Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Γεώργιος Ντίνος | -- | 1595 | 2022-08-09 21:47:15 | | | |
| 2 | Rita Xu | Meta information modification | 1595 | 2022-08-10 03:50:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kournoutou, G.G.; Dinos, G. Azithromycin. Encyclopedia. Available online: https://encyclopedia.pub/entry/25998 (accessed on 06 February 2026).
Kournoutou GG, Dinos G. Azithromycin. Encyclopedia. Available at: https://encyclopedia.pub/entry/25998. Accessed February 06, 2026.
Kournoutou, Georgia G., George Dinos. "Azithromycin" Encyclopedia, https://encyclopedia.pub/entry/25998 (accessed February 06, 2026).
Kournoutou, G.G., & Dinos, G. (2022, August 09). Azithromycin. In Encyclopedia. https://encyclopedia.pub/entry/25998
Kournoutou, Georgia G. and George Dinos. "Azithromycin." Encyclopedia. Web. 09 August, 2022.
Copy Citation
Azithromycin has become famous in the last two years, not for its main antimicrobial effect, but for its potential use as a therapeutic agent for COVID-19 infection. Initially, there were some promising results that supported its use, but it has become clear that scientific results are insufficient to support such a positive assessment.
macrolides
azithromycin
virus
coronavirus
1. Introduction
Azithromycin (Azi) belongs to the large family of macrolide antibiotics, an important class of first-line antimicrobial agents [1]. Azi belongs to the second generation of macrolides, as a semisynthetic derivative of erythromycin with a modified macrolactone ring with 15 members instead of 14 members as in erythromycin (Figure 1). Although Azi did not exhibit improved activity against Gram-positive bacteria compared to the mother compound erythromycin [2][3][4], it was selected for further development due to its enhanced pharmacokinetic profiles. In particular, it was selected for its high half-life time and the ability to accumulate at high levels within lung tissue [5][6][7][8][9]. Clarithromycin (Figure 1) is another key second-generation 14-membered macrolide with similar features and structure, and while it was initially included in a few trial schemes as a potential therapeutic drug for COVID-19, it was rapidly discontinued [10][11]. Azi, like most of the other macrolides, is not only known for its antimicrobial activity, but it also has additional actions as either anti-inflammatory or antivirus agents.
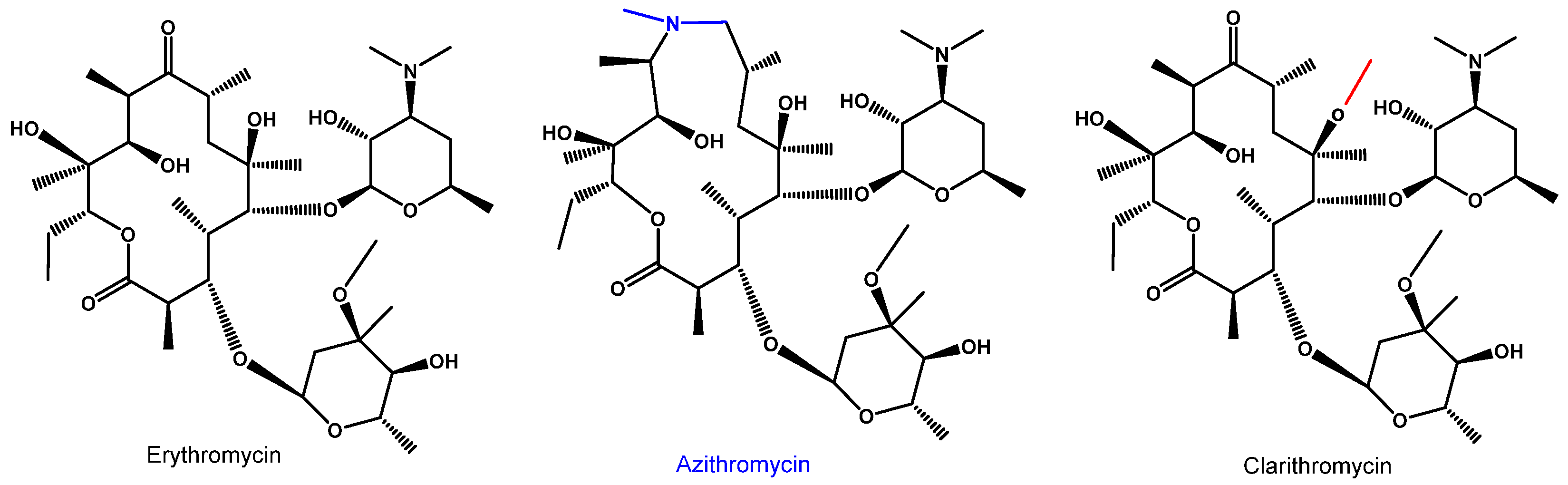
Figure 1. Molecular structure of the mother macrolide molecule erythromycin and its semisynthetic derivatives azithromycin (15-membered) and clarithromycin (14-membered). Blue and red colors in the structures represent modifications of the mother molecule (black).
2. Azithromycin as an Antimicrobial Agent
The antimicrobial activity of azithromycin results from its binding with high affinity to the entrance of the ribosomal exit tunnel of prokaryotic 70S ribosomes and strongly inhibiting the bacterial protein synthesis [12][13][14]. According to the crystal structure data, it binds to the entrance of the nascent peptide exit tunnel and partially occludes it (Figure 2).
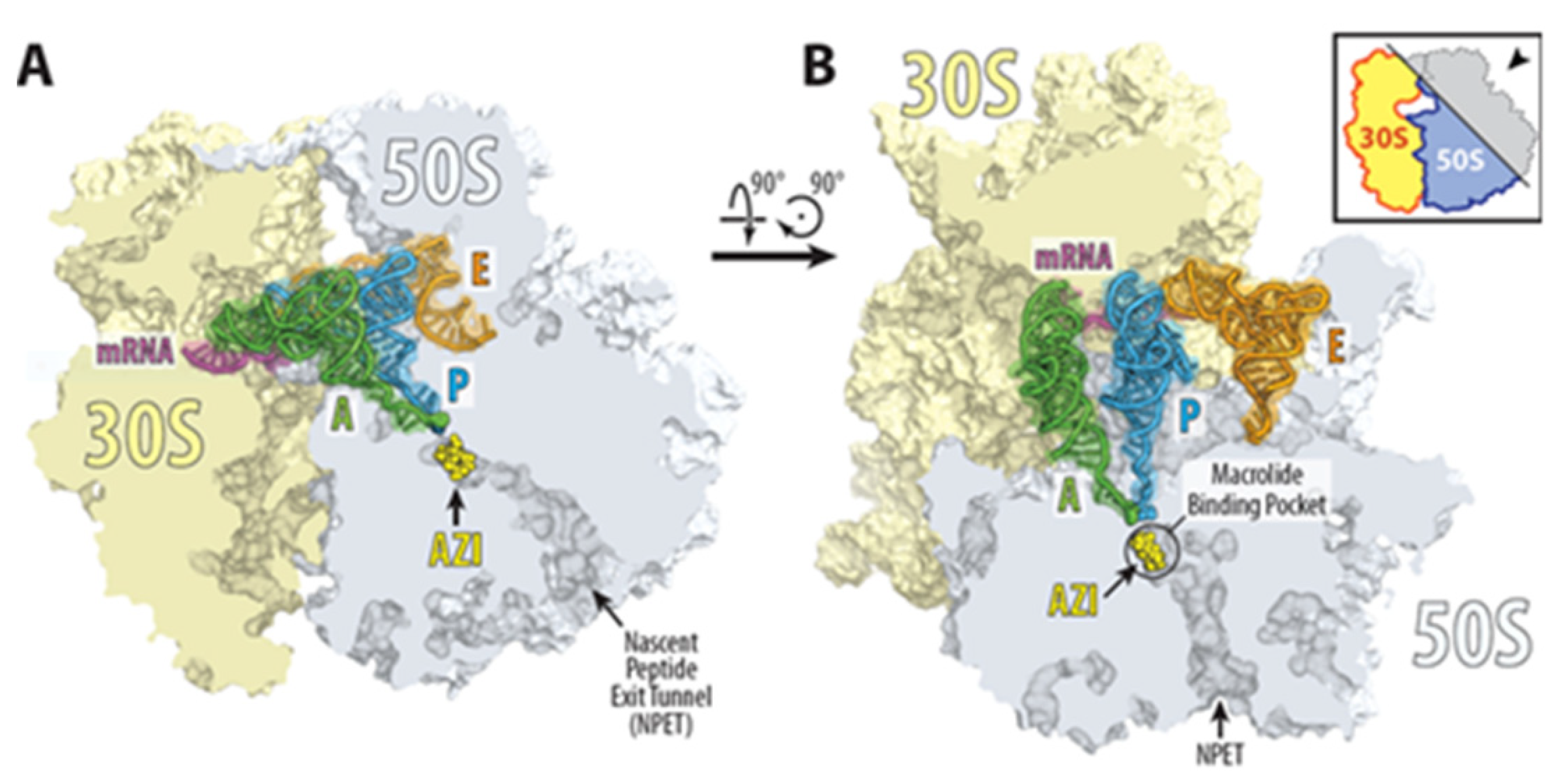
Figure 2. Structure of azithromycin in complex with the 70S ribosome carrying A-, P-, and E-site tRNAs. (A,B) Location of the ribosome-bound azithromycin (yellow) in the macrolide binding pocket at the entrance to the nascent peptide exit tunnel (NPET) of the 70S ribosome relative to tRNAs viewed as cross-cut sections through the ribosome. The 30S subunit is shown in light yellow, the 50S subunit is in light blue, the mRNA is in magenta, and the A-, P-, and E-site tRNAs are colored green, dark blue, and orange, respectively. The phenylalanyl and formyl-methionyl moieties of the A- and P-site tRNAs are shown as spheres [15].
Thus, Azi was considered as ‘tunnel plugs’ that inhibit the synthesis of every protein entering the exit tunnel [1][16]. However, more recent evidence demonstrates that macrolides selectively inhibit the translation of a subset of cellular proteins and that their action crucially depends on the nascent protein sequence and the antibiotic structure [17].
Recent studies have shown that the translation of many genes was arrested at a few distinct sites through the length of the gene after treatment with macrolide antibiotics. Analysis of the sites of the stops revealed the existence of specific sequence signatures that induce pronounced drug-induced translation arrest and lead to specific regulation of protein synthesis. Ribo-seq and toeprinting experiments have revealed leader ORFs of macrolide resistance genes carrying the +x+ motif, where + stands for positively charged amino acids lysine or arginine, and x stands for any amino acid [17][18].
Therefore, Azi emerges as a modulator of translation rather than as a global inhibitor of protein synthesis. In general, macrolide antibiotics are active mainly against Gram-positive bacteria and have a lower activity against Gram-negative bacteria [19][20]. Macrolides are very active against Gram-positive bacteria Staphylococcus, Streptococcus, and Diplococcus; among Gram-negative cocci, Neisseria gonorrhea, Haemophilus influenzae, Bordetella pertussis, and Neisseria meningitis; and are extremely active against various Mycoplasmas. Since its discovery Azi has been extensively used in the treatment of bacterial and mycobacterial infections of the respiratory, gastrointestinal, genitourinary, and cutaneous systems [21][22][23]. Azi is a member of the WHO list of essential medications [24] and is available in large quantities worldwide. Despite some mild side effects, including mainly diarrhea and QT prolongation, Azi is proven to be safe and cheap, and therefore, easily available to humans worldwide [21][22].
3. Azithromycin as an Anti-Inflammatory Agent
Beyond the antibacterial activity of azithromycin, and broadly most macrolides, their anti-inflammatory effects have been established and some of them have been used in chronic inflammatory diseases such as chronic rhinosinusitis, bronchial asthma, bronchiectasis, chronic obstructive pulmonary disease, cystic fibrosis, etc. [23][24][25][26][27]. Probably, the most striking example of their immunomodulation comes from diffuse panbronchiolitis, an idiopathic inflammation and progressively destructive disease of the bronchioles which can be converted from a lethal to a treatable disease with daily low-dose erythromycin or Azi [23][28][29]. This has been accredited to the ability of Azi to normalize the upregulated activities of IL-1β, IL-2, TNF, and GM-CSF [30]. Azi is rapidly absorbed after oral administration with a half-life time of approximately 3 days, leading to a high and constant tissue concentration [23][31]. As a result, Azi accumulates in human cells, including epithelial cells, and most notably in phagocytes where it has been concentrated hundreds to thousands of times with a focus on phagocyte lysosomes [9][31]. Its anti-inflammatory or immunomodulatory activity reported in several studies includes the most frequent effects on neutrophils, monocytes, and lymphocytes [27][29][32]. Among the usually measured immunological modified markers are the number of decreased neutrophils; the concentrations of neutrophil elastase; cytokines release; surface-expressed molecules (mainly Toll-like receptors); superoxide production; and cell homeostasis, mainly apoptosis and phagocytosis (Figure 3) [27][29][32][33]. Neutrophil function inhibition has been reported more frequently than eosinophil function inhibition. Azi stimulates neutrophil degranulation and phagocytosis-associated oxidative burst, mediated via modulation of ERK 1/2 signaling [19]. These initial stimulatory effects are followed by modulation of transcription factors activator protein (AP)-1, nuclear factor kappa B (NFκB), inflammatory cytokines, and mucin release, with overall anti-inflammatory effects [34].
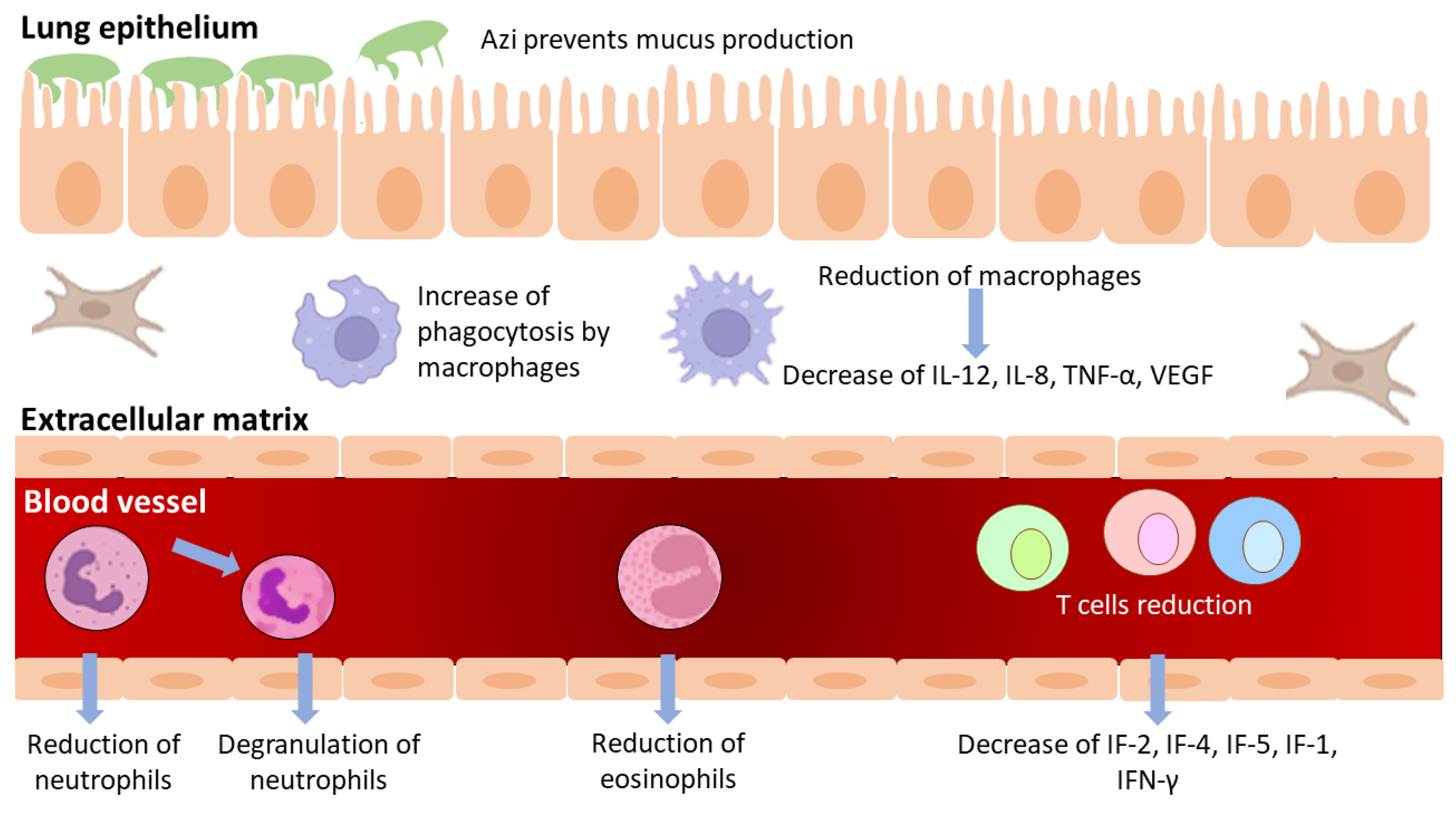
Figure 3. Immunomodulatory effects of azithromycin.
Azi inhibits lipopolysaccharide-induced pro-inflammatory cytokines; increases phagocytosis by inhibiting AP-1 [35]; improves lysosomal resistance to oxidant challenge [36]; and promotes M2 polarization of macrophages (a process in which macrophages produce distinct functional phenotypes in response to specific microenvironmental stimuli and signals) [37][38][39]. Azi can also increase the phagocytosis of apoptotic epithelial cells [40] and neutrophils by macrophages [41] further supporting its anti-inflammatory activity. Studies have shown that part of the immunomodulatory effects of macrolides could be attributed to the impairment of TLR signaling by reducing the release of PAMPs (Pathogen-Associated Molecular Patterns) and inhibiting TLR expression, either of dendritic cells or macrophages, thereby regulating the immune response [33][42].
Immunomodulatory effects, although similar to most therapeutic macrolides, are likely to differ among them. Few studies have examined the anti-inflammatory effects of macrolides on more than one macrolide, and none of the human trials have explicitly compared different macrolides. Furthermore, the majority of these trials were conducted on healthy volunteers and/or Azi was administered in varying doses at a time [27][43][44][45]. Clinical investigations in CF patients, on the other hand, revealed that Azi, but not Clarithromycin, improves respiratory function and reduces pulmonary exacerbations [46][47]. Additionally, another study showed that Azi, but not clarithromycin or roxithromycin, inhibits IL-1alpha and IL-1beta production [48]. In general, azithromycin inhibits the synthesis of pro-inflammatory cytokines by both innate and adaptive immune cells, as well as the accumulation, adhesion, and death of pulmonary neutrophils [32].
Azithromycin, like other macrolides, has very low activity against eukaryotes due to their low affinity for binding to eukaryotic ribosomes [1]. There are specific differences between eukaryotic and bacterial ribosomes (differences between rRNA bases or ribosomal proteins) that mediate the selectivity and toxicity of ribosomal drugs, as established by rRNA sequencing studies and X-ray crystallography [49].
4. Azithromycin as an Antivirus Agent
Azi’s antiviral effects have been demonstrated in vitro, albeit not all examples have been confirmed in vivo [32]. Since Azi mediated exacerbations in airway diseases, particularly in asthma [25][50], its effects were studied against viruses that cause such airway infections such as rhinoviruses (RV). Azi inhibits RV replication and releases in primary human bronchial epithelial cells in vitro [51]. The AMAZES research, the largest clinical trial of a long-term macrolide on airway diseases, found that Azi reduced asthma exacerbations by 40% in vivo [25]. The mechanism is not known, but metagenomic analysis suggested that it could be related to an antibacterial effect versus Haemophilus influenzae and possibly its abundance in inhaled air [52][53][54]. Pre-treatment with azithromycin inhibits RV replication in CF bronchial epithelial cells, probably by amplifying the antiviral response mediated by the IFN pathway [55]. Additionally, Azi showed a reduction in H1N1 viral replication in A549 cells with IC50 58 μM interfering with the internalization of viruses [56]. In experiments with the Zika virus, within glial cell lines and human astrocytes, there was a reduction in viral growth and virus-induced cytotoxicity [57]. Equally, Azi inhibited Ebola replication with EC50 5.1 μM and low toxicity; however, it did not boost survival in mice or guinea pigs when tested in vivo in a mouse model [58].
The precise mechanism of the antiviral activity of Azi remains unclear. Given that Azi is a weak base it can accumulate in acidic intracellular organelles such as endosomal vesicles and lysosomes [59]. In keeping with lysosomal accumulation, azithromycin causes lysosomal pH change [23]. This modified acidic environment caused by accumulation of Azi could also be responsible for uncoating enveloped viruses such as influenza and maybe coronavirus [59]. Data also suggest that the antiviral activity of Azi could be attributed to its ability to increase the expression of the epithelial interferon genes, leading to a reduction in viral replication [60].
Recently, Azi and spiramycin (a natural 16-membered ring macrolide) provided significant in vivo protection against enterovirus-A71 infection in mice [61]. Spiramycin was found to interfere with EV-A71 viral RNA synthesis, and it is likely that spiramycin and Azi function in concert after the viral entrance; thereby, inhibiting viral RNA synthesis either directly or indirectly.
References
- Dinos, G.P. The Macrolide Antibiotic Renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983.
- Barry, A.L.; Jones, R.N.; Thornsberry, C. In Vitro Activities of Azithromycin (CP 62,993), Clarithromycin (A-56268; TE-031), Erythromycin, Roxithromycin, and Clindamycin. Antimicrob. Agents Chemother. 1988, 32, 752–754.
- Barry, A.L.; Fuchs, P.C.; Brown, S.D. Relative Potency of Telithromycin, Azithromycin and Erythromycin against Recent Clinical Isolates of Gram-Positive Cocci. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2001, 20, 494–497.
- Fernandes, P.B.; Hardy, D.J. Comparative in Vitro Potencies of Nine New Macrolides. Drugs Exp. Clin. Res. 1988, 14, 445–451.
- Wise, R. The Development of Macrolides and Related Compounds. J. Antimicrob. Chemother. 1989, 23, 299–300.
- Foulds, G.; Shepard, R.M.; Johnson, R.B. The Pharmacokinetics of Azithromycin in Human Serum and Tissues. J. Antimicrob. Chemother. 1990, 25 (Suppl. A), 73–82.
- Retsema, J.A.; Girard, A.E.; Girard, D.; Milisen, W.B. Relationship of High Tissue Concentrations of Azithromycin to Bactericidal Activity and Efficacy in Vivo. J. Antimicrob. Chemother. 1990, 25 (Suppl. A), 83–89.
- Hardy, D.J.; Guay, D.R.P.; Jones, R.N. Clarithromycin, a Unique Macrolide. A Pharmacokinetic, Microbiological, and Clinical Overview. Diagn. Microbiol. Infect. Dis. 1992, 15, 39–53.
- Wildfeuer, A.; Laufen, H.; Zimmermann, T. Distribution of Orally Administered Azithromycin in Various Blood Compartments. Int. J. Clin. Pharmacol. Ther. 1994, 32, 356–360.
- Ayerbe, L.; Risco-Risco, C.; Forgnone, I.; Pérez-Piñar, M.; Ayis, S. Azithromycin in Patients with COVID-19: A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2022, 77, 303–309.
- Oldenburg, C.E.; Pinsky, B.A.; Brogdon, J.; Chen, C.; Ruder, K.; Zhong, L.; Nyatigo, F.; Cook, C.A.; Hinterwirth, A.; Lebas, E.; et al. Effect of Oral Azithromycin vs Placebo on COVID-19 Symptoms in Outpatients With SARS-CoV-2 Infection: A Randomized Clinical Trial. JAMA 2021, 326, 490–498.
- Schlünzen, F.; Zarivach, R.; Harms, J.; Bashan, A.; Tocilj, A.; Albrecht, R.; Yonath, A.; Franceschi, F. Structural Basis for the Interaction of Antibiotics with the Peptidyl Transferase Centre in Eubacteria. Nature 2001, 413, 814–821.
- Bulkley, D.; Innis, C.A.; Blaha, G.; Steitz, T.A. Revisiting the Structures of Several Antibiotics Bound to the Bacterial Ribosome. Proc. Natl. Acad. Sci. USA 2010, 107, 17158–17163.
- Dunkle, J.A.; Xiong, L.; Mankin, A.S.; Cate, J.H.D. Structures of the Escherichia Coli Ribosome with Antibiotics Bound near the Peptidyl Transferase Center Explain Spectra of Drug Action. Proc. Natl. Acad. Sci. USA 2010, 107, 17152–17157.
- Svetlov, M.S.; Plessa, E.; Chen, C.W.; Bougas, A.; Krokidis, M.G.; Dinos, G.P.; Polikanov, Y.S. High-Resolution Crystal Structures of Ribosome-Bound Chloramphenicol and Erythromycin Provide the Ultimate Basis for Their Competition. RNA 2019, 25, 600–606.
- Kannan, K.; Kanabar, P.; Schryer, D.; Florin, T.; Oh, E.; Bahroos, N.; Tenson, T.; Weissman, J.S.; Mankin, A.S. The General Mode of Translation Inhibition by Macrolide Antibiotics. Proc. Natl. Acad. Sci. USA 2014, 111, 15958–15963.
- Vázquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684.
- Beckert, B.; Leroy, E.C.; Sothiselvam, S.; Bock, L.V.; Svetlov, M.S.; Graf, M.; Arenz, S.; Abdelshahid, M.; Seip, B.; Grubmüller, H.; et al. Structural and Mechanistic Basis for Translation Inhibition by Macrolide and Ketolide Antibiotics. Nat. Commun. 2021, 12, 4466.
- Ishimoto, H.; Mukae, H.; Sakamoto, N.; Amenomori, M.; Kitazaki, T.; Imamura, Y.; Fujita, H.; Ishii, H.; Nakayama, S.; Yanagihara, K.; et al. Different Effects of Telithromycin on MUC5AC Production Induced by Human Neutrophil Peptide-1 or Lipopolysaccharide in NCI-H292 Cells Compared with Azithromycin and Clarithromycin. J. Antimicrob. Chemother. 2009, 63, 109–114.
- Nakayama, I. Macrolides in clinical practice. In Macrolide Antibiotics: Chemistry, Biology and Practice, 1st ed.; Omura, S., Ed.; Academic Press: Orlando, FL, USA, 2022.
- Firth, A.; Prathapan, P. Azithromycin: The First Broad-Spectrum Therapeutic. Eur. J. Med. Chem. 2020, 207, 112739.
- Taylor, W.R.; Richie, T.L.; Fryauff, D.J.; Ohrt, C.; Picarima, H.; Tang, D.; Murphy, G.S.; Widjaja, H.; Braitman, D.; Tjitra, E.; et al. Tolerability of Azithromycin as Malaria Prophylaxis in Adults in Northeast Papua, Indonesia. Antimicrob. Agents Chemother. 2003, 47, 2199–2203.
- Parnham, M.J.; Erakovic Haber, V.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of Action and Their Relevance for Clinical Applications. Pharmacol. Ther. 2014, 143, 225–245.
- WHO. WHO Model List of Essential Medicines - 22nd List. 2021. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02. (accessed on 15 February 2022).
- Gibson, P.G.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; Marks, G.B.; Baraket, M.; et al. Effect of Azithromycin on Asthma Exacerbations and Quality of Life in Adults with Persistent Uncontrolled Asthma (AMAZES): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet (Lond. Engl.) 2017, 390, 659–668.
- Hansen, M.P.; Scott, A.M.; McCullough, A.; Thorning, S.; Aronson, J.K.; Beller, E.M.; Glasziou, P.P.; Hoffmann, T.C.; Clark, J.; Del Mar, C.B. Adverse Events in People Taking Macrolide Antibiotics versus Placebo for Any Indication. Cochrane Database Syst. Rev. 2019, 18, CD011825.
- Zimmermann, P.; Ziesenitz, V.C.; Curtis, N.; Ritz, N. The Immunomodulatory Effects of Macrolides-A Systematic Review of the Underlying Mechanisms. Front. Immunol. 2018, 9, 302.
- Kudoh, S.; Azuma, A.; Yamamoto, M.; Izumi, T.; Ando, M. Improvement of Survival in Patients with Diffuse Panbronchiolitis Treated with Low-Dose Erythromycin. Am. J. Respir. Crit. Care Med. 1998, 157, 1829–1832.
- Zarogoulidis, P.; Papanas, N.; Kioumis, I.; Chatzaki, E.; Maltezos, E.; Zarogoulidis, K. Macrolides: From in Vitro Anti-Inflammatory and Immunomodulatory Properties to Clinical Practice in Respiratory Diseases. Eur. J. Clin. Pharmacol. 2012, 68, 479–503.
- Weng, D.; Wu, Q.; Chen, X.-Q.; Du, Y.-K.; Chen, T.; Li, H.; Tang, D.-L.; Li, Q.-H.; Zhang, Y.; Lu, L.-Q.; et al. Azithromycin Treats Diffuse Panbronchiolitis by Targeting T Cells via Inhibition of MTOR Pathway. Biomed. Pharmacother. 2019, 110, 440–448.
- Wilms, E.B.; Touw, D.J.; Heijerman, H.G.M. Pharmacokinetics of Azithromycin in Plasma, Blood, Polymorphonuclear Neutrophils and Sputum during Long-Term Therapy in Patients with Cystic Fibrosis. Ther. Drug Monit. 2006, 28, 219–225.
- Oliver, M.E.; Hinks, T.S.C. Azithromycin in Viral Infections. Rev. Med. Virol. 2021, 31, e2163.
- Reijnders, T.D.Y.; Saris, A.; Schultz, M.J.; van der Poll, T. Immunomodulation by Macrolides: Therapeutic Potential for Critical Care. Lancet. Respir. Med. 2020, 8, 619–630.
- Araki, N.; Yanagihara, K.; Morinaga, Y.; Yamada, K.; Nakamura, S.; Yamada, Y.; Kohno, S.; Kamihira, S. Azithromycin Inhibits Nontypeable Haemophilus Influenzae-Induced MUC5AC Expression and Secretion via Inhibition of Activator Protein-1 in Human Airway Epithelial Cells. Eur. J. Pharmacol. 2010, 644, 209–214.
- Hodge, S.; Hodge, G.; Jersmann, H.; Matthews, G.; Ahern, J.; Holmes, M.; Reynolds, P.N. Azithromycin Improves Macrophage Phagocytic Function and Expression of Mannose Receptor in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2008, 178, 139–148.
- Persson, H.L.; Vainikka, L.K.; Sege, M.; Wennerström, U.; Dam-Larsen, S.; Persson, J. Leaky Lysosomes in Lung Transplant Macrophages: Azithromycin Prevents Oxidative Damage. Respir. Res. 2012, 13, 83.
- Murphy, B.S.; Sundareshan, V.; Cory, T.J.; Hayes, D.J.; Anstead, M.I.; Feola, D.J. Azithromycin Alters Macrophage Phenotype. J. Antimicrob. Chemother. 2008, 61, 554–560.
- Legssyer, R.; Huaux, F.; Lebacq, J.; Delos, M.; Marbaix, E.; Lebecque, P.; Lison, D.; Scholte, B.J.; Wallemacq, P.; Leal, T. Azithromycin Reduces Spontaneous and Induced Inflammation in DeltaF508 Cystic Fibrosis Mice. Respir. Res. 2006, 7, 134.
- Yamauchi, K.; Shibata, Y.; Kimura, T.; Abe, S.; Inoue, S.; Osaka, D.; Sato, M.; Igarashi, A.; Kubota, I. Azithromycin Suppresses Interleukin-12p40 Expression in Lipopolysaccharide and Interferon-Gamma Stimulated Macrophages. Int. J. Biol. Sci. 2009, 5, 667–678.
- Hodge, S.; Hodge, G.; Brozyna, S.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Azithromycin Increases Phagocytosis of Apoptotic Bronchial Epithelial Cells by Alveolar Macrophages. Eur. Respir. J. 2006, 28, 486–495.
- Yamaryo, T.; Oishi, K.; Yoshimine, H.; Tsuchihashi, Y.; Matsushima, K.; Nagatake, T. Fourteen-Member Macrolides Promote the Phosphatidylserine Receptor-Dependent Phagocytosis of Apoptotic Neutrophils by Alveolar Macrophages. Antimicrob. Agents Chemother. 2003, 47, 48–53.
- Huang, S.-W.; Chen, Y.-J.; Wang, S.-T.; Ho, L.-W.; Kao, J.-K.; Narita, M.; Takahashi, M.; Wu, C.-Y.; Cheng, H.-Y.; Shieh, J.-J. Azithromycin Impairs TLR7 Signaling in Dendritic Cells and Improves the Severity of Imiquimod-Induced Psoriasis-like Skin Inflammation in Mice. J. Dermatol. Sci. 2016, 84, 59–70.
- Culić, O.; Eraković, V.; Cepelak, I.; Barisić, K.; Brajsa, K.; Ferencić, Z.; Galović, R.; Glojnarić, I.; Manojlović, Z.; Munić, V.; et al. Azithromycin Modulates Neutrophil Function and Circulating Inflammatory Mediators in Healthy Human Subjects. Eur. J. Pharmacol. 2002, 450, 277–289.
- Criqui, G.I.; Solomon, C.; Welch, B.S.; Ferrando, R.E.; Boushey, H.A.; Balmes, J.R. Effects of Azithromycin on Ozone-Induced Airway Neutrophilia and Cytokine Release. Eur. Respir. J. 2000, 15, 856–862.
- Aubert, J.D.; Juillerat-Jeanneret, L.; Fioroni, P.; Dayer, P.; Plan, P.A.; Leuenberger, P. Function of Human Alveolar Macrophages after a 3-Day Course of Azithromycin in Healthy Volunteers. Pulm. Pharmacol. Ther. 1998, 11, 263–269.
- Southern, K.W.; Barker, P.M.; Solis-Moya, A.; Patel, L. Macrolide Antibiotics for Cystic Fibrosis. Cochrane database Syst. Rev. 2012, 11, CD002203.
- Robinson, P.; Schechter, M.S.; Sly, P.D.; Winfield, K.; Smith, J.; Brennan, S.; Shinkai, M.; Henke, M.O.; Rubin, B.K. Clarithromycin Therapy for Patients with Cystic Fibrosis: A Randomized Controlled Trial. Pediatr. Pulmonol. 2012, 47, 551–557.
- Gualdoni, G.A.; Lingscheid, T.; Schmetterer, K.G.; Hennig, A.; Steinberger, P.; Zlabinger, G.J. Azithromycin Inhibits IL-1 Secretion and Non-Canonical Inflammasome Activation. Sci. Rep. 2015, 5, 12016.
- Böttger, E.C.; Springer, B.; Prammananan, T.; Kidan, Y.; Sander, P. Structural Basis for Selectivity and Toxicity of Ribosomal Antibiotics. EMBO Rep. 2001, 2, 318–323.
- Brusselle, G.G.; Vanderstichele, C.; Jordens, P.; Deman, R.; Slabbynck, H.; Ringoet, V.; Verleden, G.; Demedts, I.K.; Verhamme, K.; Delporte, A.; et al. Azithromycin for Prevention of Exacerbations in Severe Asthma (AZISAST): A Multicentre Randomised Double-Blind Placebo-Controlled Trial. Thorax 2013, 68, 322–329.
- Gielen, V.; Johnston, S.L.; Edwards, M.R. Azithromycin Induces Anti-Viral Responses in Bronchial Epithelial Cells. Eur. Respir. J. 2010, 36, 646–654.
- Sajjan, U.S.; Jia, Y.; Newcomb, D.C.; Bentley, J.K.; Lukacs, N.W.; LiPuma, J.J.; Hershenson, M.B.H. Influenzae Potentiates Airway Epithelial Cell Responses to Rhinovirus by Increasing ICAM-1 and TLR3 Expression. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 2121–2123.
- Taylor, S.L.; Leong, L.E.X.; Mobegi, F.M.; Choo, J.M.; Wesselingh, S.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; et al. Long-Term Azithromycin Reduces Haemophilus Influenzae and Increases Antibiotic Resistance in Severe Asthma. Am. J. Respir. Crit. Care Med. 2019, 200, 309–317.
- Taylor, S.L.; Ivey, K.L.; Gibson, P.G.; Simpson, J.L.; Rogers, G.B. Airway Abundance of Haemophilus Influenzae Predicts Response to Azithromycin in Adults with Persistent Uncontrolled Asthma. Eur. Respir. J. 2020, 56, 2000194.
- Schögler, A.; Kopf, B.S.; Edwards, M.R.; Johnston, S.L.; Casaulta, C.; Kieninger, E.; Jung, A.; Moeller, A.; Geiser, T.; Regamey, N.; et al. Novel Antiviral Properties of Azithromycin in Cystic Fibrosis Airway Epithelial Cells. Eur. Respir. J. 2015, 45, 428–439.
- Tran, D.H.; Sugamata, R.; Hirose, T.; Suzuki, S.; Noguchi, Y.; Sugawara, A.; Ito, F.; Yamamoto, T.; Kawachi, S.; Akagawa, K.S.; et al. Azithromycin, a 15-Membered Macrolide Antibiotic, Inhibits Influenza A(H1N1)Pdm09 Virus Infection by Interfering with Virus Internalization Process. J. Antibiot. (Tokyo). 2019, 72, 759–768.
- Retallack, H.; Di Lullo, E.; Arias, C.; Knopp, K.A.; Laurie, M.T.; Sandoval-Espinosa, C.; Mancia Leon, W.R.; Krencik, R.; Ullian, E.M.; Spatazza, J.; et al. Zika Virus Cell Tropism in the Developing Human Brain and Inhibition by Azithromycin. Proc. Natl. Acad. Sci. USA 2016, 113, 14408–14413.
- Madrid, P.B.; Panchal, R.G.; Warren, T.K.; Shurtleff, A.C.; Endsley, A.N.; Green, C.E.; Kolokoltsov, A.; Davey, R.; Manger, I.D.; Gilfillan, L.; et al. Evaluation of Ebola Virus Inhibitors for Drug Repurposing. ACS Infect. Dis. 2015, 1, 317–326.
- Damle, B.; Vourvahis, M.; Wang, E.; Leaney, J.; Corrigan, B. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin. Pharmacol. Ther. 2020, 108, 201–211.
- Menzel, M.; Akbarshahi, H.; Bjermer, L.; Uller, L. Azithromycin Induces Anti-Viral Effects in Cultured Bronchial Epithelial Cells from COPD Patients. Sci. Rep. 2016, 6, 28698.
- Zeng, S.; Meng, X.; Huang, Q.; Lei, N.; Zeng, L.; Jiang, X.; Guo, X. Spiramycin and Azithromycin, Safe for Administration to Children, Exert Antiviral Activity against Enterovirus A71 in Vitro and in Vivo. Int. J. Antimicrob. Agents 2019, 53, 362–369.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
10 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




