
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tao Han Lee | -- | 2630 | 2022-08-09 13:02:39 | | | |
| 2 | Peter Tang | Meta information modification | 2630 | 2022-08-09 13:21:59 | | | | |
| 3 | Peter Tang | -5 word(s) | 2625 | 2022-08-09 13:23:39 | | |
Video Upload Options
Hyperuricemia is described as elevated levels of serum uric acid (UA), which is more commonly known as gout, an acute, symptomatic disease where urate crystals deposit in the joint and cause subsequent inflammatory arthritis. Chronic kidney disease (CKD) is defined as abnormalities of the kidney structure or function presented for more than 3 months, according to the Kidney Disease Improving Global Outcome guidelines. Several mechanisms have been identified that explain the relationship between hyperuricemia and chronic kidney disease, including the crystal effect, renin–angiotensin–aldosterone system activation, nitric oxide synthesis inhibition, and intracellular oxidative stress stimulation, and urate-lowering therapy (ULT) has been proven to reduce renal disease progression.
1. Introduction
2. Physiology of UA
2.1. Source of UA
2.2. UA Clearance
2.3. Genetic Variations Influence the Serum UA Level
3. Hyperuricemia and Renal Disease
4. Mechanism
4.1. Crystalline Effects
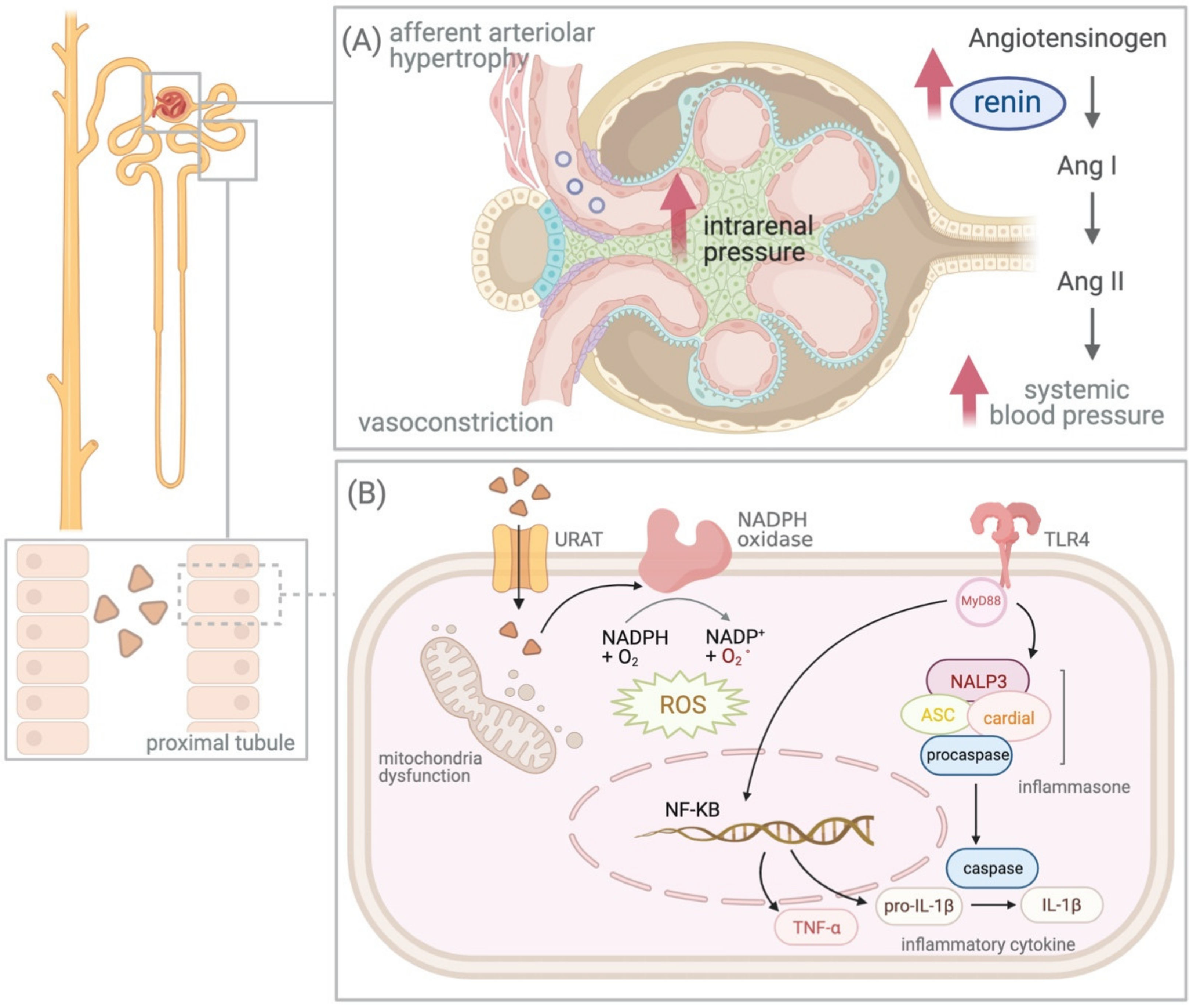
4.2. UA and Renin–Angiotensin–Aldosterone System (RAAS)
4.3. Intracellular Effect of UA
5. Treatment and Evidence of Clinical Trials
References
- Brook, R.A.; Forsythe, A.; Smeeding, J.E.; Lawrence Edwards, N. Chronic gout: Epidemiology, disease progression, treatment and disease burden. Curr. Med. Res. Opin. 2010, 26, 2813–2821.
- Kim, K.Y.; Ralph Schumacher, H.; Hunsche, E.; Wertheimer, A.I.; Kong, S.X. A literature review of the epidemiology and treatment of acute gout. Clin. Ther. 2003, 25, 1593–1617.
- Harris, M.D.; Siegel, L.B.; Alloway, J.A. Gout and hyperuricemia. Am. Fam. Physician 1999, 59, 925–934.
- Van Doornum, S.; Ryan, P.F. Clinical manifestations of gout and their management. Med. J. Aust. 2000, 172, 493–497.
- Agudelo, C.A.; Wise, C.M. Gout: Diagnosis, pathogenesis, and clinical manifestations. Curr. Opin. Rheumatol. 2001, 13, 234–239.
- Shadick, N.A.; Kim, R.; Weiss, S.; Liang, M.H.; Sparrow, D.; Hu, H. Effect of low level lead exposure on hyperuricemia and gout among middle aged and elderly men: The normative aging study. J. Rheumatol. 2000, 27, 1708–1712.
- Feig, D.I. Uric acid: A novel mediator and marker of risk in chronic kidney disease? Curr. Opin. Nephrol. Hypertens. 2009, 18, 526–530.
- Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62.
- Talbott, J.H.; Terplan, K.L. The kidney in gout. Medicine 1960, 39, 405–467.
- Clifford, A.J.; Riumallo, J.A.; Young, V.R.; Scrimshaw, N.S. Effect of Oral Purines on Serum and Urinary Uric Acid of Normal, Hyperuricemic and Gouty Humans. J. Nutr. 1976, 106, 428–434.
- Ryu, E.S.; Kim, M.J.; Shin, H.S.; Jang, Y.H.; Choi, H.S.; Jo, I.; Johnson, R.J.; Kang, D.H. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2013, 304, F471–F480.
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14.
- Loffler, W.; Grobner, W.; Medina, R.; Zollner, N. Influence of dietary purines on pool size, turnover, and excretion of uric acid during balance conditions. Isotope studies using 15N-uric acid. Res. Exp. Med. 1982, 181, 113–123.
- Merriman, T.R.; Dalbeth, N.; Johnson, R.J. Sugar-sweetened beverages, urate, gout and genetic interaction. Pac. Health Dialog. 2014, 20, 31–38.
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Cicerchi, C.; Li, N.; Roncal-Jimenez, C.A.; Ishimoto, T.; Le, M.; Garcia, G.E.; Thomas, J.B.; Rivard, C.J.; et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS ONE 2012, 7, e47948.
- Chaudhary, K.; Malhotra, K.; Sowers, J.; Aroor, A. Uric Acid—Key ingredient in the recipe for cardiorenal metabolic syndrome. Cardiorenal Med. 2013, 3, 208–220.
- Kratzer, J.T.; Lanaspa, M.A.; Murphy, M.N.; Cicerchi, C.; Graves, C.L.; Tipton, P.A.; Ortlund, E.A.; Johnson, R.J.; Gaucher, E.A. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc. Natl. Acad. Sci. USA 2014, 111, 3763–3768.
- Hyndman, D.; Liu, S.; Miner, J.N. Urate Handling in the Human Body. Curr. Rheumatol. Rep. 2016, 18, 34.
- Wright, A.F.; Rudan, I.; Hastie, N.D.; Campbell, H. A ‘complexity’ of urate transporters. Kidney Int. 2010, 78, 446–452.
- Terkeltaub, R.; Bushinsky, D.A.; Becker, M.A. Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics. Arthritis Res. Ther. 2006, 8 (Suppl. 1), S4.
- Lieske, J.C.; de la Vega, L.S.; Gettman, M.T.; Slezak, J.M.; Bergstralh, E.J.; Melton, L.J., 3rd; Leibson, C.L. Diabetes mellitus and the risk of urinary tract stones: A population-based case-control study. Am. J. Kidney Dis. 2006, 48, 897–904.
- Bjornstad, P.; Roncal, C.; Milagres, T.; Pyle, L.; Lanaspa, M.A.; Bishop, F.K.; Snell-Bergeon, J.K.; Johnson, R.J.; Wadwa, R.P.; Maahs, D.M. Hyperfiltration and uricosuria in adolescents with type 1 diabetes. Pediatr. Nephrol. 2016, 31, 787–793.
- Ichida, K.; Matsuo, H.; Takada, T.; Nakayama, A.; Murakami, K.; Shimizu, T.; Yamanashi, Y.; Kasuga, H.; Nakashima, H.; Nakamura, T.; et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012, 3, 764.
- Ferns, G.A.; Lanham, J.; Dieppe, P.; Galton, D.J. A DNA polymorphism of an apoprotein gene associates with the hypertriglyceridaemia of primary gout. Hum. Genet. 1988, 78, 55–59.
- Cameron, J.S.; Simmonds, H.A. Hereditary hyperuricemia and renal disease. Semin. Nephrol. 2005, 25, 9–18.
- Ronco, C. Hyperuricemic Syndromes: Pathophysiology and Therapy. Contrib. Nephrol. 2005, 147, 12.
- Essex, M.N.; Hopps, M.; Bienen, E.J.; Udall, M.; Mardekian, J.; Makinson, G.T. Evaluation of the Relationship Between Serum Uric Acid Levels and Cardiovascular Events in Patients with Gout: A Retrospective Analysis Using Electronic Medical Record Data. J. Clin. Rheumatol. 2017, 23, 160–166.
- Kuwabara, M.; Niwa, K.; Hisatome, I.; Nakagawa, T.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Bjornstad, P.; Jensen, T.; Sato, Y.; Milagres, T.; et al. Asymptomatic Hyperuricemia Without Comorbidities Predicts Cardiometabolic Diseases: Five-Year Japanese Cohort Study. Hypertension 2017, 69, 1036–1044.
- Bardin, T.; Richette, P. Impact of comorbidities on gout and hyperuricaemia: An update on prevalence and treatment options. BMC Med. 2017, 15, 123.
- Choi, H.K.; Ford, E.S.; Li, C.; Curhan, G. Prevalence of the metabolic syndrome in patients with gout: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007, 57, 109–115.
- Mulay, S.R.; Shi, C.; Ma, X.; Anders, H.J. Novel Insights into Crystal-Induced Kidney Injury. Kidney Dis. 2018, 4, 49–57.
- Lin, P.Y.; Lin, C.C.; Liu, H.C.; Lee, M.D.; Lee, H.C.; Ho, C.S.; Chiu, N.C.; Peng, C.C.; Huang, F.Y.; Tsai, J.D. Rasburicase improves hyperuricemia in patients with acute kidney injury secondary to rhabdomyolysis caused by ecstasy intoxication and exertional heat stroke. Pediatr. Crit. Care Med. 2011, 12, e424–e427.
- Galichon, P.; Hertig, A. Epithelial to mesenchymal transition as a biomarker in renal fibrosis: Are we ready for the bedside? Fibrogenes. Tissue Repair 2011, 4, 11.
- Grgic, I.; Duffield, J.S.; Humphreys, B.D. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr. Nephrol. 2012, 27, 183–193.
- Yamamoto, T.; Noble, N.A.; Miller, D.E.; Border, W.A. Sustained expression of TGF-beta 1 underlies development of progressive kidney fibrosis. Kidney Int. 1994, 45, 916–927.
- Sellmayr, M.; Hernandez Petzsche, M.R.; Ma, Q.; Kruger, N.; Liapis, H.; Brink, A.; Lenz, B.; Angelotti, M.L.; Gnemmi, V.; Kuppe, C.; et al. Only Hyperuricemia with Crystalluria, but not Asymptomatic Hyperuricemia, Drives Progression of Chronic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 2773–2792.
- Gasse, P.; Riteau, N.; Charron, S.; Girre, S.; Fick, L.; Petrilli, V.; Tschopp, J.; Lagente, V.; Quesniaux, V.F.; Ryffel, B.; et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am. J. Respir. Crit. Care. Med. 2009, 179, 903–913.
- Xiao, J.; Zhang, X.L.; Fu, C.; Han, R.; Chen, W.; Lu, Y.; Ye, Z. Soluble uric acid increases NALP3 inflammasome and interleukin-1beta expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int. J. Mol. Med. 2015, 35, 1347–1354.
- Zhou, Y.; Fang, L.; Jiang, L.; Wen, P.; Cao, H.; He, W.; Dai, C.; Yang, J. Uric acid induces renal inflammation via activating tubular NF-kappaB signaling pathway. PLoS ONE 2012, 7, e39738.
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241.
- Bellomo, G.; Venanzi, S.; Verdura, C.; Saronio, P.; Esposito, A.; Timio, M. Association of uric acid with change in kidney function in healthy normotensive individuals. Am. J. Kidney Dis. 2010, 56, 264–272.
- Mazzali, M.; Hughes, J.; Kim, Y.G.; Jefferson, J.A.; Kang, D.H.; Gordon, K.L.; Lan, H.Y.; Kivlighn, S.; Johnson, R.J. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001, 38, 1101–1106.
- Saito, I.; Saruta, T.; Kondo, K.; Nakamura, R.; Oguro, T.; Yamagami, K.; Ozawa, Y.; Kato, E. Serum uric acid and the renin-angiotensin system in hypertension. J. Am. Geriatr. Soc. 1978, 26, 241–247.
- Kanabrocki, E.L.; Third, J.L.; Ryan, M.D.; Nemchausky, B.A.; Shirazi, P.; Scheving, L.E.; McCormick, J.B.; Hermida, R.C.; Bremner, W.F.; Hoppensteadt, D.A.; et al. Circadian relationship of serum uric acid and nitric oxide. JAMA 2000, 283, 2240–2241.
- Bellomo, G. Uric acid and chronic kidney disease: A time to act? World J. Nephrol. 2013, 2, 17–25.
- Xu, C.; Lu, A.; Lu, X.; Zhang, L.; Fang, H.; Zhou, L.; Yang, T. Activation of Renal (Pro)Renin Receptor Contributes to High Fructose-Induced Salt Sensitivity. Hypertension 2017, 69, 339–348.
- Yu, M.A.; Sanchez-Lozada, L.G.; Johnson, R.J.; Kang, D.H. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J. Hypertens. 2010, 28, 1234–1242.
- Feig, D.I.; Soletsky, B.; Johnson, R.J. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: A randomized trial. JAMA 2008, 300, 924–932.
- Talaat, K.M.; El-Sheikh, A.R. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am. J. Nephrol. 2007, 27, 435–440.
- Kang, D.H.; Nakagawa, T.; Feng, L.; Watanabe, S.; Han, L.; Mazzali, M.; Truong, L.; Harris, R.; Johnson, R.J. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 2002, 13, 2888–2897.
- Sanchez-Lozada, L.G. The Pathophysiology of Uric Acid on Renal Diseases. Contrib. Nephrol. 2018, 192, 17–24.
- Sato, Y.; Feig, D.I.; Stack, A.G.; Kang, D.H.; Lanaspa, M.A.; Ejaz, A.A.; Sanchez-Lozada, L.G.; Kuwabara, M.; Borghi, C.; Johnson, R.J. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat. Rev. Nephrol. 2019, 15, 767–775.
- Rodriguez-Iturbe, B.; Pons, H.; Johnson, R.J. Role of the Immune System in Hypertension. Physiol. Rev. 2017, 97, 1127–1164.
- Watanabe, S.; Kang, D.H.; Feng, L.; Nakagawa, T.; Kanellis, J.; Lan, H.; Mazzali, M.; Johnson, R.J. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension 2002, 40, 355–360.
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315.
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619.
- Kang, D.H.; Park, S.K.; Lee, I.K.; Johnson, R.J. Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 2005, 16, 3553–3562.
- Kim, K.M.; Henderson, G.N.; Ouyang, X.; Frye, R.F.; Sautin, Y.Y.; Feig, D.I.; Johnson, R.J. A sensitive and specific liquid chromatography-tandem mass spectrometry method for the determination of intracellular and extracellular uric acid. J. Chromatogr. B 2009, 877, 2032–2038.
- Roncal, C.A.; Reungjui, S.; Sanchez-Lozada, L.G.; Mu, W.; Sautin, Y.Y.; Nakagawa, T.; Johnson, R.J. Combination of captopril and allopurinol retards fructose-induced metabolic syndrome. Am. J. Nephrol. 2009, 30, 399–404.
- Pittman, J.R.; Bross, M.H. Diagnosis and management of gout. Am. Fam. Physician 1999, 59, 1799–1806, 1810.




