
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Myeong-Cherl Kook | + 2467 word(s) | 2467 | 2020-10-10 07:37:52 | | | |
| 2 | Dean Liu | -1076 word(s) | 1391 | 2020-10-16 06:27:23 | | | | |
| 3 | Dean Liu | Meta information modification | 1391 | 2020-10-27 07:18:53 | | |
Video Upload Options
Authors investigated the clinicopathological features of remnant gastric cancer based on initial disease of partial gastrectomy. Pyloric metaplasia was a characteristic feature in the anastomosis area accompanied by foveolar epithelial hyperplasia, which is a characteristic of reflux gastritis. Remnant gastric cancers that occurred following benign disease were strongly associated with pyloric metaplasia of the anastomosis site, whereas malignant remnant gastric cancers were characterized by both pyloric metaplasia and intestinal metaplasia. EBV-type gastric cancer was highly represented in the benign group (31.8%), while it was present in only 3% of the malignant cases. In the benign group, remnant gastric cancer occurred after 10 years or more, and was almost located within 3 cm from the anastomosis site. These findings may be helpful to the endoscopic surveillance for remnant stomach.
1. Introduction
Early diagnosis of gastric cancer according to national screening programs and advanced surgical treatment have led to an increase in the five-year survival rates in Korea and Japan[1][2][3]. Gastric resection with lymph node dissection is the only curative treatment for these patients[4]. Metachronous cancer in the remnant stomach after partial gastrectomy has gained great interest due to the increased application of organ function-preserving surgery[5].
The hypothesized risk factors for remnant gastric cancer include Helicobacter pylori (H. pylori) infection, changes in the gastric mucosa due to bile reflux, and the type of anastomosis methods[6][7][8]. However, most of these studies are retrospective and disease incidence and pathogenesis were not investigated. Several studies have reported that the changes in gastric mucosa caused by bile reflux after subtotal gastrectomy may increase the prevalence of remnant gastric cancer. This was in contrast to the results of other studies reporting that bile reflux may decrease H. pylori infection and generated a controversy about the relevant risk factors[9][10]. In order to understand the risk factors and features of remnant gastric cancer, combined pathological analysis of the anastomosis site and background mucosa is required. However, to date, very few studies have addressed this issue.
Recent studies have suggested new molecular classifications of gastric cancer, aimed at improving prognosis and treatment[11][12]. Presumably, a better understanding of the changes occurring in the anastomosis mucosa, as well as molecular subtype classification based on immunohistochemistry, will shed light on the pathogenesis of remnant gastric cancer.
2. Remnant Gastric Cancer and Disease of Partial Gastrectomy
Numerous studies have described the clinical features of remnant gastric cancer. However, the pathogenesis and cause of the disease remains controversial. Moreover, little is known regarding the histological features of the anastomosis site and background mucosa in remnant gastric cancer. The present study investigated the clinical features of remnant gastric cancer based on initial disease of partial gastrectomy. In addition, histological analysis and molecular classification based on immunohistochemistry were conducted to investigate the pathogenesis of remnant gastric cancer. Remnant gastric cancers that occurred following benign disease were strongly associated with pyloric metaplasia of the anastomosis site, whereas malignant remnant gastric cancers were characterized by both pyloric metaplasia and intestinal metaplasia.
In accordance with previous studies, a significant difference was observed between the benign and the malignant group in the duration of the interval between the initial partial gastrectomy and the occurrence of remnant gastric cancer. Numerous studies have hypothesized that the shorter interval in the malignant group is related to the presence of a precancerous lesion, such as atrophic gastritis and intestinal metaplasia[17][18].
Reflux gastritis from bile may occur in the remnant stomach and its major histological feature is known as foveolar epithelial hyperplasia without inflammation[9]. In the present study, we analyzed the distribution and severity of reflux gastritis in detail. Pyloric metaplasia, in addition to foveolar epithelial hyperplasia, was observed around the anastomosis site. These characteristic pyloric metaplasia were frequently observed in the remnant stomach. On the other hand, the literature focusing on pyloric metaplasia in the remnant stomach is extremely poor[13]. Pyloric metaplasia is a phenomenon by which fundic-type glands transform into mucin-secreting pyloric-type glands, and has been reported to occur in two forms. One is “antralization”, in which pyloric metaplasia occurs in the fundic glands of the angle of stomach and gradually progresses toward the proximal part. As a result, the border of the fundic and pyloric mucosa (fundic-pyloric border) also moves toward the proximal part[19] This phenomenon is associated with H. pylori gastritis and is usually accompanied by intestinal metaplasia[14]. The other mechanism is “spasmolytic polypeptide-expressing metaplasia (SPEM),” which occurs separately as a spot within the fundic gland region, arises from chief cell transdifferentiation, and further progresses into intestinal metaplasia[20]. Whether these two conditions are related remains unclear. Pyloric metaplasia observed in the present study exhibited distinct characteristics from the two aforementioned mechanisms. First, it started from the anastomosis site of the remnant stomach after partial gastrectomy. Second, it was accompanied by foveolar epithelial hyperplasia, which is a characteristic of reflux gastritis. Third, it was not accompanied by intestinal metaplasia.
We demonstrated that pyloric metaplasia was the main preneoplastic change in the benign group. A previous study also reported pyloric metaplasia in the remnant stomach. However, the relationship between pyloric metaplasia and the initial disease status was not clarified, nor was the time interval from initial surgery evaluated [13]. In that study, only the presence or absence of pyloric metaplasia was recorded, but its grade was not taken into consideration. In the present study, we demonstrated that although pyloric metaplasia was present in almost all examined cases, its degree of severity was much higher in the benign group. High-grade pyloric metaplasia was prevalent in the background mucosa around the carcinomas in the benign group, while its incidence was much lower in the malignant group. This suggested that reflux-induced changes had a stronger impact on the carcinogenic background in the benign group than in the malignant group.
Notably, high-grade intestinal metaplasia was only observed in the background mucosa of the malignant cases. High-grade intestinal mucosa is the result of mucosal changes due to prolonged H. pylori gastritis. Therefore, H. pylori gastritis and reflux-induced gastritis could have a similar influence on carcinoma development in the malignant group. It can be assumed that partial gastrectomy in the benign group was performed before H. pylori gastritis could progress into the body and, therefore, background mucosa had no chance to develop.
Significant differences in EBV and CDX2 expression were found between the two groups. EBV-type gastric cancer was highly represented in the benign group (31.8%), while it was present in only 3% of the malignant cases, which is lower than the average incidence among total gastric cancers (5–10%)[21][22]. EBV-type gastric cancer exhibits unique molecular alterations[16]. However, the mechanism of its development is not well-established. EBV-type gastric cancer is highly prevalent in the cardia and body among remnant gastric cancers, but it has no relationship with H. pylori infection[23][24]. In a recent study, the EBV infection rate was higher in patients with remnant gastric cancer compared with those with conventional gastric cancer. It has been reported that Billroth II anastomosis, carcinoma at the anastomosis site, diffuse type, and EBV genome polymorphisms are related to the EBV-associated remnant gastric cancer[25]. CDX2 was less expressed in the benign group than malignant group, and this could be related to the low grade of intestinal metaplasia in the background mucosa of benign cases[26]. CDX2 is a homeobox gene expressed in the intestinal mucosa and has a role in the differentiation of intestinal epithelium[27]. CDX2 is expressed in all gastric carcinomas that develop an intestinal phenotype, but it is also present in a proportion of cases with gastric phenotype[28]. The clinical relevance of the different molecular subtypes of gastric carcinoma is not clear at present, but carcinogenic factors might differ depending on the initial disease.
Annual endoscopic surveillance after gastrectomy in the malignant group should be recommended even after the 5- year follow-up because the tumors seems to occur at a constant rate without changing over the years. In the benign group, remnant gastric cancer occurs after 10 years or more, and at the time of diagnosis, about 50% is stage II or higher (Figure S1). Meanwhile high-grade pyloric metaplasia was mainly distributed within 3 cm from the anastomosis site in the benign group (Figure S2). Considering the data of the present study, it could be suggested to carefully observe the anastomosis area (within 3 cm) in the endoscopic surveillance 10 years from the partial gastrectomy performed for the benign cause. (Figure 2)
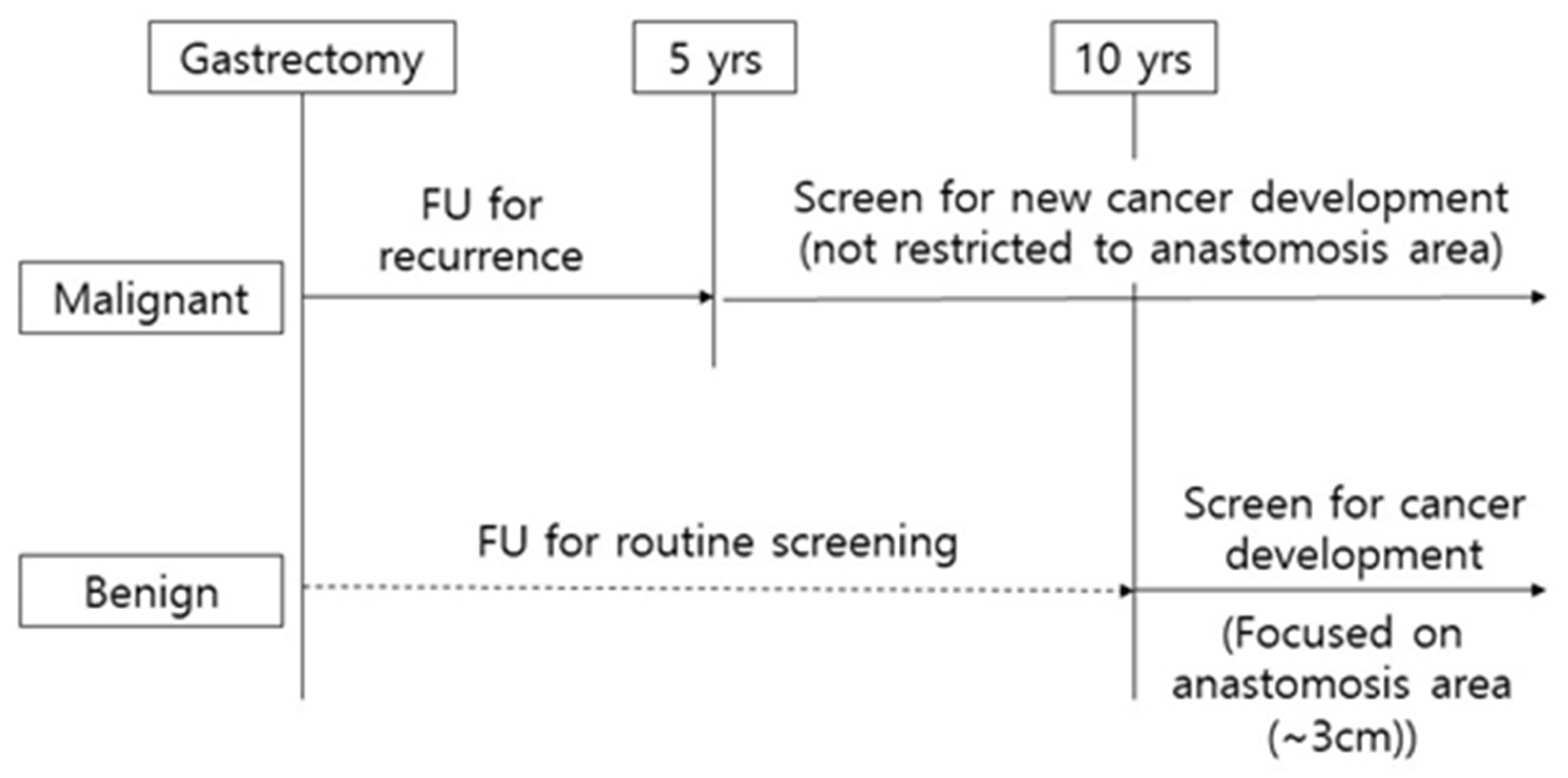
Figure 2. Scheme of clinical surveillance for remnant gastric cancer.
References
- Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014. J. Gastric Cancer 2016, 16, 131–140.
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Niksic, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Esteve, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075.
- Katai, H.; Ishikawa, T.; Akazawa, K.; Isobe, Y.; Miyashiro, I.; Oda, I.; Tsujitani, S.; Ono, H.; Tanabe, S.; Fukagawa, T.; et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: A retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer 2017, 14, 144–154.
- Development Working Group & Review Panel Guideline Committee of the Korean Gastric Cancer Association (KGCA) Korean Practice Guideline for Gastric Cancer 2018: An Evidence-based, Multi-disciplinary Approach. J. Gastric Cancer 2019, 19, 1–48.
- Ohyama, S.; Tokunaga, M.; Hiki, N.; Fukunaga, T.; Fujisaki, J.; Seto, Y.; Yamaguchi, T. A clinicopathological study of gastric stump carcinoma following proximal gastrectomy. Gastric Cancer 2009, 12, 88–94.
- Lee, Y.; Tokunaga, A.; Tajiri, T.; Masuda, G.; Okuda, T.; Fujita, I.; Kiyama, T.; Yoshiyuki, T.; Kato, S.; Matsukura, N.; et al. Inflammation of the gastric remnant after gastrectomy: Mucosal erythema is associated with bile reflux and inflammatory cellular infiltration is associated with Helicobacter pylori infection. J. Gastroenterol. 2004, 39, 520–526.
- Choi, I.J.; Kook, M.-C.; Kim, Y.-I.; Cho, S.-J.; Lee, J.Y.; Kim, C.G.; Park, B.; Nam, B.H. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N. Engl. J. Med. 2018, 378, 1085–1095.
- Chan, D.-C.; Fan, Y.-M.; Lin, C.-K.; Chen, C.-J.; Chen, C.-Y.; Chao, Y.-C. Roux-en-Y Reconstruction after Distal Gastrectomy to Reduce Enterogastric Reflux and Helicobacter pylori Infection. J. Gastrointest. Surg. 2007, 11, 1732–1740.
- Dixon, M.F.; O’Connor, H.J.; Axon, A.T.; King, R.F.; Johnston, D. Reflux gastritis: Distinct histopathological entity? J. Clin. Pathol. 1986, 39, 524–530.
- Onoda, N.; Maeda, K.; Sawada, T.; Wakasa, K.; Arakawa, T.; Chung, K.H.-Y.-S. Prevalence of Helicobacter pylori infection in gastric remnant after distal gastrectomy for primary gastric cancer. Gastric Cancer 2001, 4, 87–92.
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456.
- Setia, N.; Agoston, A.T.; Han, H.S.; Mullen, J.T.; Duda, D.G.; Clark, J.W.; Deshpande, V.; Mino-Kenudson, M.; Srivastava, A.; Lennerz, J.K.; et al. A protein and mRNA expression-based classification of gastric cancer. Mod. Pathol. 2016, 29, 772–784.
- Yamaguchi, H.; Goldenring, J.R.; Kaminishi, M.; Lee, J.R. Identification of spasmolytic polypeptide expressing metaplasia (SPEM) in remnant gastric cancer and surveillance postgastrectomy biopsies. Dig. Dis. Sci. 2002, 47, 573–578.
- Xia, H.H.-X.; Kalantar, J.S.; Talley, N.J.; Wyatt, J.M.; Adams, S.; Chueng, K.; Mitchell, H.M.; Cheung, K. Antral-type mucosa in the gastric incisura, body, and fundus (antralization): A link between Helicobacter pylori infection and intestinal metaplasia? Am. J. Gastroenterol. 2000, 95, 114–121.
- Kim, S.S.; Kook, M.-C.; Shin, O.-R.; Kim, H.S.; Bae, H.-I.; Park, Y.; Choi, I.J.; Kim, Y.-I.; Nam, B.H.; Kim, S.; et al. Factors to improve the interobserver agreement for gastric atrophy and intestinal metaplasia: Consensus of definition and criteria. Histopathology 2018, 72, 838–845.
- The Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209.
- Sowa, M.; Kato, Y.; Onoda, N.; Kubo, T.; Maekawa, H.; Yoshikawa, K.; Nishimura, M.; Nakanishi, I.; Chung, Y.S. Early cancer of the gastric remnant with special reference to the importance of follow-up of gastrectomized patients. Eur. J. Surg. Oncol. 1993, 19, 43–49.
- Ohira, M.; Toyokawa, T.; Sakurai, K.; Kubo, N.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Onoda, N.; Hirakawa, K. Current status in remnant gastric cancer after distal gastrectomy. World J. Gastroenterol. 2016, 22, 2424–2433.
- Kimura, K. Chronological Transition of the Fundic-Pyloric Border Determined by Stepwise Biopsy of the Lesser and Greater Curvatures of the Stomach. Gastroenterology 1972, 63, 584–592.
- Goldenring, J.R.; Nam, K.T.; Wang, T.C.; Mills, J.C.; Wright, N.A. Spasmolytic Polypeptide-Expressing Metaplasia and Intestinal Metaplasia: Time for Reevaluation of Metaplasias and the Origins of Gastric Cancer. Gastroenterology 2010, 138, 2207–2210.e1.
- Kim, H.S.; Shin, S.-J.; Beom, S.-H.; Jung, M.; Choi, Y.Y.; Son, T.; Kim, H.-I.; Cheong, J.-H.; Hyung, W.J.; Noh, S.H.; et al. Comprehensive expression profiles of gastric cancer molecular subtypes by immunohistochemistry: Implications for individualized therapy. Oncotarget 2016, 7, 44608–44620.
- Birkman, E.-M.; Mansuri, N.; Kurki, S.; Ålgars, A.; Lintunen, M.; Ristamäki, R.; Sundström, J.; Carpén, O. Gastric cancer: Immunohistochemical classification of molecular subtypes and their association with clinicopathological characteristics. Virchows Arch. 2017, 472, 369–382.
- Lee, J.H.; Kim, S.-H.; Han, S.-H.; An, J.-S.; Lee, E.-S.; Kim, Y.-S. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: A meta-analysis. J. Gastroenterol. Hepatol. 2009, 24, 354–365.
- Murphy, G.; Pfeiffer, R.; Camargo, M.C.; Rabkin, C.S. Meta-analysis Shows That Prevalence of Epstein–Barr Virus-Positive Gastric Cancer Differs Based on Sex and Anatomic Location. Gastroenterology 2009, 137, 824–833.
- Lu, C.; Zhang, H.; Zhou, W.; Wan, X.; Li, L.; Yu, C. Epstein–Barr virus infection and genome polymorphisms on gastric remnant carcinoma: A meta-analysis. Cancer Cell Int. 2020, 20, 1–9.
- Almeida, R.; Silva, E.; Santos-Silva, F.; Silberg, D.G.; Wang, J.; De Bolós, C.; David, L. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J. Pathol. 2002, 199, 36–40.
- Mizoshita, T.; Inada, K.-I.; Tsukamoto, T.; Kodera, Y.; Yamamura, Y.; Hirai, T.; Kato, T.; Joh, T.; Itoh, M.; Tatematsu, M. Expression of Cdx1 and Cdx2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa--with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric Cancer 2001, 4, 185–191.
- Mizoshita, T.; Tsukamoto, T.; Inada, K.-I.; Ogasawara, N.; Hirata, A.; Kato, S.; Joh, T.; Itoh, M.; Yamamura, Y.; Tatematsu, M. Immunohistochemically detectable Cdx2 is present in intestinal phenotypic elements in early gastric cancers of both differentiated and undifferentiated types, with no correlation to non-neoplastic surrounding mucosa. Pathol. Int. 2004, 54, 392–400.




