Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zaira Gadzhimagomedova | -- | 2680 | 2022-08-05 12:41:00 | | | |
| 2 | Zaira Gadzhimagomedova | -2 word(s) | 2678 | 2022-08-05 17:04:22 | | | | |
| 3 | Catherine Yang | Meta information modification | 2678 | 2022-08-08 03:35:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gadzhimagomedova, Z.; Pankin, I.; Zolotukhin, P.; Kit, O.; Kirsanova, D.; Soldatov, A.; Gadzhimagomedova, Z. Nanoparticles Used in X-Ray Photodynamic Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/25903 (accessed on 07 February 2026).
Gadzhimagomedova Z, Pankin I, Zolotukhin P, Kit O, Kirsanova D, Soldatov A, et al. Nanoparticles Used in X-Ray Photodynamic Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/25903. Accessed February 07, 2026.
Gadzhimagomedova, Zaira, Ilia Pankin, Peter Zolotukhin, Oleg Kit, Daria Kirsanova, Alexander Soldatov, Zaira Gadzhimagomedova. "Nanoparticles Used in X-Ray Photodynamic Therapy" Encyclopedia, https://encyclopedia.pub/entry/25903 (accessed February 07, 2026).
Gadzhimagomedova, Z., Pankin, I., Zolotukhin, P., Kit, O., Kirsanova, D., Soldatov, A., & Gadzhimagomedova, Z. (2022, August 05). Nanoparticles Used in X-Ray Photodynamic Therapy. In Encyclopedia. https://encyclopedia.pub/entry/25903
Gadzhimagomedova, Zaira, et al. "Nanoparticles Used in X-Ray Photodynamic Therapy." Encyclopedia. Web. 05 August, 2022.
Copy Citation
Photodynamic therapy (PDT) has long been known as an effective method for treating surface cancer tissues. Deeper penetration of X-rays into tissues has been implemented, which is now known as X-ray photodynamic therapy (XPDT). The two methods differ in the photon energy used, thus requiring the use of different types of scintillating nanoparticles. These nanoparticles are known to convert the incident energy into the activation energy of a photosensitizer, which leads to the generation of reactive oxygen species.
X-ray photodynamic therapy
photodynamic therapy
nanophosphor
scintillating nanoparticles
photosensitizer
1. Nanoparticles as Photosensitizers
TiO2:Ce. Single TiO2 is not effective enough in the generation of ROS. For example, Chun-Chen Yang and his colleagues [3] doped the anatase lattice of TiO2 with Ce, which was more effective than a single TiO2 for better photosensitivity and ROS generation results.
Cerium was used to dope the TiO2 anatase structures to increase photochemical reactions owing to its strong catalytic potential and light response extension. Furthermore, it could suppress the recombination of electron–hole pairs and increase their lifetime. Additionally, Ce has a greater X-ray photon interaction cross-section than that of biological tissues, which leads to the intense X-ray interaction with control materials and ROS generation [4]. The X-ray radiation dose was approximately 0.133 Gy for 100 s.
TiO2:C. Chun-Chen Young and his co-authors [4] investigated nanoparticles with a narrow bandgap and ROS generation under the influence of soft X-ray radiation to destroy tumor cells with less damage to healthy tissues. In this experiment, anatase lattice was doped with C, and then it was activated by X-rays to produce more reactive oxygen species and destroy tumor cells. After X-ray radiation and the introduction of TiO2:C to tumor cells, the size of the tumor gradually reduced. The X-ray radiation dose was approximately 0.133 Gy for 100 s.
(n-Bu4N)2(Mo6I8(OCOCF3)6). Kaplan Kirakci et al. [5] studied the nanoparticles made of the octahedral molybdenum cluster compound or (n-Bu4N)2(Mo6I8(OCOCF3)6). These nanoparticles generate singlet oxygen O2(1Δg) from blood under X-ray irradiation. The (n-Bu4N)2(Mo6I8(OCOCF3)6) nanoparticles were prepared by nanoprecipitation.
(n-Bu4N)2(Mo6I8(OCOCF3)6) nanoparticles efficiently absorbed X-rays because they consist of heavy elements, which in turn leads to the generation of the excited triplet states of photosensitizer interacting with molecular oxygen to produce singlet oxygen O2(1Δg).
Some specific concentrations of the obtained nanoparticles are non-toxic and quite stable in water. The simple structure of nanoparticles leads to their significantly higher activity at lower concentrations. This fact is mainly based on the energy transfer between scintillating nanoparticles and photosensitizers. In addition, the small size of the nanoparticles provides a large energy transfer. The smaller the particles are, the closer they can approach the target. This means that the value of the transferred energy from the nanoparticle to the molecular oxygen is higher. As a result, it leads to the formation of a higher number of ROS. Thus, the enhancement effectiveness of (n-Bu4N)2(Mo6I8(OCOCF3)6) nanoparticles could be achieved by their size. The X-ray dose reported as 1 Gy and 2 Gy for 100 s.
Pollen-structured gold cluster. The pollen-structured gold cluster (PSGC) were designed for mediating X-ray-induced PDT for radiotherapy enhancement [6]. PSGCs were made of mesoporous silica and then their core was passivated by a rich layer of gold nanoparticles (Figure 1). PSGCs were synthesized with a deposition–precipitation process. The architecture of PSGCs provides a large surface area, which in turn leads to the generation of ROS under X-ray irradiation.
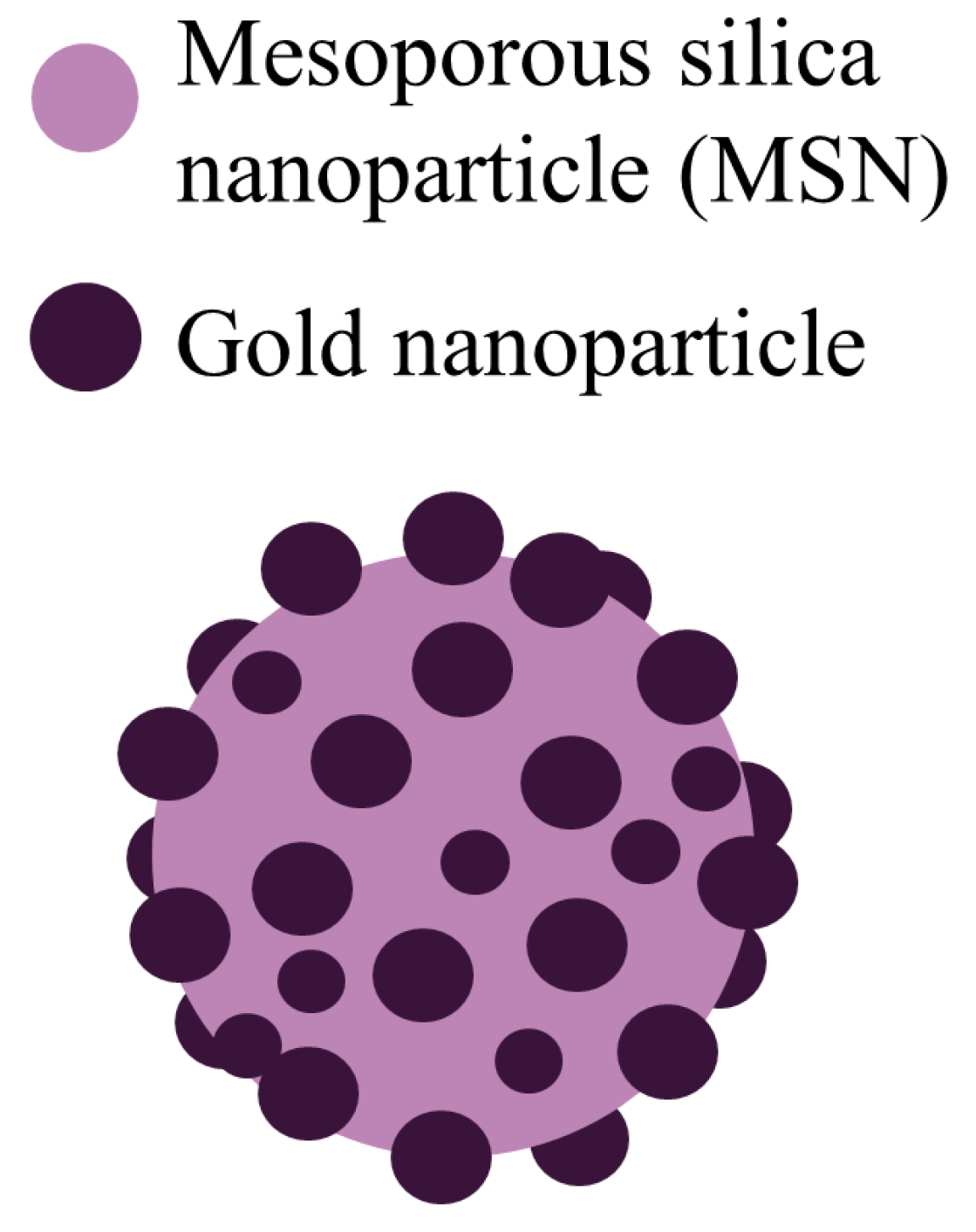
Figure 1. Schematic illustration of the pollen-structured gold cluster. The cluster consists of a mesoporous silica core and a gold active shell [7].
The obtained nanoparticles were exposed to a variety of irradiation doses: 0.5, 1, 2, and 5 Gy. A single dose of X-ray irradiation was provided with the voltage of the tube equal to 160 kV.
PSGCs have a significantly higher ability to generate ROS and they offer a better treatment of breast cancer cells [6], demonstrating the potential clinical application in the treatment of deep tumors.
2. Scintillating Nanoparticles
Y2O3:Eu and Y3Al5O12:Eu. Yang C. et al. in [8] investigated the luminescent properties of Y2O3:Eu and Y3Al5O12:Eu particles as scintillating nanoparticles irradiated with X-rays in PDT. The nanoparticles can be used for cancer treatment in combination with some kind of photosensitizers that selectively accumulate in tumor cells. Two sets of finely dispersed Y2O3:Eu and Y3Al5O12:Eu phosphors were synthesized by the Pechini method and self-propagating high-temperature synthesis. The data in [8] show that the use of this X-ray activated phosphor in combination with the photosensitizers results in the destruction of tumor cells under irradiation thus confirming a high efficiency of the suggested approach.
Y2O3. Jonathan P. Scaffidi et al. in [9] investigated the combination of yttrium oxide (Y2O3) scintillating nanoparticles, a part of the HIV-1 TAT (Human immunodeficiency viruses – 1 trans-activator of transcription) peptide, and psoralen was investigated by X-ray radiation. The X-ray radiation is absorbed by the Y2O3 nanoparticles, which then emit UV light. The absorption of UV photons by the nanoparticles connected with psoralen has the potential to cross-link adenine and thymine residues in DNA.
NaGdF4: Gd3+, Eu3+. Some lanthanide-doped nanoparticles can absorb X-ray radiation. There was a series of Gd3+ and Eu3+ compositions in lanthanide fluorides for optimizing the emission from Eu3+ under X-ray excitation [10]. In addition, the optimum concentration of Eu3+ that produced the most intense emission in NaGdF4 was 15% molar concentration. Moreover, an attempt to include a sensitizer (Ce3+) in NaGdF4:Eu3+ resulted in a reduction in the emission following X-ray excitation.
LiGa5O8:Cr. Hongmin Chen in [11] used nanoscintillators based on LiGa5O8:Cr, which emit persistent near-infrared luminescence. This allows to obtain optical images of deep tissues that can be used to control exposure. In particular, LiGa5O8:Cr nanoparticles and a 2,3-naphthalocyanine photosensitizer were encapsulated in mesoporous silica nanoparticles. Nanoconjugates can be efficiently accumulated in lung tumors, as evidenced by monitoring X-ray luminescence from LiGa5O8:Cr. It should be noted that LiGa5O8:Cr nanoparticles were prepared by a polystyrene sphere-assisted sol–gel method.
3. Nanocomposites: Photosensitizers and Scintillating Nanoparticles
AIE-Au. Wenjing Sun et al. in their work [12] investigated such conjugates of photosensitizers as aggregation-induced emission heterogeneous Au clustoluminogens (AIE-Au) which consist of glutathione-protected gold clusters (GCs) to achieve efficient low-dose XPDT.
When irradiated, AIE-Au strongly absorbed X-rays to generate hydroxyl radicals, which increased the radiotherapy effect by damaging DNA. Furthermore, the aggregates of glutathione-protected GCs increased the X-ray excited luminescence. The AIE-Au transformed X-rays into optical luminescence and excited the rose bengal (RB) photosensitizers, which oxidizes lipid membranes. In other words, AIE-Au clustoluminogens triggered the generation of reactive oxygen species. X-ray doses used in this investigation were 1 Gy.
LiYF4@SiO2@ZnO. Qien et al. in [13] reported herein on the integration of a scintillator and a semiconductor as an XPDT agent. These conjugates are core-shell CeIII-doped LiYF4@SiO2@ZnO nanoparticles (LSZNPs).
In this structure, the ultraviolet fluorescence from the CeIII-doped LiYF4 nanoscintillator under irradiation gives rise to the formation of electron–hole pairs in ZnO nanoparticles leading to the formation of biotoxic hydroxyl radicals.
This method demonstrates a reduced dependence on the intracellular oxygen levels by integrating the scintillator and semiconductor as a photosensitizer. These nanoparticles have been used as the scintillation nanoparticles in the down-conversion of X-rays to match the energy gap of a semiconductor to produce ROS from water molecules for an effective explosion.
The volume of the tumor (the HeLa cell line was used) with the introduced LSZNPs and exposed to an X-ray radiation of 8 Gy was almost completely inhibited in 15 days. In addition, late-stage apoptosis featuring karyopyknosis, karyorrhexis and the significant formation of apoptotic bodies was observed after the irradiation of these tissues. The application of these nanoparticles demonstrates antitumor therapeutic effectiveness. X-ray irradiation was used at 3 Gy.
RGD-ZSM-RB. Zn- and Mn-incorporated silica (ZSM) nanoscintillators were conjugated with photosensitizer rose bengal and arginylglycylaspartic acid (RGD) peptide, due to the fact that they are intrinsically dual modal targeted imaging probes as it was shown in [14]. The proposed RGD-ZSM-RB nanosensitizer shows excellent deep-tumor therapy with low dose X-ray irradiation (1 Gy). After the conjugation of RGD peptides with silicate scintillators, the nanosensitizer could show effective accumulation in cancer cells and local inhibition. Moreover, Zn and Mn dopants are significant elements in the human body. XPDT treatment therefore minimizes the potential adverse effects of local radiotherapy due to the application of low radiation doses like 1 Gy, while the typical dose for a solid epithelial tumor varies from 60 to 80 Gy.
LaF3. Lanthanum fluoride is a transparent material that could allow radioluminescence at a set of wavelengths through the simple substitution of lanthanum ions with other luminescent lanthanides. For example, in [15] Kudinov K. et al. prepared lanthanum fluoride nanoparticles doped with cerium, terbium, or both, which had good spectral overlap with Rose Bengal photosensitizer molecules.
LaF3:Tb. Lanthanum fluoride nanoparticles have been improved and doped with rare-earth element Tb3+ in [16]. They were Tb3+-doped LaF3 scintillating nanoparticles (LaF3:Tb) combined with meso-tetra(4-carboxyphenyl)porphyrin photosensitizer, followed by activation with soft X-rays. Scintillating LaF3:Tb nanoparticles were obtained, sized approximately 25 nm. They had good dispersibility in aqueous solutions and demonstrated great biocompatibility.
CeF3:Tb3+. In the studies of K. Popovich et al. [16] CeF3:Tb3+-based nanoparticles modified with SiO2 and protoporphyrin IX (PpIX) were investigated. Nanopowder CeF3:Tb3+ was prepared via the sol–gel method, with further surface coating by SiO2 layer and the conjugation with photosensitizer PpIX. Radioluminescence spectra showed an energy transfer from Ce3+ to Tb3+ ions and from Tb3+ to the molecules of PpIX photosensitizer. The samples were exposed with X-rays using a tube with a Cu anode (voltage 40 kV, current 30 mA, average wavelength Kα1,2 = 0.15418 nm).
Emission bands of Tb3+ overlap well with the absorption lines of protoporphyrin PpIX. Therefore, the efficient energy transfer from donor to acceptor and the production of singlet oxygen were expected. This combination of nanoparticles generated ROS. Nanocomposites under investigation may be quite good candidates for the application in XPDT.
CeF3:Tb3+, Gd3+. Another promising nanoparticle is CeF3 doped with Gd3+ and Tb3+ (CGT) investigated in [17]. CGT nanoparticles were synthesized with a hydrothermal process. Co-doped CeF3:Gd3+, Tb3+ scintillating nanoparticles were then coated with mesoporous silica. Such doping with Gd and Tb boosted the scintillation properties of the CeF3 nanoparticles.
The enhancement of the X-ray-excited optical luminescence was due to the fact that the energy levels of Gd3+ lie between the activator (Ce3+) and the luminescent center (Tb3+), giving rise to efficient energy transfer. The irradiation of nanoparticles was carried out with single X-ray dose 3 Gy.
ZnGa2O4:Cr (ZGO:Cr/W). Song L. and others reported in [18] on a low-dose X-ray-activated persistent luminescence nanoparticle (PLNP)-mediated PDT nanoplatform for treating deep cancer tumors. The high persistent luminescence and long persistent luminescence time were the key points for effective cancer treatment. In order to achieve this goal, WVI-doped ZnGa2O4:Cr (ZGO:Cr/W) PLNPs were synthesized. In comparison with the traditional ZnGa2O4:Cr PLNPs, ZGO:Cr/W PLNPs show a higher persistent luminescence intensity and a longer persistent luminescence time after the same irradiation dose. Then, by coupling with PS Zn(II) phthalocyanine tetrasulfonic acid (ZnPcS4), the PDT nanoplatform (ZGO:Cr/W–ZnPcS4) was constructed. The constant luminescence could continue exciting the coupled PS after the X-ray irradiation was removed, leading to the reduced X-ray dose (~0.18 Gy for 2 min) to minimize the side effects of XPDT.
ZnS:Cu,Co. Like Lun Ma et al. in [19] synthesized copper- and cobalt-doped ZnS (ZnS:Cu,Co) scintillating nanoparticles which were then used in combination with tetrabromorhodamine-123 (TBrRh123) photosensitizer. It was determined that in the conjugates there was an efficient energy transfer from nanoparticles to photosensitizers. As a result, singlet oxygen was generated for the treatment of cancer cells.
Furthermore, ZnS:Cu, Co nanoparticles have a long X-ray excited afterglow used as a continuous light source to activate XPDT. ZnS: Cu, Co-TBrRh123 X-ray conjugates are very effective for the destruction of cancer cells. Besides ZnS:Cu, Co nanoparticles irradiated by X-rays (2 Gy) can also be used to visualize cells. All this indicates that ZnS:Cu, Co nanoparticles are promising for biomedical applications.
GdEuC12 micelles. It should be mentioned that sometimes lanthanide micelles are used as nanoparticles in XPDT. For instance, Slávka Kaščáková et al. designed a liponanoparticle based on GdEuC12 micelles including hypericin as a photosensitizer in their hydrophobic core, which provides singlet oxygen production upon X-ray irradiation [20]. The micelles were composed of amphiphilic lanthanide chelates and incorporated hypericin as photosensitizer in the hydrophobic core.
Tb2O3. Tb2O3 has recently become the center of attention. Anne-Laure Bulin et al. in [21] used Tb2O3 coated with a polysiloxane layer. This nanosystem was combined with porphyrin molecules that were able to generate singlet oxygen, which is a major cytotoxic agent in X-ray photodynamic therapy. This combination was suitable for singlet oxygen formation under X-ray irradiation.
The BaGdF5 nanophosphors doped with different Eu:Gd ratios in the range from 0.01 to 0.50 were synthesized by new microwave route [22] in [23]. Different coatings with amorphous SiO2 and citrates were studied. Micro-CT imaging demonstrate supe-rior X-ray attenuation and sufficient contrast in liver and spleen after intravenous injection of citric coated nanoparticles. Nitrogen adsorption/desorption analysis revealed mesoporous SiO2 formation characterized by the slit-shaped type of pores, that should be accessible for Methylene Blue photosensitizer molecules. It was shown that SiO2 coating subsequently facilitate Methylene Blue conjugation and result to the formation of BaGdF5:10%Eu3+@SiO2@MB nanocomposite (Figure 2) as a promising candidate for appli-cation in XPDT.
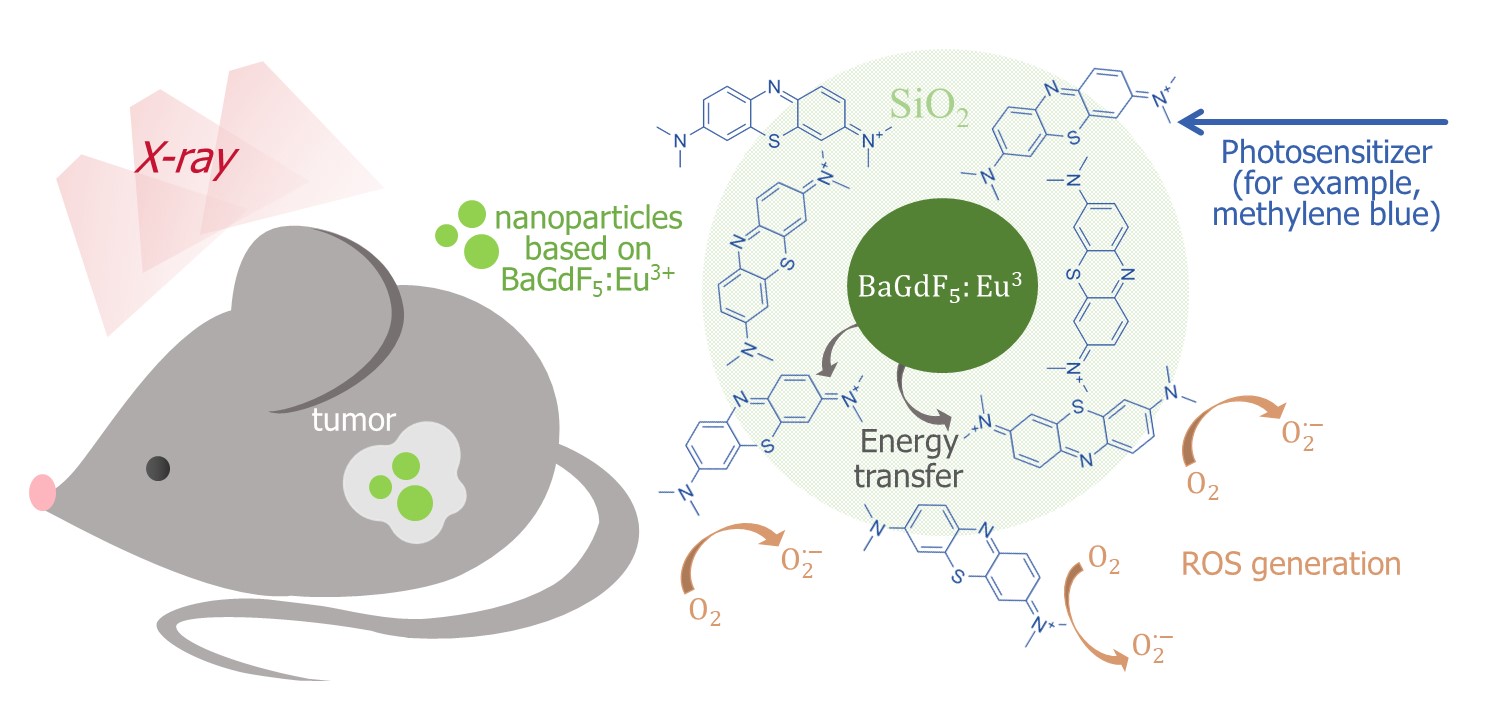
Figure 2. Schematic illustration of the BaGdF5:Eu3+-assisted XPDT concept and possible morphology of the corresponding composite material based on the BaGdF5:Eu3+ agent coupled with the methylene blue photosensitizer [23].
4. Metal–Organic Frameworks as Photosensitizers
Metal–organic frameworks (MOFs) are crystalline porous compounds that consist of organic linkers and metal ions. The demonstrate a great potential in drug delivery applications due to their high surface area and drug-loading capacity, tailorable pore structure as well as their functionality [24][25][26][27][28][29]. Many MOFs have already been used as nanoparticles in photodynamic therapy. However, MOFs are also often used as nanoparticles in X-ray photodynamic therapy. Let us consider several structures of MOFs that turned out to be the most successful in various studies.
Hf-DBB-Ru. Recently nanoscale metal–organic frameworks (nMOFs)-Hf-DBB-Ru have been discovered[30]. Hf-DBB-Ru is (Hf6(µ3-O)4(µ3-OH)4(DBB-Ru)6)12+ where DBB-Ru = bis(2,2′-bipyridine)(5,5′-di(4-benzoato)-2,2′-bipyridine)ruthenium (II) chloride.
Hf-DBB-Ru was synthesized by solvothermal reaction between HfCl4 and H2DBBRu with trifluoroacetic acid as the modulator. Hf-DBB-Ru was irradiated with 1, 2, 3, 5 or 10 Gy X-ray.
This MOF was designed from Ru-based photosensitizers. The cationic framework demonstrated strong targeting to mitochondria. Under X-ray irradiation, Hf-DBB-Ru successfully generated hydroxyl radicals from Hf6 secondary building units (SBUs) and singlet oxygen from the DBB-Ru photosensitizers. Mitochondria-targeted particles depolarized the mitochondrial membrane to activate the apoptosis of cancer cells, which resulted in the significant regression of colorectal tumor cells. When the volume of the tumor reached 100−150 mm3, the tissue was irradiated by light with energy 180 J/cm2 (650 nm).
Hf6-DBA and Hf12-DBA. Kaiyuan Ni et al. in [31] investigated two more nMOF structures based on Hf-Hf6-DBA (Hf-Hf6-DBA = Hf6(μ3-O)4(μ3-OH)4(DBA)6) and Hf12-DBA (Hf12-DBA = Hf12(μ3-O)8(μ3-OH)8(μ2-OH)6(DBA)9), where DBA = 2,5-di(p-benzoato) anthracene. These nMOFs were synthesized via solvothermal reactions.
nMOFs were constructed as radio-enhancers by taking advantage of the electron-dense Hf6O4(OH)4 and Hf12O8(OH)14 SBUs as X-ray absorbers to produce ROS.
Under X-ray irradiation, nMOFs convert energy to anthracene-based bridging ligands, DBA, to emit radioluminescence in the visible spectrum range. For example, in [31] Hf12-DBA and Hf6-DBA (incubated in CT26 cell line) were irradiated with 2 Gy X-rays dose. It was shown that Hf12-DBA gave a much brighter radioluminescence signal with respect to Hf6-DBA.
Hf12-DBA demonstrated great radio-enhancement over Hf6-DBA. It has been suggested that this is due to enhanced X-ray absorption by the electron-dense Hf12 clusters and hydroxyl radical diffusion through the porous nanoplates. It should be noted that under γ-rays Hf12-DBA also exhibited greater radio-sensitization than HfO2 and Hf6-DBA.
References
- Samana Shrestha; Jing Wu; Bindeshwar Sah; Adam Vanasse; Leon N Cooper; Lun Ma; Gen Li; Huibin Zheng; Wei Chen; Michael P. Antosh; et al. X-ray induced photodynamic therapy with copper-cysteamine nanoparticles in mice tumors. Proceedings of the National Academy of Sciences 2019, 116, 16823-16828, 10.1073/pnas.1900502116.
- Lei Shi; Pei Liu; Jing Wu; Lun Ma; Han Zheng; Michael P Antosh; Haiyan Zhang; Bo Wang; Wei Chen; Xiuli Wang; et al. The effectiveness and safety of X-PDT for cutaneous squamous cell carcinoma and melanoma. Nanomedicine 2019, 14, 2027-2043, 10.2217/nnm-2019-0094.
- Chun-Chen Yang; Yu-Jun Sun; Pei-Hsuan Chung; Wei-Yao Chen; Wojciech Swieszkowski; Weiming Tian; Feng-Huei Lin; Development of Ce-doped TiO2 activated by X-ray irradiation for alternative cancer treatment. Ceramics International 2017, 43, 12675-12683, 10.1016/j.ceramint.2017.06.149.
- Chun-Chen Yang; Min-Hsiung Tsai; Keng-Yuan Li; Chun-Han Hou; Feng-Huei Lin; Carbon-Doped TiO2 Activated by X-Ray Irradiation for the Generation of Reactive Oxygen Species to Enhance Photodynamic Therapy in Tumor Treatment. International Journal of Molecular Sciences 2019, 20, 2072, 10.3390/ijms20092072.
- Kaplan Kirakci; Jaroslav Zelenka; Michaela Rumlová; Jiří Martinčík; Martin Nikl; Tomáš Ruml; Kamil Lang; Octahedral molybdenum clusters as radiosensitizers for X-ray induced photodynamic therapy. Journal of Materials Chemistry B 2018, 6, 4301-4307, 10.1039/c8tb00893k.
- Lih Shin Tew; Meng-Ting Cai; Leu-Wei Lo; Yit Lung Khung; Nai-Tzu Chen; Pollen-Structured Gold Nanoclusters for X-ray Induced Photodynamic Therapy. Materials 2018, 11, 1170, 10.3390/ma11071170.
- Zaira Gadzhimagomedova; Peter Zolotukhin; Oleg Kit; Daria Kirsanova; Alexander Soldatov; Nanocomposites for X-Ray Photodynamic Therapy. International Journal of Molecular Sciences 2020, 21, 4004, 10.3390/ijms21114004.
- Vadim V. Bakhmetyev; Tamara S. Minakova; Sergey V. Mjakin; Lev A. Lebedev; Анна Власенко; Anna A. Nikandrova; Irina Ekimova; Nina S. Eremina; Maxim M. Sychov; Armelle Ringuede; et al. Synthesis and surface characterization of nanosized Y2O3:Eu and YAG:Eu luminescent phosphors which are useful in photodynamic therapy of cancer. European Journal of Nanomedicine 2016, 8, 173-184, 10.1515/ejnm-2016-0020.
- Jonathan P. Scaffidi; Molly K. Gregas; Benoit Lauly; Yan Zhang; Tuan Vo-Dinh; Activity of Psoralen-Functionalized Nanoscintillators against Cancer Cells upon X-ray Excitation. ACS Nano 2011, 5, 4679-4687, 10.1021/nn200511m.
- L. Sudheendra; Gautom K. Das; C. Li; Simon R. Cherry; Ian M. Kennedy; Lanthanide-doped nanoparticles for hybrid x-ray/optical imaging. SPIE Proceedings 2013, 8596, 85960D-85960D-8, 10.1117/12.2005250.
- Hongmin Chen; Xilin Sun; Geoffrey D. Wang; Koichi Nagata; Zhonglin Hao; Andrew Wang; Zibo Li; Jin Xie; Baozhong Shen; LiGa5O8:Cr-based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumors. Materials Horizons 2017, 4, 1092-1101, 10.1039/c7mh00442g.
- Wenjing Sun; Li Luo; Yushuo Feng; Yuting Cai; Yixi Zhuang; Rong‐Jun Xie; Xiaoyuan Chen; Hongmin Chen; Aggregation‐Induced Emission Gold Clustoluminogens for Enhanced Low‐Dose X‐ray‐Induced Photodynamic Therapy. Angewandte Chemie International Edition 2019, 59, 9914-9921, 10.1002/anie.201908712.
- Chen Zhang; Kuaile Zhao; Wenbo Bu; Dalong Ni; Yanyan Liu; Jingwei Feng; Jianlin Shi; Marriage of Scintillator and Semiconductor for Synchronous Radiotherapy and Deep Photodynamic Therapy with Diminished Oxygen Dependence. Angewandte Chemie International Edition 2014, 54, 1770-1774, 10.1002/anie.201408472.
- Wenjing Sun; Tianhang Shi; Li Luo; Xiaomei Chen; Peng Lv; Ying Lv; Yixi Zhuang; JinJie Zhu; Gang Liu; Xiaoyuan Chen; et al.Hongmin Chen Monodisperse and Uniform Mesoporous Silicate Nanosensitizers Achieve Low‐Dose X‐Ray‐Induced Deep‐Penetrating Photodynamic Therapy. Advanced Materials 2019, 31, e1808024, 10.1002/adma.201808024.
- Konstantin Kudinov; Devesh Bekah; Daniel Cooper; Sathvik Shastry; Colin Hill; Stephen Bradforth; Jay Nadeau; Lanthanum fluoride nanoparticles for radiosensitization of tumors. Colloidal Nanoparticles for Biomedical Applications XI 2016, 9722, 97220V, 10.1117/12.2213374.
- Min-Hua Chen; Yi-Jhen Jenh; Sheng-Kai Wu; Yo-Shen Chen; Nobutaka Hanagata; Feng-Huei Lin; Non-invasive Photodynamic Therapy in Brain Cancer by Use of Tb3+-Doped LaF3 Nanoparticles in Combination with Photosensitizer Through X-ray Irradiation: A Proof-of-Concept Study. Nanoscale Research Letters 2017, 12, 1-6, 10.1186/s11671-017-1840-3.
- Farooq Ahmad; Xiaoyan Wang; Zhao Jiang; Xujiang Yu; Xinyi Liu; Rihua Mao; Xiaoyuan Chen; Wanwan Li; Codoping Enhanced Radioluminescence of Nanoscintillators for X-ray-Activated Synergistic Cancer Therapy and Prognosis Using Metabolomics. ACS Nano 2019, 13, 10419-10433, 10.1021/acsnano.9b04213.
- Liang Song; Pei-Pei Li; Wen Yang; Xia-Hui Lin; Hong Liang; Xiao-Feng Chen; Gang Liu; Juan Li; Huang-Hao Yang; Low-Dose X-ray Activation of W(VI)-Doped Persistent Luminescence Nanoparticles for Deep-Tissue Photodynamic Therapy. Advanced Functional Materials 2018, 28, 1707496, 10.1002/adfm.201707496.
- Lun Ma; Xiaoju Zou; Brian Bui; Wei Chen; Kwang Hyun Song; Timothy Solberg; X-ray excited ZnS:Cu,Co afterglow nanoparticles for photodynamic activation. Applied Physics Letters 2014, 105, 013702, 10.1063/1.4890105.
- Slávka Kaščáková; Alexandre Giuliani; Sara Lacerda; Agnès Pallier; Pascal Mercère; Eva Toth; Matthieu Réfrégiers; X-ray-induced radiophotodynamic therapy (RPDT) using lanthanide micelles: Beyond depth limitations. Nano Research 2015, 8, 2373-2379, 10.1007/s12274-015-0747-5.
- Anne-Laure Bulin; Charles Truillet; Rima Chouikrat; François Lux; Céline Frochot; David Amans; Gilles Ledoux; Olivier Tillement; Pascal Perriat; Muriel Barberi-Heyob; et al.Christophe Dujardin X-ray-Induced Singlet Oxygen Activation with Nanoscintillator-Coupled Porphyrins. The Journal of Physical Chemistry C 2013, 117, 21583-21589, 10.1021/jp4077189.
- D. Yu. Kirsanova; V. V. Butova; V. A. Polyakov; P. V. Zolotukhin; A. A. Belanova; V. M. Legostaev; E. A. Kuchma; Z. M. Gadzhimagomedova; A. V. Soldatov; X-RAY NANOPHOSPHORS BASED ON BaGdF5 FOR X-RAY PHOTODYNAMIC THERAPY IN ONCOLOGY. Nanotechnologies in Russia 2020, 15, 105-111, 10.1134/s1995078020010164.
- Zaira Gadzhimagomedova; Vladimir Polyakov; Ilia Pankin; Vera Butova; Daria Kirsanova; Mikhail Soldatov; Darya Khodakova; Anna Goncharova; Elizaveta Mukhanova; Anna Belanova; et al.Aleksey MaksimovAlexander Soldatov BaGdF5 Nanophosphors Doped with Different Concentrations of Eu3+ for Application in X-ray Photodynamic Therapy. International Journal of Molecular Sciences 2021, 22, 13040, 10.3390/ijms222313040.
- Dongmei Yang; Xiaojiao Kang; Ping'an Ma; Yunlu Dai; Zhiyao Hou; Ziyong Cheng; Chunxia Li; Jun Lin; Hollow structured upconversion luminescent NaYF4:Yb3+, Er3+ nanospheres for cell imaging and targeted anti-cancer drug delivery. Biomaterials 2013, 34, 1601-1612, 10.1016/j.biomaterials.2012.11.004.
- S. Rojas; T. Devic; P. Horcajada; Metal organic frameworks based on bioactive components. Journal of Materials Chemistry B 2017, 5, 2560-2573, 10.1039/c6tb03217f.
- Chi Van Nguyen; Yu-Te Liao; Ting-Cih Kang; Jeffrey E. Chen; Takuya Yoshikawa; Yuta Nakasaka; Takao Masuda; Kevin C.-W. Wu; A metal-free, high nitrogen-doped nanoporous graphitic carbon catalyst for an effective aerobic HMF-to-FDCA conversion. Green Chemistry 2016, 18, 5957-5961, 10.1039/c6gc02118b.
- Inhar Imaz; Marta Rubio-Martínez; Jihyun An; Isabel Solé-Font; Nathaniel L. Rosi; Daniel Maspoch; Metal–biomolecule frameworks (MBioFs). Chemical Communications 2011, 47, 7287-7302, 10.1039/c1cc11202c.
- Inhar Imaz; Marta Rubio-Martínez; Jihyun An; Isabel Solé-Font; Nathaniel L. Rosi; Daniel Maspoch; Metal–biomolecule frameworks (MBioFs). Chemical Communications 2011, 47, 7287-7302, 10.1039/c1cc11202c.
- Mei-Ru Song; Dong-Yao Li; Fu-Yu Nian; Jin-Ping Xue; Juan-Juan Chen; Zeolitic imidazolate metal organic framework-8 as an efficient pH-controlled delivery vehicle for zinc phthalocyanine in photodynamic therapy. Journal of Materials Science 2017, 53, 2351-2361, 10.1007/s10853-017-1716-z.
- Guangxu Lan; KaiYuan Ni; Samuel S. Veroneau; Xuanyu Feng; Geoffrey T. Nash; Taokun Luo; ZiWan Xu; Wenbin Lin; Titanium-Based Nanoscale Metal–Organic Framework for Type I Photodynamic Therapy. Journal of the American Chemical Society 2019, 141, 4204-4208, 10.1021/jacs.8b13804.
- KaiYuan Ni; Guangxu Lan; Christina Chan; Bryan Quigley; Kuangda Lu; Theint Aung; Nining Guo; Patrick La Riviere; Ralph R. Weichselbaum; Wenbin Lin; et al. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nature Communications 2018, 9, 1-12, 10.1038/s41467-018-04703-w.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
08 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




