
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | I-Ching Wang | -- | 1945 | 2022-08-02 21:58:56 | | | |
| 2 | Dean Liu | -44 word(s) | 1901 | 2022-08-08 03:55:39 | | | | |
| 3 | Dean Liu | -43 word(s) | 1858 | 2022-08-10 07:47:27 | | |
Video Upload Options
Accurate measurement of negative gingival recession (GR) is essential to accurately determine the clinical attachment loss, which leads to an accurate diagnosis and optimal therapy of periodontal disease.
1. Introduction
2. Normal Anatomy
Preservation of an intact dentogingival unit with the gingival margin slightly coronal to the CEJ in a state of optimal health is a hallmark feature for an intact, healthy dentition. The biological interface between the gingiva and the tooth that forms the initial barrier to underlying tissues is known as the “dentogingival junction”(DGJ) [6]. The average dimension of sulcus depth, epithelial attachment, and the connective tissue attachment was reported to be 0.69 mm, 0.97 mm, and 1.07 mm, respectively [7]. Today, the latter two a functional unit, is termed the “supracrestal tissue attachment” [8] which was previously known as the “biologic width”.
The apical migration of the DGJ after dentition completes the active phase of eruption is called passive eruption [9]. Gargiulo et al. described the changes that occur in the location of DGJ in relation to the CEJ in four stages of passive eruption [7]. In stage I of the passive eruption, which represents a physiologically healthy state, the location of epithelial attachment (today called junctional epithelium) is entirely on the enamel with the most apical termination at the CEJ. The average gingival dimension coronal to the CEJ (negative GR) at stage I that comprises of sulcus depth and epithelial attachment is 2.15 mm [7]. Along with the apical shift of the dentogingival junction, which is considered a consequence of pathological periodontal destruction, the epithelial attachment is on the enamel and cementum at stage II and entirely located on the cementum at stage III until the epithelial attachment and gingival margin lie apical to the CEJ at stage IV [7][10][11]. The average gingival dimension coronal to the CEJ (negative GR) is 1.29 mm (sulcus and part of epithelial attachment) and 0.6 mm (purely sulcus depth) at stages II and III, respectively. In stage IV and beyond, the gingival margin is at the level of or apical to the CEJ. To sum up, the dimension described by Gargiulo et al. (stage I–III of passive eruption) is in accordance with the common understanding that the facial gingiva margin is approximately 0.5 to 2 mm coronal to the CEJ. This was based on the observation of the distance from free gingiva margin to the free gingival groove and the latter corresponds to the bottom of the gingiva crevice (the base of the epithelial attachment) that is often located at CEJ [12].
Normally, the scalloped osseous crest parallels the CEJ circumferentially. The osseous scallop (defined by the distance from the crestal bone level at the mid-buccal/lingual site to the crestal bone level at the interproximal site) is greatest at the maxillary anterior teeth, averaging 3.5 mm, and gradually flattens out posteriorly [13]. The extent of the osseous scallop is strongly associated with the bone morphotype and can range from 2.1 mm in a flat type to as high as 4.1 mm in a pronounced scalloped type [14]. On average, the distance from the gingival margin to the bone crest is about 3 mm at the mid-facial sites and ranges from 3 to 4.5 mm at interproximal sites in periodontally healthy patients depending on the amount of gingival scallop in relation to the underlying interproximal osseous scallop [15]. Considering that the average distance from CEJ to the alveolar crest is 1.5 mm (range: 1.08 to 1.71 mm) in stages I to III of passive eruption [7], the interproximal papilla height coronal to the interproximal CEJ is between 2–3.5 mm on average, and is greater in patients with a thick phenotype.
3. Clinical Assessment Approaches
3.1. Manual Instrumentation
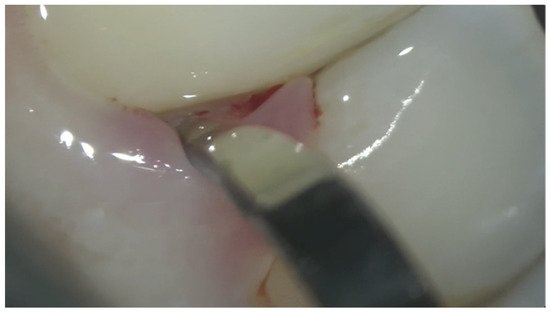
3.2. Automated Instrumentation
3.3. Imaging Technologies
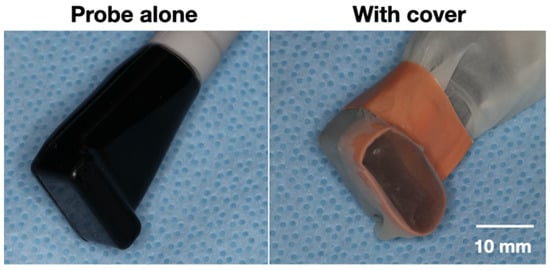
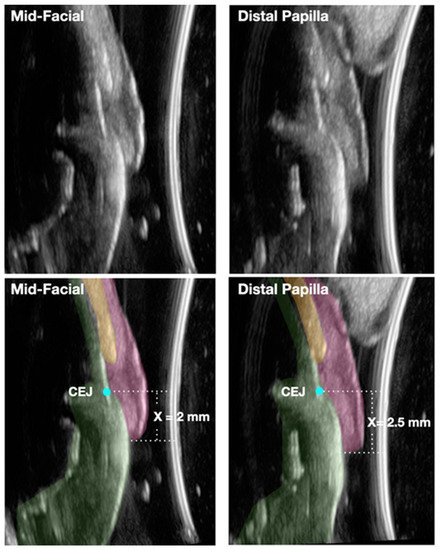
4. Conclusion
-
Measuring the amount of gingiva recession, both negative and positive, is an integral part of determining the clinical attachment loss.
-
Detecting the CEJ has proven to be a challenge clinically when the gingival margin is coronal to the CEJ. To properly diagnose the amount of negative gingival recession, understanding the normal site-specific anatomy is the first step.
-
With the aid of novel noninvasive and chairside ultrasound imaging and the high- magnification operating microscope, accurate and reproducible assessment of the negative gingival recession can become a reality that allows for early detection and intervention of periodontitis. These technologies could also prove to be valuable clinical and research tools in accurately detecting the amount of clinical attachment gain resulting from periodontal therapeutic modalities.
References
- Listgarten, M.A. Re: Periodontal terminology. J. Periodontol. 1993, 64, 918.
- Ramfjord, S.P. Indices for prevalence and incidence of periodontal disease. J. Periodontol. 1959, 30, 51–59.
- Vandana, K.; Gupta, I. The location of cemento enamel junction for CAL measurement: A clinical crisis. J. Indian Soc. Periodontol. 2009, 13, 12–15.
- Badersten, A.; Nilvéaus, R.; Egelberg, J. Reproducibility of probing attachment level measurements. J. Clin. Periodontol. 1984, 11, 475–485.
- Hug, H.U.; Van’t Hof, M.A.; Spanauf, A.J.; Renggli, H.H. Validity of clinical assessments related to the cemento-enamel junction. J. Dent. Res. 1983, 62, 825–829.
- Sicher, H. Changing concepts of the supporting dental structures. Oral Surg. Oral Med. Oral Pathol. 1959, 12, 31–35.
- Gargiulo, A.W.; Wentz, F.M.; Orban, B. Dimensions and relations of the dentogingival junction in humans. J. Periodontol. 1961, 32, 261–267.
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S219–S229.
- Gottlieb, B.; Orban, B. Active and passive continuous eruption of teeth. J. Dent. Res. 1933, 13, 214.
- Mele, M.; Felice, P.; Sharma, P.; Mazzotti, C.; Bellone, P.; Zucchelli, G. Esthetic treatment of altered passive eruption. Periodontology 2000 2018, 77, 65–83.
- Newman, M.G.; Takei, H.; Klokkevold, P.R.; Carranza, F.A. Newman and Carranza’s Clinical Periodontology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018.
- Ainamo, J.; Löe, H. Anatomical characteristics of gingiva. A clinical and microscopic study of the free and attached gingiva. J. Periodontol. 1966, 37, 5–13.
- Ash, M.M.; Nelson, S.J. Wheeler’s Dental Anatomy, Physiology and Occlusion, 8th ed.; Saunder Elsevier: St. Louis, MO, USA, 2003; p. 259.
- Becker, W.; Ochsenbein, C.; Tibbetts, L.; Becker, B.E. Alveolar bone anatomic profiles as measured from dry skulls: Clinical ramifications. J. Clin. Periodontol. 1997, 24, 727–731.
- Kois, J.C. Altering gingival levels: The restorative connection part I: Biologic variables. Int. J. Periodontics Restor. Dent. 1994, 6, 3–7.
- Goodson, J.M. Clinical measurements of periodontitis. J. Clin. Periodontol. 1986, 13, 446–460.
- Hill, E.G.; Slate, E.H.; Wiegand, R.E.; Grossi, S.G.; Salinas, C.F. Study design for calibration of clinical examiners measuring periodontal parameters. J. Periodontol. 2006, 77, 1129–1141.
- Glavind, L.; Löe, H. Errors in the clinical assessment of periodontal destruction. J. Periodontal. Res. 1967, 2, 180–184.
- Kingman, A.; Löe, H.; Ånerud, Å.; Boysen, H. Errors in measuring parameters associated with periodontal health and disease. J. Periodontol. 1991, 62, 477–486.
- Watts, T. Constant force probing with and without a stent in untreated periodontal disease: The clinical reproducibility problem and possible sources of error. J. Clin. Periodontol. 1987, 14, 407–411.
- Barendregt, D.S.; van der Velden, U.; Timmerman, M.F.; Bulthuis, H.M.; van der Weijden, F. Detection of the cemento-enamel junction with three different probes: An “in vitro” model. J. Clin. Periodontol. 2009, 36, 212–218.
- Corraini, P.; Baelum, V.; Lopez, R. Reliability of direct and indirect clinical attachment level measurements. J. Clin. Periodontol. 2013, 40, 896–905.
- Jeffcoat, M.K.; Jeffcoat, R.L.; Jens, S.C.; Captain, K. A new periodontal probe with automated cemento-enamel junction detection. J. Clin. Periodontol. 1986, 13, 276–280.
- Jeffcoat, M.K.; Jeffcoat, R.L.; Captain, K. A periodontal probe with automated cemento--enamel junction detection-design and clinical trials. IEEE Trans. Biomed. Eng. 1991, 38, 330–333.
- Preshaw, P.M.; Kupp, L.; Hefti, A.F.; Mariotti, A. Measurement of clinical attachment levels using a constant-force periodontal probe modified to detect the cemento-enamel junction. J. Clin. Periodontol. 1999, 26, 434–440.
- Karpinia, K.; Magnusson, I.; Gibbs, C.; Yang, M.C. Accuracy of probing attachment levels using a CEJ probe versus traditional probes. J. Clin. Periodontol. 2004, 31, 173–176.
- Deepa, R.; Prakash, S. Accuracy of probing attachment levels using a new computerized cemento-enamel junction probe. J. Indian Soc. Periodontol. 2012, 16, 74–79.
- Wang, S.F.; Leknes, K.N.; Zimmerman, G.J.; Sigurdsson, T.J.; Wikesjö, U.M.; Selvig, K.A. Intra-and inter-examiner reproducibility in constant force probing. J. Clin. Periodontol. 1995, 22, 918–922.
- Hefti, A.F. Periodontal Probing. Crit. Rev. Oral Biol. Med. 1997, 8, 336–356.
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. 1), S46–S73.
- Quirynen, M.; Callens, A.; van Steenberghe, D.; Nys, M. Clinical evaluation of a constant force electronic probe. J. Periodontol. 1993, 64, 35–39.
- Perry, D.A.; Taggart, E.J.; Leung, A.; Newburn, E. Comparison of a conventional probe with electronic and manual pressure-regulated probes. J. Periodontol. 1994, 65, 908–913.
- Bareja, H.; Bansal, M.; Naveen Kumar, P.G. Comparative assessment of conventional periodontal probes and CEJ handpiece of electronic probes in the diagnosis and primary care of periodontal disease. J. Fam. Med. Prim. Care 2021, 10, 692–698.
- Brezniak, N.; Goren, S.; Zoizner, R.; Shochat, T.; Dinbar, A.; Wasserstein, A.; Heller, M. The accuracy of the cementoenamel junction identification on periapical films. Angle Orthod. 2004, 74, 496–500.
- Patcas, R.; Markic, G.; Müller, L.; Ullrich, O.; Peltomäki, T.; Kellenberger, C.J.; Karlo, C.A. Accuracy of linear intraoral measurements using cone beam CT and multidetector CT: A tale of two CTs. Dentomaxillofac. Radiol. 2012, 41, 637–644.
- Ghorayeb, S.R.; Bertoncini, C.A.; Hinders, M.K. Ultrasonography in dentistry. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 1256–1266.
- Lynch, J.; Hinders, M. Ultrasonic device for measuring periodontal attachment levels. Rev. Sci. Instrum. 2002, 73, 2686–2693.
- Tsiolis, F.I.; Needleman, I.G.; Griffiths, G.S. Periodontal ultrasonography. J. Clin. Periodontol. 2003, 30, 849–854.
- Chifor, R.; HedeÅŸiu, M.; Bolfa, P.; Catoi, C.; Crisan, M.; Serbanescu, A.; Badea, A.F.; Moga, I. The evaluation of 20 MHz ultrasonography, computed tomography scans as compared to direct microscopy for periodontal system assessment. Med. Ultrason. 2011, 13, 120–126.
- Nguyen, K.-C.T.; Le, L.H.; Kaipatur, N.R.; Zheng, R.; Lou, E.H.; Major, P.W. High-resolution ultrasonic imaging of dento-periodontal tissues using a multi-element phased array system. Ann. Biomed. Eng. 2016, 44, 2874–2886.
- Chifor, R.; Badea, M.E.; Hedesiu, M.; Serbanescu, A.; Badea, A.F. Experimental model for measuring and characterisation of the dento-alveolar system using high frequencies ultrasound techniques. Med. Ultrason. 2010, 12, 127–132.
- Mahmoud, A.M.; Ngan, P.; Crout, R.; Mukdadi, O.M. High-resolution 3D ultrasound jawbone surface imaging for diagnosis of periodontal bony defects: An in vitro study. Ann. Biomed. Eng. 2010, 38, 3409–3422.
- Chan, H.-L.; Sinjab, K.; Chung, M.-P.; Chiang, Y.-C.; Wang, H.-L.; Giannobile, W.V.; Kripfgans, O.D. Non-invasive evaluation of facial crestal bone with ultrasonography. PLoS ONE 2017, 12, e0171237.
- Zimbran, A.; Dudea, S.M.; Dudea, D. Evaluation of periodontal tissues using 40MHz ultrasonography. preliminary report. Med. Ultrason. 2013, 15, 6–9.
- Salmon, B.; Le Denmat, D. Intraoral ultrasonography: Development of a specific high-frequency probe and clinical pilot study. Clin. Oral Investig. 2012, 16, 643–649.
- Nguyen, K.-C.T.; Yan, Y.; Kaipatur, N.R.; Major, P.W.; Lou, E.H.; Punithakumar, K.; Le, L.H. Computer-Assisted Detection of Cemento-Enamel Junction in Intraoral Ultrasonographs. Appl. Sci. 2021, 11, 5850.
- Tattan, M.; Sinjab, K.; Lee, E.; Arnett, M.; Oh, T.J.; Wang, H.L.; Chan, H.L.; Kripfgans, O.D. Ultrasonography for chairside evaluation of periodontal structures: A pilot study. J. Periodontol. 2020, 91, 890–899.
- Siqueira, R.; Sinjab, K.; Pan, Y.C.; Soki, F.; Chan, H.L.; Kripfgans, O. Comprehensive peri-implant tissue evaluation with ultrasonography and cone-beam computed tomography: A pilot study. Clin. Oral Implant. Res. 2021, 32, 777–785.
- Chan, H.L.; Sinjab, K.; Li, J.; Chen, Z.; Wang, H.L.; Kripfgans, O.D. Ultrasonography for noninvasive and real-time evaluation of peri-implant tissue dimensions. J. Clin. Periodontol. 2018, 45, 986–995.
- Magnusson, I.; Listgarten, M.A. Histological evaluation of probing depth following periodontal treatment. J. Clin. Periodontol. 1980, 7, 26–31.
- Fowler, C.; Garrett, S.; Crigger, M.; Egelberg, J. Histologic probe position in treated and untreated human periodontal tissues. J. Clin. Periodontol. 1982, 9, 373–385.
- Armitage, G.C.; Svanberg, G.K.; Löe, H. Microscopic evaluation of clinical measurements of connective tissue attachment levels. J. Clin. Periodontol. 1977, 4, 173–190.
- Armitage, G.C. Manual periodontal probing in supportive periodontal treatment. Periodontology 2000 1996, 12, 33–39.




