Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria J. Barrero | -- | 2270 | 2022-08-05 10:12:37 | | | |
| 2 | Camila Xu | Meta information modification | 2270 | 2022-08-05 10:30:30 | | | | |
| 3 | Camila Xu | Meta information modification | 2270 | 2022-08-05 10:31:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Martinez-Delgado, B.; Barrero, M.J. Epigenetic Aspects of Rare Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/25891 (accessed on 07 February 2026).
Martinez-Delgado B, Barrero MJ. Epigenetic Aspects of Rare Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/25891. Accessed February 07, 2026.
Martinez-Delgado, Beatriz, Maria J. Barrero. "Epigenetic Aspects of Rare Diseases" Encyclopedia, https://encyclopedia.pub/entry/25891 (accessed February 07, 2026).
Martinez-Delgado, B., & Barrero, M.J. (2022, August 05). Epigenetic Aspects of Rare Diseases. In Encyclopedia. https://encyclopedia.pub/entry/25891
Martinez-Delgado, Beatriz and Maria J. Barrero. "Epigenetic Aspects of Rare Diseases." Encyclopedia. Web. 05 August, 2022.
Copy Citation
Epigenetics plays an important role in pathogenicity since it regulates basic cellular functions, such as gene expression, DNA damage, chromatin topology, and chromosomal organization. Rare diseases affect more than 300 million people worldwide.
molecular diagnosis
rare diseases
epigenetics
transcriptomics
1. Introduction
Epigenetics plays an important role in pathogenicity since it regulates basic cellular functions, such as gene expression, DNA damage, chromatin topology, and chromosomal organization. DNA in the eukaryotic cell nucleus is wrapped around two copies of each of the core histones (H2A, H2B, H3, and H4) to form chromatin. Among other epigenetic mechanisms, modifications of DNA and histones play critical roles in gene expression regulation. The level of chromatin compaction has important consequences for gene transcription as it influences the accessibility of DNA sequences to transcription factors and other regulatory proteins. Modifications of DNA and histones regulate the level of chromatin compaction, either directly or by facilitating the binding of remodeling proteins that recognize modified sites.
Genetic alterations can have an important impact on epigenetic regulation. Mutations might affect the function of genes involved in histone or DNA modifications or even affect histone genes. These alterations typically have a broad impact on gene expression. Alternatively, mutations can be located in regulatory elements or alter the conformation of chromatin affecting the expression of particular genes.
A disease is considered rare if it affects fewer than 1 in 2000 people [1]. Despite the low individual incidence, rare diseases affect altogether 350 million people in the world [2]. More than 8000 rare diseases have been described [3]. The large variabilities and complexities of symptoms often complicate their diagnoses, which can take up to several years for some patients [4]. Many rare diseases are associated with epigenetic alterations that cause changes in gene expression and can be used to aid diagnosis [5].
2. Epigenetic Aspects of Rare Diseases
Alterations in chromatin properties and structure are common in rare diseases and can be used as diagnostic tools. These alterations can be caused directly by mutations in genes that encode proteins involved in the regulation of chromatin. In addition, other alterations not involving epigenetic factors directly can affect the epigenome. For example, chromatin-related factors are very often recruited to chromatin through transcription factors and, therefore, mutations in transcription factors, their binding sites, or components of signal transduction pathways that control their activity can also lead to alterations in the cellular epigenetic landscape (Figure 1).
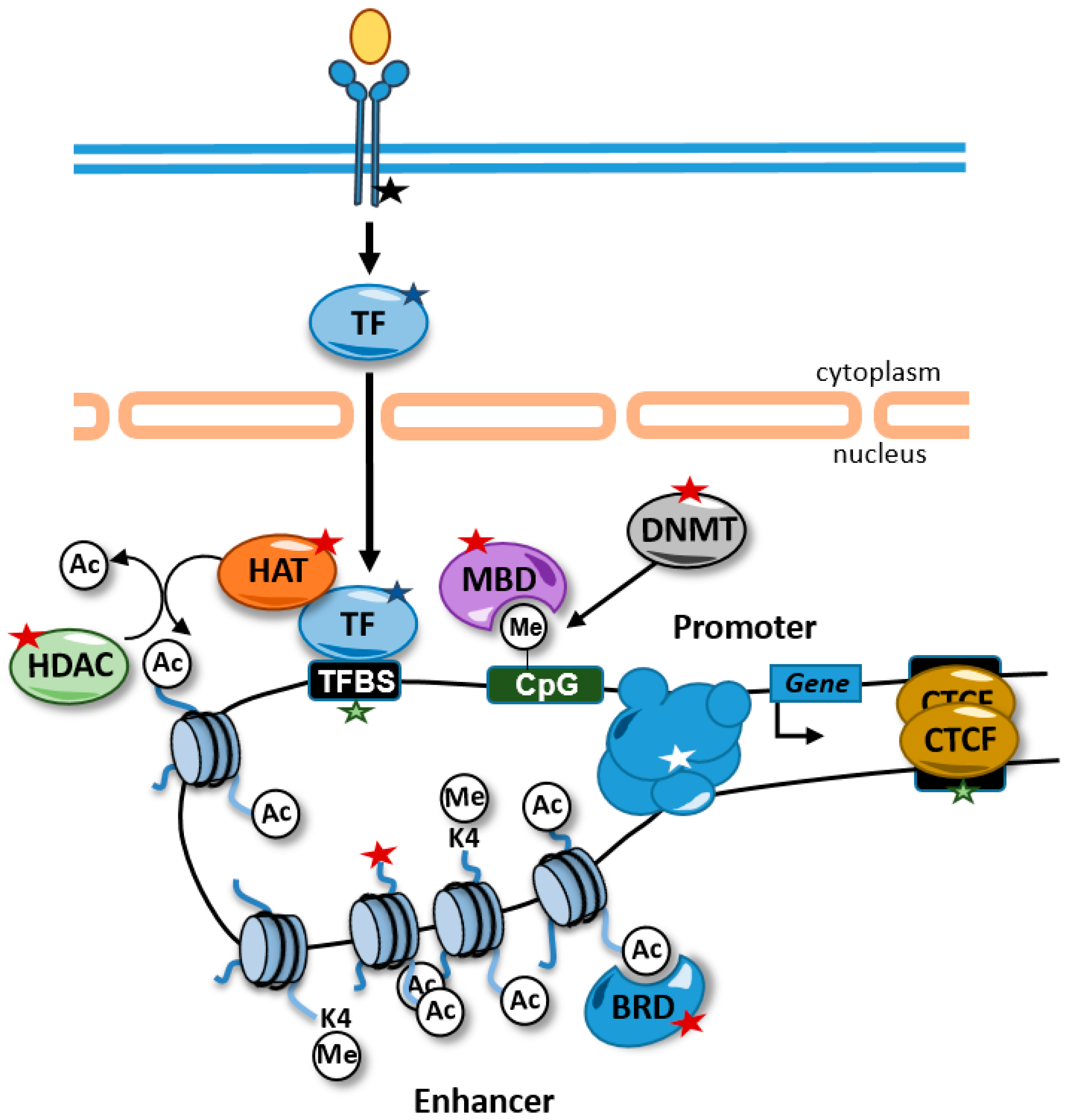 Figure 1. Alterations causing rare diseases that disrupt the epigenome and affect gene expression. Alterations in signal transduction pathways that regulate transcription factor activity (black star), transcription factors (blue star), transcription factor binding sites (green stars), chromatin-related activities (red stars), and promoter–enhancer interactions (white star) can affect gene expression. Some alterations, such as mutations in transcription factor binding sites, are likely to affect the expression of one gene, but other alterations, such as alterations in transcription factors and histone modifying enzymes, are predicted to have genome-wide impacts on the epigenome and in the expression of genes. For example, disruptions of transcription factor activity might interfere with the recruitment of HATs to the chromatin and maintain the proper levels of histone acetylation at enhancers. TFBS, transcription factor binding site; HDAC, histone deacetylases; HAT, histone acetyltransferases; BRD, bromodomain-containing protein; MBD, methyl CpG binding protein; DNMT, DNA methyltransferase; TF, transcription factor; Ac, acetylated residue; Me, methylated cytosine.
Figure 1. Alterations causing rare diseases that disrupt the epigenome and affect gene expression. Alterations in signal transduction pathways that regulate transcription factor activity (black star), transcription factors (blue star), transcription factor binding sites (green stars), chromatin-related activities (red stars), and promoter–enhancer interactions (white star) can affect gene expression. Some alterations, such as mutations in transcription factor binding sites, are likely to affect the expression of one gene, but other alterations, such as alterations in transcription factors and histone modifying enzymes, are predicted to have genome-wide impacts on the epigenome and in the expression of genes. For example, disruptions of transcription factor activity might interfere with the recruitment of HATs to the chromatin and maintain the proper levels of histone acetylation at enhancers. TFBS, transcription factor binding site; HDAC, histone deacetylases; HAT, histone acetyltransferases; BRD, bromodomain-containing protein; MBD, methyl CpG binding protein; DNMT, DNA methyltransferase; TF, transcription factor; Ac, acetylated residue; Me, methylated cytosine.Haploinsufficiency in chromatin-related factors frequently causes neurodevelopmental syndromes. Although most of these proteins are ubiquitously expressed, the nervous system appears to be particularly vulnerable to the alteration of their activities. Next, critical aspects of epigenetic regulation and its alterations are reviewed (Table 1).
Table 1. DNA methylation-related genes known to cause rare diseases according to OMIM (https://www.omim.org/ accessed on 25 June 2022).
| Function | Gene Symbol | Disease | MIM Phenotype |
|---|---|---|---|
| DNMT | DNMT1 | Cerebellar ataxia, deafness, narcolepsy, autosomal dominant | 604121 |
| Neuropathy, hereditary sensory, type IE | 614116 | ||
| DNMT3A | Heyn–Sproul–Jackson syndrome | 618724 | |
| Tatton–Brown–Rahman syndrome | 615879 | ||
| DNMT3B | Facioscapulohumeral muscular dystrophy 4, digenic | 619478 | |
| Immunodeficiency–centromeric instability–facial anomalies syndrome 1 | 242860 | ||
| MBD- containing protein |
MECP2 | Rett syndrome | 312750 |
| MBD5 | Intellectual developmental disorder, autosomal dominant 1 | 156200 | |
| GATAD2B | GAND syndrome | 615074 |
2.1. DNA Methylation
DNA methylation is catalyzed by DNA methyltransferases (DNMTs), typically at cytosines (5mC) [6]. Despite being a relatively stable mark, it can be reversed by the action of ten-eleven translocation (TET) enzymes that oxidize the methyl group of 5mC to yield 5-hydroxymethylcytosine (5hmC) [7]. DNA methylation is essential for normal development and is associated with a number of key processes, including genomic imprinting, X-chromosome inactivation, and gene repression. In particular, methylation of CpG islands, 500–2000 bp CpG-rich areas typically found near the transcription start site of genes, is an important mechanism for gene silencing [6]. The 5hmC residues are found in active genes and are emerging as regulators of gene activation and cellular differentiation during embryonic development and brain maturation [8].
The DNA-methyltransferase enzymes (DNMT1, DNMT3A, and DNMT3B) maintain normal patterns of DNA methylation. In addition, 5mC and 5hmC can be recognized by methyl binding proteins (MECP2, MBD1, MBD2, MBD3, MBD4, MBD5, and MBD6) that possess a methyl-binding domain (MBD) and act as methylation-sensitive transcriptional repressors. Both mutations in DNMTs and methyl binding proteins can cause rare syndromes (Table 1). Mutations in DNMT1 are associated with neuropathies, mutations in DNMT3A cause overgrowth syndromes with intellectual disability, and DNMT3B mutations are involved in immunodeficiency and intellectual disability [9]. Loss-of-function mutations in MECP2 cause Rett syndrome, a rare neurodevelopmental disorder, and alterations in other MBD-containing proteins have been described in autism spectrum disorders [10]. Since all these factors are involved in gene repression, it is expected that their loss-of-function results in the overexpression of certain genes that likely contribute to the disease. However, how the induction of genes contributes to the phenotype is not completely understood. In addition, other chromatin functionalities might be compromised. For example, mutations in DNMT3B cause centromeric instability and increased frequency of somatic recombination [11].
Mutations in factors controlling DNA methylation can also be involved in imprinting disorders. In humans, around 100 autosomal genes are preferentially expressed from only one of the two parental chromosomes as a result of differential DNA methylation during gametogenesis in the male and female germ lines [12]. Alterations in the methylation status of these genes, most commonly loss but also acquirement of DNA methylation at the non-imprinted locus, might be driven by genetic changes in a cis-acting element or trans-acting factor involved in the establishment or maintenance of imprinted methylation [13]. A number of alterations may also be caused by random environment-driven errors [13]. Most individuals with imprinting disorders exhibit altered DNA methylation at several imprinted loci, a condition that is referred to as multilocus imprinting disturbance (MLID). The molecular basis of these disorders is complex with few pathological variants likely involved in the establishment and maintenance of imprinting identified [14]. Genetic alterations that affect cis-acting elements might include deletions, duplications, and translocations, but perhaps are more common cases of uniparental disomy in which two copies of a given imprinted region are from one progenitor. Due to the dynamic regulation of DNA methylation in cells, it is relatively common for patients to show mosaicisms with variable levels of DNA methylation at imprinted regions between or within tissues, which might complicate the diagnosis. Emerging new technologies now allow the detection of allele-specific expression in single cells and are contributing to improving the understanding of how DNA methylation and epigenetics in general contribute to mosaicisms in rare diseases [15].
2.2. Histone Modifications
Dysregulation of histone methylation and acetylation have been involved in rare diseases [16]. Histone lysine methylation plays an essential role in gene expression and its deregulation has been linked to different neurodevelopmental conditions. Lysine methylation is a complex modification that affects gene expression in different ways depending on the modified residue [17]. Lysine methylation occurring at residues 4 and 36 of histone H3 is generally associated with active chromatin. Tri-methylation of histone H3 at lysine 4 (H3K4me3) is usually located at the transcription start sites (TSS) of actively transcribed genes while tri-methylation of histone H3 at lysine 36 (H3K36me3) is usually found at the gene bodies. Tri-methylation at lysine 9 and 27 of histone H3 (H3K9me3 and H3K27me3), and lysine 20 of histone H4 (H4K20me3) are typically associated with inactive or repressed chromatin. H3K27me3 is mediated by the polycomb repressive complex and is generally associated with facultative heterochromatin, while H3K9me3 marks constitutive heterochromatin. The levels of histone lysine methylation at a particular genomic location are dynamically controlled by the actions of histone lysine methyltransferases (KMTs) and demethylases (KDMs). Haploinsufficiency of KMTs or KDMs manifests in numerous neurodevelopmental disorders (Table 2) [18]. The overlap of symptoms caused by mutations in diverse histone modifiers and distinct symptoms caused by genes belonging to the same family of proteins suggests the existence of a complex network of gene expression regulation in the brain. The Kabuki syndrome can be caused by the loss of function of KMT2D (also called MLL2) or KDM6A (also called UTX). This overlap might be explained by the participation of both factors in the activation of the same genes, KDM2D by mediating H3K4 methylation and KDM6A by removing the repressive H3K27me mark. More striking, patients with characteristics of Kleefstra syndrome harbor alterations in EHMT1 or KMT2C genes, involved in gene repression and gene activation, respectively. In a similar way, mutations in NSD1 or EZH2 cause overgrowth syndromes. This overlap in phenotype is in contrast with alterations in the different members of the MLL family of H3K4 methyltransferases (KMT2A-D, SET1A, and SET1B) that cause different symptoms, suggesting that they play crucial yet non-redundant roles in the brain. Finally, both gain and loss-of-function mutations in NSD2 have been found in patients with intellectual disabilities [19].
Table 2. Genes involved in histone methylation known to cause rare diseases according to OMIM (https://www.omim.org/ accessed on 25 June 2022).
| Function | Gene Symbol | Disease | MIM Phenotype |
|---|---|---|---|
| H3K4 KMT | KMT2A | Wiedemann–Steiner syndrome | 605130 |
| KMT2D | Kabuki syndrome type 1 | 147920 | |
| KMT2C | Kleefstra syndrome 2 | 617768 | |
| KMT2B | Dystonia 28, childhood-onset | 617284 | |
| SET1A | Epilepsy, early-onset, with or without developmental delay | 618832 | |
| Neurodevelopmental disorder with speech impairment and dysmorphic facies | 619056 | ||
| SET1B | Intellectual developmental disorder with seizures and language delay | 619000 | |
| ASH1L | Intellectual developmental disorder, autosomal dominant 52 | 617796 | |
| H3K9 KMT | EHMT1 | Kleefstra syndrome 1 | 610253 |
| H3K27 KMT | EZH2 | Weaver syndrome | 277590 |
| H3K36 KMT | NSD1 | Sotos syndrome | 117550 |
| NSD2 | Rauch–Steindl syndrome | 619695 | |
| SETD2 | Luscan–Lumish | 616831 | |
| H4K20 KMT | KMT5B | Intellectual developmental disorder, autosomal dominant 51 | 617788 |
| H3K4 KDM | KDM1A | Cleft palate, psychomotor retardation, and distinctive facial features | 616728 |
| KDM5C | Intellectual developmental disorder, X-linked syndromic, Claes–Jensen type | 300534 | |
| H3K27 KDM | KDM6A | Kabuki syndrome type 2 | 300867 |
| H3K9 KDM | PHF8 | Intellectual developmental disorder, X-linked, syndromic, Siderius type | 300263 |
Histone acetylation is involved in transcriptional activation, and it is controlled by the action of histone acetyltransferases (HATs) and histone deacetylases (HDACs). The acetylated lysine residues of histones are recognized by bromodomain (BRD)-containing proteins that function as effectors of the acetylation signal through the recruitment of factors that mediate transcription. Alterations in activities related to histone acetylation also cause neurodevelopmental disorders, including the loss of function of HATs, HDACs, BRD-containing proteins, and structural components of HAT complexes (Table 3) [16]. Similar to KMTs and despite the fact that multiple HATs seem to acetylate the same residues in histone tails, some non-overlapping symptoms have been described, suggesting that their functions are non-redundant. In addition, it is important to take into account that histone-modifying enzymes might also modify non-histone proteins, such as transcription factors that impact the epigenome.
Table 3. Genes involved in histone acetylation known to cause rare diseases according to OMIM (https://www.omim.org/ accessed on 25 June 2022).
| Function | Gene Symbol | Disease | MIM Phenotype |
|---|---|---|---|
| HATs | KAT6A | Arboleda–Tham syndrome | 616268 |
| KAT6B | Genitopatellar syndrome | 606170 | |
| SBBYSS syndrome | 603736 | ||
| CREBBP/ EP300 |
Rubinstein–Taybi syndrome | 180849 | |
| Menke–Hennekam syndrome 2 | 618333 | ||
| BRD-containing protein | BRPF1 | Intellectual developmental disorder with dysmorphic facies and ptosis | 617333 |
| HDAC | HDAC4 | Neurodevelopmental disorder with central hypotonia and dysmorphic facies | 619797 |
| HDAC8 | Cornelia de Lange syndrome 5 | 300882 | |
| BRAF complex subunit | PHF21A | Intellectual developmental disorder with behavioral abnormalities and craniofacial dysmorphism with or without seizures | 618725 |
| HAT complex subunit | TRRAP | Developmental delay with or without dysmorphic facies and autism | 618454 |
In addition to histone modifications and its effector readers, gene expression and repression entail the remodeling of chromatin, making it more or less accessible to transcription factors and the transcriptional machinery. Chromatin remodelers utilize energy from ATP hydrolysis to alter nucleosome spacing/density or to facilitate histone variant exchange. Several activities with ATP-remodeling activity or that are components of ATP remodeling complexes have been identified in patients with rare diseases, the most well-known being the Coffin–Siris syndrome caused by loss-of-function mutations of different subunits of the SWI/SNF chromatin remodeling complex involved in transcriptional activation (Table 4).
Table 4. Genes involved in chromatin remodeling known to cause rare diseases according to OMIM (https://www.omim.org/ accessed on 25 June 2022).
| Function | Gene Symbol | Disease | MIM Phenotype |
|---|---|---|---|
| SWI/SNF complex | ARID1A | Coffin–Siris syndrome 2 | 614607 |
| ARID1B | Coffin–Siris syndrome 1 | 135900 | |
| ARID2 | Coffin–Siris syndrome 6 | 617808 | |
| SMARCB1 | Coffin–Siris syndrome 3 | 614608 | |
| SMARCA4 | Coffin–Siris syndrome 4 | 614609 | |
| SMARCE1 | Coffin–Siris syndrome 5 | 616938 | |
| ARID2 | Coffin–Siris syndrome 6 | 617808 | |
| DPF2 | Coffin–Siris syndrome 7 | 618027 | |
| SMARCC2 | Coffin–Siris syndrome 8 | 618362 | |
| SMARCD1 | Coffin–Siris syndrome 11 | 618779 | |
| SMARCD2 | Specific granule deficiency 2 | 617475 | |
| ATRX | Alpha-thalassemia/mental retardation syndrome | 301040 | |
| Intellectual disability-hypotonic facies syndrome, X-linked | 309580 | ||
| ISWI complex | BPTF | Neurodevelopmental disorder with dysmorphic facies and distal limb anomalies | 617755 |
| CHD family | CHD2 | Developmental and epileptic encephalopathy 94 | 615369 |
| CHD7 | CHARGE syndrome | 214800 | |
| Hypogonadotropic hypogonadism 5 with or without anosmia | 612370 | ||
| CHD8 | Intellectual developmental disorder with autism and macrocephaly | 615032 | |
| CHD5 | Parenti–Mignot neurodevelopmental syndrome | 610771 | |
| CHD1 | Pilarowski–Bjornsson syndrome | 617682 | |
| CHD3 | Snijders Blok–Campeau syndrome | 618205 | |
| CHD4 | Sifrim–Hitz–Weiss syndrome | 617159 |
Recently, it has been described that mutations in histone H3 tails can also contribute to rare neurologic dysfunctions and congenital anomalies. These mutations likely cause disruptions of H3 interactions with DNA, other histones, and histone chaperone proteins, and result in altered histone modification patterns [20].
References
- EURORDIS. What Is a Rare Disease? Available online: https://www.eurordis.org/content/what-rare-disease (accessed on 25 June 2022).
- Nguengang Wakap, S.; Lambert, D.M.; Olry, A.; Rodwell, C.; Gueydan, C.; Lanneau, V.; Murphy, D.; le Cam, Y.; Rath, A. Estimating Cumulative Point Prevalence of Rare Diseases: Analysis of the Orphanet Database. Eur. J. Hum. Genet. 2019, 28, 165–173.
- Haendel, M.; Vasilevsky, N.; Unni, D.; Bologa, C.; Harris, N.; Rehm, H.; Hamosh, A.; Baynam, G.; Groza, T.; McMurry, J.; et al. How Many Rare Diseases Are There? Nat. Rev. Drug Discov. 2019, 19, 77–78.
- Bauskis, A.; Strange, C.; Molster, C.; Fisher, C. The Diagnostic Odyssey: Insights from Parents of Children Living with an Undiagnosed Condition. Orphanet J. Rare Dis. 2022, 17, 233.
- Rastegar, M.; Yasui, D.H. Editorial: Epigenetic Mechanisms and Their Involvement in Rare Diseases. Front. Genet. 2021, 12, 755076.
- Jones, P.A. Functions of DNA Methylation: Islands, Start Sites, Gene Bodies and Beyond. Nat. Rev. Genet. 2012, 13, 484–492.
- Kohli, R.M.; Zhang, Y. TET Enzymes, TDG and the Dynamics of DNA Demethylation. Nature 2013, 502, 472–479.
- Shi, D.Q.; Ali, I.; Tang, J.; Yang, W.C. New Insights into 5hmC DNA Modification: Generation, Distribution and Function. Front. Genet. 2017, 8, 100.
- Velasco, G.; Francastel, C. Genetics Meets DNA Methylation in Rare Diseases. Clin. Genet. 2019, 95, 210–220.
- Du, Q.; Luu, P.L.; Stirzaker, C.; Clark, S.J. Methyl-CpG-Binding Domain Proteins: Readers of the Epigenome. Epigenomics 2015, 7, 1051–1073.
- Ehrlich, M.; Jackson, K.; Weemaes, C. Immunodeficiency, Centromeric Region Instability, Facial Anomalies Syndrome (ICF). Orphanet J. Rare Dis. 2006, 1, 2.
- Barlow, D.P. Gametic Imprinting in Mammals. Science 1995, 270, 1610–1613.
- Monk, D.; Mackay, D.J.G.; Eggermann, T.; Maher, E.R.; Riccio, A. Genomic Imprinting Disorders: Lessons on How Genome, Epigenome and Environment Interact. Nat. Rev. Genet. 2019, 20, 235–248.
- Sanchez-Delgado, M.; Riccio, A.; Eggermann, T.; Maher, E.R.; Lapunzina, P.; Mackay, D.; Monk, D. Causes and Consequences of Multi-Locus Imprinting Disturbances in Humans. Trends Genet. 2016, 32, 444–455.
- Varrault, A.; Dubois, E.; le Digarcher, A.; Bouschet, T. Quantifying Genomic Imprinting at Tissue and Cell Resolution in the Brain. Epigenomes 2020, 4, 21.
- Fallah, M.S.; Szarics, D.; Robson, C.M.; Eubanks, J.H. Impaired Regulation of Histone Methylation and Acetylation Underlies Specific Neurodevelopmental Disorders. Front. Genet. 2021, 11, 1734.
- Martin, C.; Zhang, Y. The Diverse Functions of Histone Lysine Methylation. Nat. Rev. Mol. Cell Biol. 2005, 6, 838–849.
- Husmann, D.; Gozani, O. Histone Lysine Methyltransferases in Biology and Disease. Nat. Struct. Mol. Biol. 2019, 26, 880.
- Popp, B.; Brugger, M.; Poschmann, S.; Bartolomaeus, T.; Radtke, M.; Hentschel, J.; di Donato, N.; Rump, A.; Gburek-Augustat, J.; Graf, E.; et al. A Novel Syndrome Caused by the Constitutional Gain-of-Function Variant p.Glu1099Lys in NSD2. medRxiv 2022.
- Bryant, L.; Li, D.; Cox, S.G.; Marchione, D.; Joiner, E.F.; Wilson, K.; Janssen, K.; Lee, P.; March, M.E.; Nair, D.; et al. Histone H3.3 beyond Cancer: Germline Mutations in Histone 3 Family 3A and 3B Cause a Previously Unidentified Neurodegenerative Disorder in 46 Patients. Sci. Adv. 2020, 6, eabc9207.
More
Information
Subjects:
Genetics & Heredity
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
906
Revisions:
3 times
(View History)
Update Date:
05 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




