. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials 2010, 31, 6738–6745.
- Zhang, Z.; Niu, G.; San Choi, J.; Giegengack, M.; Atala, A.; Soker, S. Bioengineered multilayered human corneas from discarded human corneal tissue. Biomed. Mater. 2015, 10, 035012.
- Yam, G.H.-F.; Yusoff, N.Z.B.M.; Goh, T.-W.; Setiawan, M.; Lee, X.-W.; Liu, Y.-C.; Mehta, J.S. Decellularization of human stromal refractive lenticules for corneal tissue engineering. Sci. Rep. 2016, 6, 26339.
- He, Z.; Forest, F.; Bernard, A.; Gauthier, A.-S.; Montard, R.; Peoc’h, M.; Jumelle, C.; Courrier, E.; Perrache, C.; Gain, P. Cutting and decellularization of multiple corneal stromal lamellae for the bioengineering of endothelial grafts. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6639–6651.
- Del Barrio, J.L.A.; El Zarif, M.; Azaar, A.; Makdissy, N.; Khalil, C.; Harb, W.; el Achkar, I.; Jawad, Z.A.; de Miguel, M.P.; Alió, J.L. Corneal stroma enhancement with decellularized stromal laminas with or without stem cell recellularization for advanced keratoconus. Am. J. Ophthalmol. 2018, 186, 47–58.
- Fernández-Pérez, J.; Ahearne, M. Decellularization and recellularization of cornea: Progress towards a donor alternative. Methods 2020, 171, 86–96.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.4K
Revisions:
2 times
(View History)
Update Date:
11 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





