
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Monjurul Hoque | -- | 4410 | 2022-08-10 10:53:47 | | | |
| 2 | Vivi Li | Meta information modification | 4410 | 2022-08-11 08:18:56 | | |
Video Upload Options
Suitable packaging material in combination with high-pressure processing (HPP) can retain nutritional and organoleptic qualities besides extending the product’s shelf life of food products. However, the selection of appropriate packaging materials suitable for HPP is tremendously important because harsh environments like high pressure and high temperature during the processing can result in deviation in the visual and functional properties of the packaging materials. Traditionally, fossil-based plastic packaging is preferred for the HPP of food products, but these materials are of serious concern to the environment. Therefore, bio-based packaging systems are proposed to be a promising alternative to fossil-based plastic packaging. Some studies have scrutinized the impact of HPP on the functional properties of biopolymer-based packaging materials.
1. Introduction
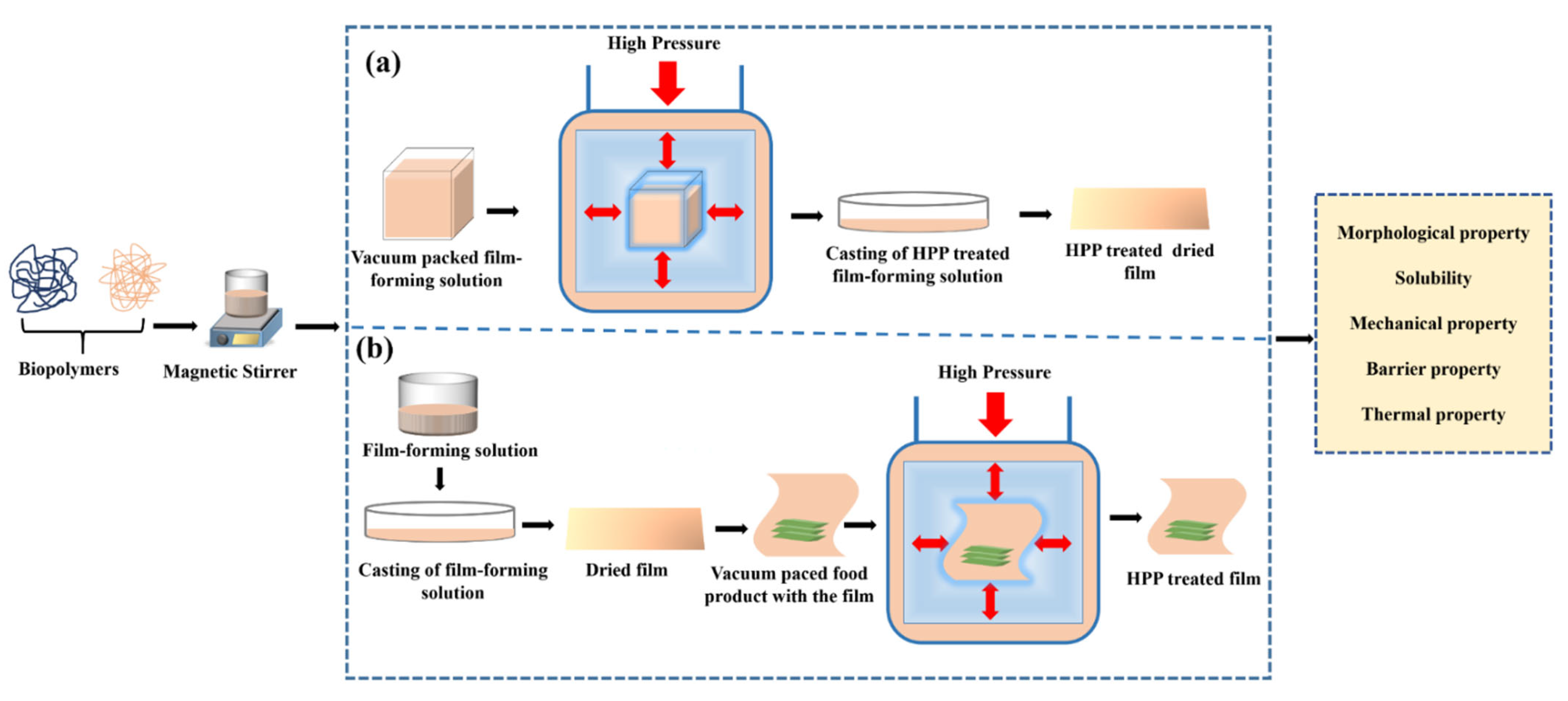
2. Impact of HPP When Applied to the Film-Forming Solution (FFS)
2.1. Mechanical Attributes
| Film Matrix | Processing Conditions | Water Solubility (WS) | Barrier Property (WVP/OP) | Mechanical Property | Thermal Properties | R | |
|---|---|---|---|---|---|---|---|
| TS | EAB | ||||||
| Buckwheat starch (BS) | 600 MPa at 20 °C for 20 min | WS of the thermally processed BS film was 19.85 ± 0.33% significantly decreased to 11.67 ± 0.69% upon application of 600 MPa | WVP of the thermally processed BS film 3.10 × 10−9 g/m s Pa significantly decreased to 2.10 × 10−9 g/m s Pa, upon application of 600 MPa | TS of the thermally processed BS film 13.61 ± 1.06 MPa significantly increased to 18.29 ± 1.05 MPa upon application of 600 MPa | EAB of the thermally processed BS film 5.65 ± 0.23% significantly increased to 7.92 ± 0.58% upon application of 600 MPa | To, Tm, and ΔH of thermally processed BS film 70.52 °C, 112.75 °C, and 78.64 J/g increased to 76.16 °C, 120.64 °C, and 79.30 J/g, respectively; upon application of 600 MPa | [30] |
| Tapioca-starch (TPS) | 600 MPa at 20 °C for 20 min | WS of the thermally processed TPS film 28.53 ± 0.68% significantly decreased to 17.53 ± 0.51% upon application of 600 MPa | No significant variation in WVP for TPS film when treated with HPP | TS of the thermally processed TPS film 24.67 ± 1.03 MPa significantly increased to 26.92 ± 0.43 MPa upon application of 600 MPa | EAB of the thermally processed TPS film 5.04 ± 0.56% significantly increased to 5.71 ± 0.20% when subjected to 600 MPa | To, and ΔH of thermally processed TPS increased from 70.92 °C and 56.92 J/g to 84.32 °C and 78.40 J/g, respectively but Tm decreased from 124.62 to 122.07 °C; upon application of 600 Mpa | |
| PVA, chitosan (CHI), and nano-TiO2 | 200, 400, and 600 MPa at 23 ± 2 °C for 15 min | -- | WVP of PVA–CHI–TiO2 (0.10%) (4.36 ± 0.308) × 10−12 g·cm/cm2·s·Pa significantly decreased to (3.60 ± 0.137) × 10−12, (3.47 ± 0.139) × 10−12, and (3.92 ± 0.0433) × 10−12 g·cm/cm2·s·Pa when subjected to 200, 400, and 600 MPa, respectively; OP of the film 1.34 ± 0.05 cm3 m−2·s−1·Pa−1 showed no significant variation when treated with 200 MPa but OP significantly decreased to 1.30 ± 0.05 and 1.25 ± 0.05 cm3 m−2·s−1·Pa−1 when treated with 400 and 600 MPa | TS of PVA–CHI–TiO2 (0.10%) 8.24 ± 0.27 MPa significantly increased to 13.67 ± 0.41, 13.98 ± 0.33, and 17.15 ± 0.97 when subjected to 200, 400, and 600 MPa, respectively | EAB of PVA–CHI–TiO2 (0.10%) 64.82 ± 1.10% significantly increased to 68.48 ± 1.66, 68.12 ± 1.94, and 67.92 ± 2.73% when subjected to 200, 400, and 600 MPa, respectively | -- | [36] |
| Chitosan | 100, 200, 300, 400, and 500 MPa for 15 min | -- | WVP and OP of the chitosan film decreased continuously when the pressure increased from 100 to 500 MPa | TS of film increased 35.2% as compared to the untreated film when treated at 400 MPa for 15 min but further increase in the pressure decreased the TS | EAB of the chitosan film decreased continuously as the pressure increased from 100 to 500 MPa | -- | [37] |
| Pigskin gelatin | 0.1, 300, and 600 MPa at 20, 40, and 60 °C for 5, 17.5, and 30 min | -- | WVTR of the untreated film 65.56 ± 1.2 g/(day m2) significantly decreased to 63.47 ± 0.9 g/(day m2), when subjected to 600 MPa for 30 min at 20.5 °C | TS of the untreated film 25.7 ± 2.2 MPa significantly increased to 28.7 ± 2.5 MPa when subjected to 600 MPa for 30 min at 20.5 °C | EAB of the untreated film 8.6 ± 0.6% insignificantly increased to 10.1 ± 1.5% when subjected to 600 MPa for 30 min at 20.5 °C | Tg, and Tm of the untreated film 58.8 ± 0.4, and 131.5 ± 0.7 °C increased to 60.7 ± 4.5, and 138.2 ± 0.5 °C, respectively, but ∆Hm decreased from 46.4 ± 0.8 to 36.5 ± 3.3 J/g, subjected to 600 MPa for 30 min at 20.5 °C | [38] |
| Amaranth protein | 200, 400, and 600 for 5 min | WS of the untreated film 79.9 ± 2.1% significantly decreased to 56.4 ± 5.5, 46.1 ± 0.5, and 46.1 ± 2.5% when treated with 200, 400, and 600 for 5 min, respectively | WVP of the untreated film (5.6 ± 0.5) × 10−12 g H2O/Pa m s significantly decreased to (4.8 ± 0.4) × 10−12, (4.6 ± 0.1) × 10−12, and (3.2 ± 0.6) × 10−12 g H2O/Pa m, when treated with 200, 400, and 600 for 5 min, respectively | TS of the control film increased by 26%, 101%, and 165% when subjected to 200, 400, and 600 for 5 min, respectively | No significant variation in EAB under high-pressure treatment | -- | [35] |
| Nisin-soy-protein-isolate | 100, 200, 300, 400, and 500 MPa at 20 °C for 10 min | -- | WVP of the untreated film significantly decreased as the pressure level increased from 100 to 500 MPa | TS of the untreated film significantly increased as the pressure level increased from 100 to 500 MPa | EAB of the untreated film significantly decreased as the pressure level increased from 100 to 500 MPa | -- | [29] |
| Whey protein concentrate, thyme (TEO) | 600 MPa at 70 °C, for 20 min | -- | WVP of thermally treated WPC-TEO film was (24.867 ± 2.855) × 10−10 g/s.m.Pa significantly decreased to (10.178 ± 1.690) × 10−10 g/s.m.Pa, when subjected to 600 MPa at 70 °C, for 20 min | -- | -- | -- | [39] |
| Poly (lactic acid) and Ag (5%) | 0, 200, and 400 MPa for 15 min at 25 °C | -- | WVP of untreated PLA/Ag-5% film (4.3 ± 0.3) × 10−10 (g·m/m2·s·Pa) significantly decrease to (2.8 ± 0.1) × 10−10 and (3.2 ± 0.2) × 10−10 (g·m/m2·s·Pa), when subjected to 200 and 400 MPa for 15 min | TS of untreated PLA/Ag-5% film 34 ± 2 MPa significantly increased to 36 ± 2 MPa at 400 MPa for 15 min | EAB of untreated PLA/Ag-5% film 170 ± 8% significantly decreased to 161 ± 14 and 119 ± 14%, when subjected to 400 MPa for 15 min | Tg, and Tc of PLA/Ag-5% film 50.1 ± 0.2, and 110.4 ± 0.4 °C significantly decreased to 51.9 ± 0.2, and 112.9 ± 0.5 °C, respectively, when treated with 400 MPa for 15 min; Tm showed no significant variation between treated and untreated film | [31] |
| Poly (lactic acid) and ZnO (0, 2.5, 5.0 and 10.0 % of PLA) | 0, 200 and 400 MPa for 10 min | -- | OP of the untreated PLA/ZnO-5% film 4.83 ± 0.13 (cm3 24 h−1 m−2) × (cm bar−1) slightly decreased to 3.02 ± 0.29 (cm3 24 h−1 m−2) × (cmbar−1) when subjected to 400 MPa for 10 min.; WVP of the PLA/ZnO-5% film decreased significantly when subjected to 400 MPa for 10 min. |
TS of untreated PLA/ZnO-5% film 35.8 ± 1.48 MPa, increased to 41.9 ± 1.43, and 42.9 ± 1.08 MPa when subjected to 200, and 400 MPa for 10 min, respectively | EAB of untreated PLA/ZnO-5% film 8.19 ± 0.17% decreased to 7.90 ± 0.34, and 7.61 ± 0.58% when treated with 200, and 400 MPa for 10 min, respectively | Tg and Tc of untreated PLA/ZnO-5% film 46.7 ± 1.82 and 95.9 ± 0.30 °C significantly increased to 49.8 ± 1.50 and 100.9 ± 0.70 °C and showed no significant variation in Tc when subjected to 400 MPa for 10 min | [40] |
2.2. Water Solubility (WS)
3. Effect of HPP on the Properties of Flexible Biopolymer-Based Packaging Materials
3.1. Surface Attributes (Morphological Characteristics)
3.2. Barrier Properties
3.3. Mechanical Properties
References
- Li, T.; Zhao, L.; Wang, Y.; Wu, X.; Liao, X. Effect of High Pressure Processing on the Preparation and Characteristic Changes of Biopolymer-Based Films in Food Packaging Applications. Food Eng. Rev. 2021, 13, 454–464.
- Huang, H.-W.; Hsu, C.-P.; Wang, C.-Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2020, 28, 1–13.
- Srinivas, M.S.; Madhu, B.; Srinivas, G.; Jain, S. High pressure processing of foods: A Review. Agric. J. 2018, 65, 467–476.
- Marangoni Júnior, L.; Cristianini, M.; Padula, M.; Anjos, C.A.R. Effect of high-pressure processing on characteristics of flexible packaging for foods and beverages. Food Res. Int. 2019, 119, 920–930.
- United States Department of Agriculture/Food Safety & Inspection Service (USDA-FSIS). 2021FSIS directives for Verification Activities for High Pressure Processing, Irradiation and Microwave Tempering. USDA-FSI: Washington, DC, USA; FSIS DIRECTIVE - 5000.15; SERIES TYPE - 5,000 Series: Program Services; Issue date Aug 02, 2021. Available online: https://www.fsis.usda.gov/policy/fsis-directives/5000.15 (accessed on 23 June 2022).
- Huang, H.-W.; Wu, S.-J.; Lu, J.-K.; Shyu, Y.-T.; Wang, C.-Y. Current status and future trends of high-pressure processing in food industry. Food Control 2017, 72, 1–8.
- Considine, K.M.; Kelly, A.L.; Fitzgerald, G.F.; Hill, C.; Sleator, R.D. High-pressure processing—effects on microbial food safety and food quality. FEMS Microbiol. Lett. 2008, 281, 1–9.
- Juliano, P.; Koutchma, T.; Sui, Q.; Barbosa-Cánovas, G.V.; Sadler, G. Polymeric-Based Food Packaging for High-Pressure Processing. Food Eng. Rev. 2010, 2, 274–297.
- Koutchma, T. Adapting High Hydrostatic Pressure (HPP) for Food Processing Operations; Academic Press: Cambridge, MA, USA, 2014.
- Food Safety Authority of Ireland, FSAI, 2020. Report of the Scientific Committee of the Food Safety Authority of Ireland for Appraisal of new and emerging food processing technologies and their potential risks to food safety. Issued date 2022. Available online: https://www.fsai.ie/Appraisalofnewemerging_foodprocessingtechnologies_foodsafety/ (accessed on 20 June 2022).
- Galotto, M.; Ulloa, P.; Guarda, A.; Gavara, R.; Miltz, J. Effect of High-Pressure food processing on the physical properties of synthetic and biopolymer films. J. Food Sci. 2009, 74, E304–E311.
- Zaghloul, M.M.Y.; Zaghloul, M.Y.M.; Zaghloul, M.M.Y. Experimental and modeling analysis of mechanical-electrical behaviors of polypropylene composites filled with graphite and MWCNT fillers. Polym. Test. 2017, 63, 467–474.
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Influence of flame retardant magnesium hydroxide on the mechanical properties of high density polyethylene composites. J. Reinf. Plast. Compos. 2017, 36, 1802–1816.
- Zaghloul, M.M.Y.M. Mechanical properties of linear low-density polyethylene fire-retarded with melamine polyphosphate. J. Appl. Polym. Sci. 2018, 135, 46770.
- López-Rubio, A.; Lagarón, J.M.; Hernández-Muñoz, P.; Almenar, E.; Catalá, R.; Gavara, R.; Pascall, M.A. Effect of high pressure treatments on the properties of EVOH-based food packaging materials. Innov. Food Sci. Emerg. Technol. 2005, 6, 51–58.
- Marangoni Junior, L.; Alves, R.M.V.; Moreira, C.Q.; Cristianini, M.; Padula, M.; Anjos, C.A.R. High-pressure processing effects on the barrier properties of flexible packaging materials. J. Food Processing Preserv. 2020, 44, e14865.
- Hoque, M.; McDonagh, C.; Tiwari, B.K.; Kerry, J.P.; Pathania, S. Effect of Cold Plasma Treatment on the Packaging Properties of Biopolymer-Based Films: A Review. Appl. Sci. 2022, 12, 1346.
- Gasti, T.; Dixit, S.; Hiremani, V.D.; Chougale, R.B.; Masti, S.P.; Vootla, S.K.; Mudigoudra, B.S. Chitosan/pullulan based films incorporated with clove essential oil loaded chitosan-ZnO hybrid nanoparticles for active food packaging. Carbohydr. Polym. 2022, 277, 118866.
- Chakraborty, P.; Nath, D.; Hoque, M.; Sarkar, P.; Hati, S.; Mishra, B.K. Biopolymer-based antimicrobial coatings for aquatic food products: A review. J. Food Processing Preserv. 2022, 46, e16465.
- Agarwal, S.; Hoque, M.; Bandara, N.; Pal, K.; Sarkar, P. Synthesis and characterization of tamarind kernel powder-based antimicrobial edible films loaded with geraniol. Food Packag. Shelf Life 2020, 26, 100562.
- Hoque, M.; Sarkar, P.; Ahmed, J. Preparation and characterization of tamarind kernel powder/ZnO nanoparticle-based food packaging films. Ind. Crops Prod. 2022, 178, 114670.
- Hoque, M.; Gupta, S.; Santhosh, R.; Syed, I.; Sarkar, P. 3-Biopolymer-based edible films and coatings for food applications. In Food, Medical, and Environmental Applications of Polysaccharides; Pal, K., Banerjee, I., Sarkar, P., Bit, A., Kim, D., Anis, A., Maji, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 81–107.
- Agarwal, S.; Hoque, M.; Mohapatra, N.; Syed, I.; Dhumal, C.V.; Bose, S.; Biswas, P.K.; Kar, P.; Bishoyi, N.; Sarkar, P. Chapter 19-Oil-entrapped films. In Biopolymer-Based Formulations; Pal, K., Banerjee, I., Sarkar, P., Kim, D., Deng, W.-P., Dubey, N.K., Majumder, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 425–444.
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Effects of high pressure processing on gelation properties and molecular forces of myosin containing deacetylated konjac glucomannan. Food Chem. 2019, 291, 117–125.
- Cadesky, L.; Walkling-Ribeiro, M.; Kriner, K.T.; Karwe, M.V.; Moraru, C.I. Structural changes induced by high-pressure processing in micellar casein and milk protein concentrates. J. Dairy Sci. 2017, 100, 7055–7070.
- Martínez, M.A.; Velazquez, G.; Cando, D.; Núñez-Flores, R.; Borderías, A.J.; Moreno, H.M. Effects of high pressure processing on protein fractions of blue crab (Callinectes sapidus) meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 323–329.
- Leite, T.S.; de Jesus, A.L.T.; Schmiele, M.; Tribst, A.A.L.; Cristianini, M. High pressure processing (HPP) of pea starch: Effect on the gelatinization properties. LWT-Food Sci. Technol. 2017, 76, 361–369.
- Devi, A.F.; Buckow, R.; Hemar, Y.; Kasapis, S. Modification of the structural and rheological properties of whey protein/gelatin mixtures through high pressure processing. Food Chem. 2014, 156, 243–249.
- Wei, J.; Zhang, Z.; Cai, Q.; Peng, B. Effects of high hydrostatic pressure on structural and physical properties of nisin-SPI film. Int. J. Biol. Macromol. 2018, 111, 976–982.
- Kim, S.; Yang, S.-Y.; Chun, H.H.; Song, K.B. High hydrostatic pressure processing for the preparation of buckwheat and tapioca starch films. Food Hydrocoll. 2018, 81, 71–76.
- Chi, H.; Xue, J.; Zhang, C.; Chen, H.; Li, L.; Qin, Y. High Pressure Treatment for Improving Water Vapour Barrier Properties of Poly(lactic acid)/Ag Nanocomposite Films. Polymers 2018, 10, 1011.
- Fan, C.; Cui, R.; Lu, W.; Chen, H.; Yuan, M.; Qin, Y. Effect of high pressure treatment on properties and nano–Ag migration of PLA-based food packaging film. Polym. Test. 2019, 76, 73–81.
- Larrea-Wachtendorff, D.; Sousa, I.; Ferrari, G. Starch-Based Hydrogels Produced by High-Pressure Processing (HPP): Effect of the Starch Source and Processing Time. Food Eng. Rev. 2021, 13, 622–633.
- Mulla, M.Z.; Subramanian, P.; Dar, B.N. Functionalization of legume proteins using high pressure processing: Effect on technofunctional properties and digestibility of legume proteins. LWT 2022, 158, 113106.
- Condés, M.C.; Añón, M.C.; Mauri, A.N. Amaranth protein films prepared with high-pressure treated proteins. J. Food Eng. 2015, 166, 38–44.
- Lian, Z.; Zhang, Y.; Zhao, Y. Nano-TiO2 particles and high hydrostatic pressure treatment for improving functionality of polyvinyl alcohol and chitosan composite films and nano-TiO2 migration from film matrix in food simulants. Innov. Food Sci. Emerg. Technol. 2016, 33, 145–153.
- Niu, Y.Q.; Chen, S.S.; Gao, Y.P.; Ma, Z.S. The properties of ultra-high pressure treated chitosan edible films. In Advanced Materials Research; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2012.
- Molinaro, S.; Cruz-Romero, M.; Sensidoni, A.; Morris, M.; Lagazio, C.; Kerry, J.P. Combination of high-pressure treatment, mild heating and holding time effects as a means of improving the barrier properties of gelatin-based packaging films using response surface modeling. Innov. Food Sci. Emerg. Technol. 2015, 30, 15–23.
- Bleoancă, I.; Enachi, E.; Borda, D. Thyme Antimicrobial Effect in Edible Films with High Pressure Thermally Treated Whey Protein Concentrate. Foods 2020, 9, 855.
- Cui, R.; Fan, C.; Dong, X.; Fang, K.; Lin, L.; Qin, Y. Effect of ultrahigh-pressure treatment on the functional properties of poly(lactic acid)/ZnO nanocomposite food packaging film. J. Sci. Food Agric. 2021, 101, 4925–4933.
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356.
- Yang, Z.; Chaib, S.; Gu, Q.; Hemar, Y. Impact of pressure on physicochemical properties of starch dispersions. Food Hydrocoll. 2017, 68, 164–177.
- Katopo, H.; Song, Y.; Jane, J.-l. Effect and mechanism of ultrahigh hydrostatic pressure on the structure and properties of starches. Carbohydr. Polym. 2002, 47, 233–244.
- Kim, H.-S.; Choi, H.-S.; Kim, B.-Y.; Baik, M.-Y. Characterization of Acetylated Corn Starch Prepared under Ultrahigh Pressure (UHP). J. Agric. Food Chem. 2010, 58, 3573–3579.
- Ahmed, J.; Mulla, M.Z.; Vahora, A.; Bher, A.; Auras, R. Morphological, barrier and thermo-mechanical properties of high-pressure treated polylactide graphene oxide reinforced composite films. Food Packag. Shelf Life 2021, 29, 100702.
- Richter, T.; Sterr, J.; Jost, V.; Langowski, H.-C. High pressure-induced structural effects in plastic packaging. High Press. Res. 2010, 30, 555–566.
- Fleckenstein, B.S.; Sterr, J.; Langowski, H.C. The effect of high pressure processing on the integrity of polymeric packaging–analysis and categorization of occurring defects. Packag. Technol. Sci. 2014, 27, 83–103.
- Sansone, L.; Aldi, A.; Musto, P.; Di Maio, E.; Amendola, E.; Mensitieri, G. Assessing the suitability of polylactic acid flexible films for high pressure pasteurization and sterilization of packaged foodstuff. J. Food Eng. 2012, 111, 34–45.
- Bahrami, R.; Zibaei, R.; Hashami, Z.; Hasanvand, S.; Garavand, F.; Rouhi, M.; Jafari, S.M.; Mohammadi, R. Modification and improvement of biodegradable packaging films by cold plasma; a critical review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1936–1950.
- Ahmed, J.; Mulla, M.Z.; Vohra, A. High-pressure treatment of water-filled co-extruded polylactide films: Effect on microstructure, barrier, thermal, and rheological properties. J. Food Sci. 2022, 87, 1754–1766.
- Gonçalves, S.M.; Chávez, D.W.H.; Oliveira, L.M.d.; Sarantópoulos, C.I.G.d.L.; Carvalho, C.W.P.d.; Melo, N.R.d.; Rosenthal, A. Effects of high hydrostatic pressure processing on structure and functional properties of biodegradable film. Heliyon 2020, 6, e05213.
- Chi, H.; Li, W.; Fan, C.; Zhang, C.; Li, L.; Qin, Y.; Yuan, M. Effect of High Pressure Treatment on Poly(lactic acid)/Nano–TiO2 Composite Films. Molecules 2018, 23, 2621.
- Ahmed, J.; Mulla, M.; Arfat, Y.A. Application of high-pressure processing and polylactide/cinnamon oil packaging on chicken sample for inactivation and inhibition of Listeria monocytogenes and Salmonella Typhimurium, and post-processing film properties. Food Control 2017, 78, 160–168.




