1. Decellularized Extracellular Matrices
As an alternative to artificial scaffolds, decellularized extracellular matrices are a natural source for scaffolds. Decellularization is the total elimination of cells from tissue by physical or chemical methods. After cell removal, the obtained scaffold consists of natural basic structural ECM proteins and glycosaminoglycans
[1]. The lack of cellular components eliminates the risk of adverse side effects, such as an inflammatory response or immunological rejection. If the obtained 3D structure is not warranted, matrices might pass through drying and pulverization for further reconstitution into molds or shapes of interest
[2][3][4].
Data from preclinical and clinical evaluation of acellular biologic ECM scaffolds in cardiac pump dysfunction and ischemic heart failure showed reduced fibrotic tissue, improved perfusion of infarcted myocardium, and reverse structural remodeling
[5]. A decellularized pericardial matrix colonized with human viable Wharton’s jelly-derived mesenchymal stromal cells was implanted in patients with non-revascularizable myocardial scars and has shown a reduction in scar mass after three months
[6]. A previous study using human decellularized pulmonary heart valves engineered with autologous EPC in pediatric patients with pulmonary valve pathology reported that these valves have the potential to remodel and grow along with the somatic growth of the patient
[7].
A potential disadvantage of decellularized extracellular matrices is the existence of remaining cells from the native tissue in the scaffold. These remaining cells can be dangerous if they trigger a patient’s immune response
[1]. In addition, an alternative to decellularized extracellular matrices is the use of artificial scaffolds.
2. Biofabrication Approaches Used to Develop Artificial Scaffolds
Different techniques are used to create artificial scaffolds to improve their physical and biological properties
[8]. Artificial biodegradable scaffolds hold the inherent advantage of being easily crafted into any shape and engineered for desired mechanical properties
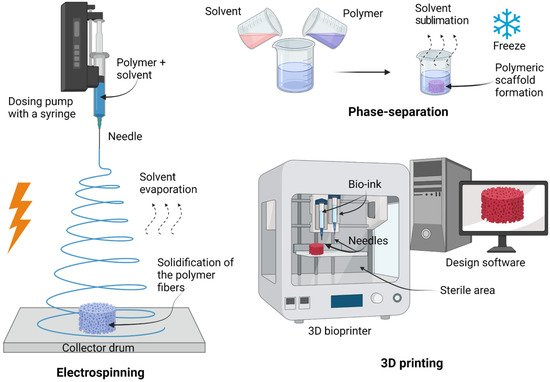
[4]. Various techniques have been developed to fabricate artificial scaffolds that mimic the ECM-native myocardium. These include, but are not limited to, electrospinning, phase separation, and 3D printing (
Figure 1)
[8]. Understandably, each technique has its advantages and disadvantages, which the researchers will discuss in general from the most commonly used artificial scaffold biofabrication methods, in this section.
Figure 1. Scheme of principal preparation techniques for scaffolds. Electrospinning consists of the formation of fibers using a voltage source. This involves the generation of a charged polymer jet that is ejected through a high-voltage. 3D printing generates a scaffold using computer design software, printing the scaffold layer by layer. Created with
BioRender.com accessed on 1 June 2022.
2.1. Electrospinning
Electrospinning is a technique used for micro- and nanoscale fiber production from an electrically forced solution/melt of polymeric biomaterials
[9]. The four basic elements required to implement electrospinning are a dosing pump with a syringe (containing the polymer solution), a needle (Taylor cone), a collector drum (can be a plate, metal screen, or rotating mandrel), and a high-voltage power supply (up to 30 kV). Briefly, electrospinning involves the generation of a charged polymeric biomaterial jet that is ejected through a high-voltage electric field; randomly rotating polymeric fibers rest on a grounded complex to create a scaffold. The solvent evaporates, and the polymer fibers solidify
[10][11]. It is important to consider every single parameter, polymer molecular weight, voltage, the distance between the capillary and collector, polymer concentration, solution conductivity, and solvent volatility, all of which impact the characteristics and properties of the fibers
[12]. An important parameter in cardiac tissue engineering is the diameter of the fiber. The diameter of the fiber in electrospinning plays an important role in the conductivity and properties that are needed in cardiac regeneration. According to Abedi et al., the reduction of average fiber diameter from 225 to 110 nm in a scaffold based on chitosan and PVA with multi-wall carbon nanotubes increased electrical conductivity from 8 × 10
−5 S/m to 9 × 10
−3 S/m
[13].
-
Advantages: simple methodology, relatively low preparation cost, sample uniformity, generation of aligned fibers smaller than a micrometer, scaffold incrementation of surface-to-volume ratio (it improves cell attachment, proliferation, and differentiation), tunable porosity interconnectivity over 80%, unique pore shapes, and superior mechanical properties
[9][10][14][15];
-
Disadvantages: the need for high-voltage appliances and the use of toxic solvents
[15].
2.2. Phase-Separation
Phase separation, also called thermally induced phase separation, is a simple method that comprises solution preparation with polymeric biomaterials and solvents, followed by freezing. Once the mix is prepared, the solvent is removed without degrading the polymer by sublimation via freeze-drying to obtain scaffolds with high porosity and interconnectivity
[16]. Freezing temperature, concentration, and the nature of solvent and solute can be tunable to modify some pore characteristics
[17].
-
Advantages: simplicity of the technique, and preservation of the scaffold structure because the process does not involve high temperatures
[15];
-
Disadvantages: lengthy process, poor architecture, limited control of size range, irregular porosity, unsuitable mechanical properties for replacing human tissue, and remaining solvents are potentially toxic
[15].
2.3. 3D Printing
Another novel method for fabricating artificial scaffolds is 3D printing. Its use involves bio-printers, needles, bio-inks, and design software
[18]. A 3D model was generated using computer-aided design (CAD) software for conversion to STL format. This 3D format of the scaffold is sliced into 2D layers to be successively printed and bound layer by layer. Hence, the resolution level depends on the diameter of the needles used
[19][20]. Some current bio-printer models mimic a sterile environment, such as a biosafety cabinet, using HEPA filters and UV lamps for sterilization. This process can be observed because of their clear windows
[11].
-
Advantages: probably the most attractive technique in terms of micro-architecture, allows the use of a wide range of biomaterials, and excellent controllability over structural properties such as porosity, pore size, and pore interconnectivity
[18];
-
Disadvantages: initial investment in the instrument, the use of toxic solvents, and mechanical instability
[11].
2.4. Solvent Casting and Solvent Casting/Particulate Leaching
The solvent casting method is based on polymer and organic solvent mixing, casting into a 3D mold, or immersing the mold in a polymeric solution to get a scaffold once the solvent has been removed by simple evaporation, vacuum drying, or lyophilization. After solvent removal, the scaffold is washed with water, leaving the porous structure. The internal size of pores is related to and controlled by salt granules
[15][17][21].
-
Advantages: simple methodology, mechanically stable, and does not need sophisticated instruments
[15][17][21];
-
Disadvantages: maintaining desired porosity and uniform salt dispersion is a challenge, a long time for solvent evaporation, inefficient complete salt leach out, and inefficient complete removal of the solvent
[15][17][21].
2.5. Emulsion Templating
-
Emulsion templating is a promising biofabrication approach for scaffolds with up to 99% porosity and high interconnectivity. The technique is based on two steps. The first step is the preparation of an emulsion of two immiscible liquids; one phase is the internal or dispersed phase, and the other one is the external or continuous phase. The second step is the solidification of the continuous phase of the emulsion, while the dispersed phase (which works as a template by its addition drop-by-drop) is removed to obtain a porous scaffold. Emulsion can be given by water-in-oil (
w/
o) or oil-in-water (
o/
w)
[22].
3. Materials Used for Artificial Scaffold Construction
Biomaterials can be defined as any material or combination of materials that can be used for any period for the total or partial replacement of any tissue. Biomaterial’s goal is to maintain or improve the quality of a patient’s life. Polymeric biomaterials can be classified by their synthetic or natural origin
[23]. Polymeric biomaterials are an attractive option for tissue engineering since they can monitor their development, thus controlling topological, mechanical, and structural properties with the benefit of functionalizing or adding other materials (composites) to enhance attachment
[24][25]. Over time, many scaffold formulations have been developed by mixing two or more different biomaterials to mimic the ECM native myocardium and its mechanical and biological properties
[26].
3.1. Synthetic Materials for Artificial Scaffolds
Although not all synthetic materials are directly biodegradable, non-biodegradable biomaterials can be engineered to biodegrade. Biodegradable materials have a great advantage. They have the capability to be replaced when mature cells produce their own ECM. Using synthetic materials in cardiac tissue engineering has grown in recent years, with emphasis on developing patches for cardiac repair with the use of biodegradable polymers, such as poly(ε-caprolactone) (PCL), poly(glycerol sebacate) (PGS), polyethylene glycol (PEG), poly(l-lactide acid) (PLLA), poly l-lactic-co-ε-caprolactone (PLCL), and poly(lactic-co-glycolic acid) (PLGA)
[27]. In the early part of the 21st century, Matsubayashi et al. developed a porous PCL cardiac patch seeded with VSMC with the intent of repairing an aneurysm and designed to prevent ventricular dilation post-myocardial infarction
[28]. Moreover, Morgan et al. developed other porous structures using PGS to control the orientation of cardiac cells
[29]. Improving cardiac orientation, Hu et al. developed patches using PGS copolymerized with an aniline trimer to obtain electroactive material, significantly enhancing cell interactions
[30]. An interesting part of heart mending is valve repair, wherein materials such as PEG-based hydrogels are used as scaffolds. By cross-linking PEG with peptides, biomimetic properties were obtained; the resulting hydrogels influenced elongation. The de novo ECM deposition and hydrogel degradation behavior of encapsulated valvular cells potentiated the use of these materials for developing future heart valves
[31]. In another interesting approach, the implantation of tissue-engineered vascular grafts (PLLA and PLCL) seeded with autologous bone marrow mononuclear cells was used as an extracardiac total cavopulmonary conduit in pediatric univentricular physiology. No evidence of aneurysmal formation, graft rupture, graft infection, or calcification was reported, and seven (28%) patients had asymptomatic graft stenosis
[32].
3.2. Natural Biomaterials for Artificial Scaffolds
In the search for natural biomaterials, researchers have investigated the individual components of the ECM as platforms. Many biomaterials for scaffold construction, such as collagen, fibrinogen, silk, alginate, and chitosan, are under investigation
[27][33][34][35][36].
3.2.1. Collagen
Collagen is commonly used in myocardial tissue engineering, given that it is a major component of the myocardium ECM
[37] (
Table 1). In the heart, collagen type I is the major constituent of the ECM, representing 75–85%
[38], with the added advantage of low immunogenicity. Collagen type I comprises two alpha-1 chains and one alpha-2 chain, creating long fibers whose properties depend on density and spatial alignment
[24], albeit collagens can divide into fibrillar and non-fibrillar components. These non-fibrillar components can form networks or associate with fibrillar collagens or membranes
[39][40]. Recent research has focused on utilizing collagen-based biomaterials for pathologies, such as myocardial infarction. These materials can deliver particulates, such as growth factors or peptides, to induce differentiation and patterning
[41]. Researchers have based initial approaches to delivering these materials on intramyocardial injection, as this permits direct and precise delivery to the affected area. However, with this approach, surgery becomes a must, and there is a second issue of potential leaking of the material to other areas
[42]. An alternative to this delivery system is the formation of so-called “cardiac patches”. Even though they are not exclusive to collagen, cardiac patches have unique properties. One such feature is their potential to cultivate cells ex vivo to promote proper invasion of the patch; further, these patches can go into models and have high engraftment levels
[33]. Lastly, a 3D collagen type I matrix seeded with autologous bone marrow mononuclear cells (for the regeneration of ischemic myocardium) showed increased thickness of the infarct scar with viable tissue and helped normalize cardiac wall stress in injured regions, limiting ventricular remodeling and improving diastolic function
[43].
3.2.2. Fibrinogen
Previous work has used collagen and fibrin/fibrinogen patches to enhance cardiac cell maturation by simulating cardiac muscle development
[33][34]. One clear advantage of these materials is their help in promoting electrical conductivity, particularly in differentiating cells and other stimulants. Certain maturation properties are achieved, such as the induction of Purkinje-like cells
[33][48][49]. Fibrin is a naturally occurring biomaterial, a biopolymer formed during coagulation
[38][50]. It has interested many researchers, either alone or in combination. Fibrin/fibrinogen helps with certain properties, including biocompatibility and biodegradability, when used as a scaffold. Fibrin comprises a randomly organized 3D structure with high interconnectivity, yet the threads forming the network are soft, allowing deformation without a break. Fibrinogen decomposition (fibrinopeptides), which depends on the amount of thrombin used and re-polymerization, regulates the mechanical properties of fibrin gels
[50]. In clinical applications, fibrin is obtained from plasma for autologous applications, such as treating osteoarthritis
[36]. It is important to mention that fibrin glue is another important application typically used in surgical procedures to replace sutures
[51]. Other useful applications include repairing the urinary tract, eye, liver, lung, spleen, heart valves, and filling bone cavities
[38][51][52][53].
3.2.3. Silk
The use of silk as a novel biomaterial for tissue engineering has been studied in recent years (
Table 2). Silk has been studied not only because it has several similarities to other materials, such as fibrin/fibronectin (architecture, mechanical properties, and degradation rates) but also because silk, in comparison with fibronectin, does not contribute to pathological hypertrophy
[54]. In animal models, silk-based scaffolds have shown therapeutic effects and the capacity to maintain the differentiation of cells into cardiac lineages
[55]. It is important to note that orientation is key, as defined in cardiac tissue studies, to demonstrating the maintenance and development of sarcomeres, particularly an upregulation in the synthesis of titin protein
[56].
3.2.4. Alginate
Alginate is a natural derivative of the cell walls of brown marine algae or bacteria. It is a polysaccharide with properties of biocompatibility, solubility in postmodified salts or esters, porosity, biodegradability, and viscosity tunability. Besides their use in tissue engineering, alginate has been used in medical applications, such as drug and protein delivery, wound healing, and implants. Disadvantages in the medical use of alginate in cardiac tissue engineering include inadequate mechanical stability and poor biological stability (inhibition of proliferation and instable biodegradability). As an option for solving these disadvantages, composite scaffolds are preferred by the combination of alginate with other polymers (
Table 3)
[58].
3.2.5. Chitosan
Chitosan (CS) is a polysaccharide obtained by the deacetylation of chitin. Normally found in insects and crustaceans, such as shrimp, crabs, and lobsters. It is a natural polymer with a linear structure consisting of β (1–4) glycosidic bonds linked to
d-glucosamine residues with a variable number of randomly located N-acetyl-
d-glucosamine (NAG) groups. It is soluble in dilute or weak acids but insoluble in aqueous solutions above pH 6.5
[39]. Recently, chitosan has been widely used in tissue engineering because of its capacity to protonate amino groups in an acidic medium, providing high biocompatibility, non-toxicity, anti-thrombogenic, biodegradability properties, and possessing a hydrophilic surface
[60]. The combination of chitosan scaffolds with stem cells has shown positive results in delivering stem cells to infarcted myocardium and increasing cell retention while preserving cardiac function
[61]. These properties make chitosan scaffolds suitable for cell attachment and proliferation (
Table 4). However, chitosan scaffolds alone have limitations because they have weak mechanical properties and a high degradation rate
[62].
There is no evidence of the clinical use of chitosan-based tissue engineering therapies. Nevertheless, recent research has been devoted to studying the potential use of chitosan-based biomaterials as an injectable therapy delivering progenitor cells
[65]. Because of its hemostatic properties, chitosan has been used in chitosan-based pads to improve hemostasis following transradial arterial access in a couple of clinical studies
[66][67] and a few more ongoing clinical trials (NCT04380883, NCT04857385, NCT03522077, NCT02837744).