Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | José Sánchez-Fernández | -- | 3359 | 2022-07-29 18:36:23 | | | |
| 2 | Camila Xu | Meta information modification | 3359 | 2022-08-01 03:36:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Santamaria-Garcia, V.J.; Flores-Hernandez, D.R.; Contreras-Torres, F.F.; Cué-Sampedro, R.; Sánchez-Fernández, J.A. Photoresponsive Supramolecular Systems. Encyclopedia. Available online: https://encyclopedia.pub/entry/25678 (accessed on 06 February 2026).
Santamaria-Garcia VJ, Flores-Hernandez DR, Contreras-Torres FF, Cué-Sampedro R, Sánchez-Fernández JA. Photoresponsive Supramolecular Systems. Encyclopedia. Available at: https://encyclopedia.pub/entry/25678. Accessed February 06, 2026.
Santamaria-Garcia, Vivian J., Domingo R. Flores-Hernandez, Flavio F. Contreras-Torres, Rodrigo Cué-Sampedro, José Antonio Sánchez-Fernández. "Photoresponsive Supramolecular Systems" Encyclopedia, https://encyclopedia.pub/entry/25678 (accessed February 06, 2026).
Santamaria-Garcia, V.J., Flores-Hernandez, D.R., Contreras-Torres, F.F., Cué-Sampedro, R., & Sánchez-Fernández, J.A. (2022, July 29). Photoresponsive Supramolecular Systems. In Encyclopedia. https://encyclopedia.pub/entry/25678
Santamaria-Garcia, Vivian J., et al. "Photoresponsive Supramolecular Systems." Encyclopedia. Web. 29 July, 2022.
Copy Citation
Photosensitive supramolecular systems have garnered attention due to their potential to catalyze highly specific tasks through structural changes triggered by a light stimulus. The tunability of their chemical structure and charge transfer properties provides opportunities for designing and developing smart materials for multidisciplinary applications. Photoswitchable systems designed to catalyze chemical reactions must incorporate the appropriate photochromic units into the system to translate the structural switching states into a different chemical reactivity.
photoresponsive
supramolecular structures
photoswitchable systems
1. Introduction
Nanoscience and its related fields have the potential to lead humanity to the understanding and mastery of learning the highly efficient design principles found in nature to resolve a plethora of current technological challenges. Stimuli-responsive materials have attracted significant attention in material science. In response to external stimuli, they can undergo substantial micro- or nano-scale changes with exquisite spatial and temporal control, thus they are considered “smart materials”. The stimuli disturbing these materials are of a different nature. Among the most studied are those with a chemical nature, such as pH [1], ionic solvent [2], and chemical agents [3], and others with a physical nature, such as temperature, mechanical force, light [4][5], and electric or magnetic fields [6]. However, biochemical stimuli are also frequently applied [7]. The wide practical application of smart materials involves sensing, drug delivery, additive manufacturing, and photochromic devices [8].
Light is a worthy ubiquitous stimulus whose intensity and wavelength can be easily controlled according to application requirements. The light-triggered response of a photosensitive system may be reversible or not. However, it has been proved that the reversible responses allow the system to shift, commonly between two states that provide a way to switch the system on or off and hence to catalyze and control a reaction. Most of the reversible photo-induced reactions are described by the photochromism phenomenon. This involves a reversible toggling between two isomers of the photochromic molecule, i.e., reversible photoisomerization [9][10]. Similar to other photochemical reactions, photochromism corresponds to an equilibrium state; thus, the product does not appear as a separate phase but results as distinguishable due to the reversibility attribute. Each of the two states exhibits a characteristic absorption spectrum that results from electromagnetic radiation in at least one direction.
Azobenzene is a well-studied photochromic unit that governs the photoisomerization processes reported in the literature. It is a T-type of a photochrome that shows the capability of going from an E(trans) to a Z(cis) conformation when being irradiated with ultraviolet (UV) light, which exerts structural stresses on the molecule [11][12]. Some of the structural changes imply modifications in bonding angles, dipole moments, and even conjugation properties [13]. Moieties such as azobenzenes have commonly enabled highly efficient transformations in the forward and reverse directions by altering their steric and electronic properties [14].
Photoswitchable systems designed to catalyze chemical reactions must incorporate the appropriate photochromic units into the system to translate the structural switching states into a different chemical reactivity [9]. Therefore, one must optimize the intrinsic switching properties of the photochrome in a photoisomerization process to attend to a specific target task by activating or deactivating an initial material, the product, or a dye mediator [15][16]. The vast majority of the photoswitchable systems fall into one of the following categories, which will be discussed later: metal–organic cages (MOCs), metal–organic frameworks, or hydrogen-bonded organic frameworks (HOFs).
Metal–ligand interactions have been exploited for the construction of photoswitchable cages and frameworks. The coordination of organic ligands to metal ions gives rise to well-defined cavities that confer functional properties to modulate reactions in a very similar fashion to enzymes. On the other hand, hydrogen bonding is a donor–acceptor interaction specifically involving hydrogen atoms interacting with an acceptor carrying nonbonding electron lone pairs. Hydrogen bonding is an essential component of the structure and function of biological molecules since they highly influence molecular packing and solvation, and even alter conformation. They can serve as molecular interactions that control and stabilize the supramolecular organization through self-assembly, thus directly impacting functionality aspects [10]. A key factor in the formation of hierarchical structures with asymmetrical and/or anisotropic organization emerges in nanoarchitectonics-based material constructions, such as in most biological functional systems or bio-like highly functional materials, which are fundamental for creating rational flows of energy and efficient photocatalysts [17][18].
2. Supramolecular Structures with Photoresponsive Units
Substantial advances in materials science would not have been possible without the application and understanding of chemistry. The impact of chemistry greatly agglutinates to the transformation and control of the use of electromagnetism on photoreactions and photochromism phenomena, creating countless possibilities for developing new photoswitchable systems. The coupling of photosensitive systems to nanoparticles is a convenient route for the design of specialized materials. It is well known that most of the relevant properties of nanoparticles depend on their size and morphology. For example, for nanomedicine applications, particles must have dimensions of 10–100 nm for optimum circulation in the bloodstream and to avoid renal and lymphatic clearance. In this regard, Manners and co-workers explored the possibilities of creating π conjugated polymer nanoparticles sensitive to light and focused on self-assembly methods [19]. The reported strategies conceptualize a photoresponsive, spiropyran-coated, nanostructured surface that enables the highly efficient release of cancer cells [20]. Great progress in the photoresponsive system for in vivo application might be achieved by exploring the self-illuminating induced photocleavage of linkers based on abnormal disease microenvironments [21].
In the quest to attain the best performance of carbazole moieties in carbazole-based copolymers (CPs) in organic field-effect transistor (OFET) memory devices, a highly valued aspect is the search for the best possible conjugation state. The electronic polarization of the carbazole ring enhances the dipole−dipole interactions [22]. The research of the structure−property relationship of the carbazole-based copolymers has shown the relation of the donor−acceptor system with the modulation of the performance of photoresponsive OFET memory devices [23]. In response to these findings, Ono et al. postulated the integration of chiral moieties into carbazole-cored molecules to enhance the photosensitive material properties [24].
Experiments with various donor–acceptor-type CPs such as poly(1,8-carbazole)-benzothiadiazole copolymer (PCzBT) and a poly(1,8-carbazole)-dithienylbenzothiadiazole copolymer (PCzDTBT) with high photostability as well as diameters as small as 3.0–4.5 nm were described by Piwonski et al. [25]. Under the optimal biological regulatory mechanisms, a variety of reversible assembly/disassembly strategies for targeting switchable photocatalytic systems may be successfully extended for supramolecular designs that can facilitate nonparallel stacking of electron-donor (D) and electron-acceptor (A) moieties in constructing indistinguishable photoredox catalytic systems [26][27]. The phenomenon of an intramolecular charge-transfer interaction between an electron-rich carbazole fragment and an electron-deficient iminium double bond leads to a broad absorption in the visible spectrum. A charge transfer complex can be irradiated to trigger a single electron transfer process from the carbazole to the iminium ion, developing a chiral radical intermediate [26].
Biomimetics may be combined with engineering techniques to create bioengineered materials against bacterial infections. An interesting approach is the conjugation of molecules or proteins to natural or semisynthetic polymers, developing bioengineered polymer–drug conjugates [28]. In the same line, several of the architectonic characteristics of biomolecules, in particular nucleic acids, proteins, and sugars, that use diverse chemical interactions to form well-defined architectures with distinctive functions have a clear advantage because of their self-assembling properties [29]. The importance of self-assemble colloidal crystals requires a deeper scientific understanding of ligand–particle and particle–fluid interactions at multiple scales and governed by various conditions according to their nature [30].
Photoactive molecules such as viologenic compounds can be found within organic electrochromic materials in different states. The three common redox forms of viologens are the usually colorless di-cation, the highly colored radical cation, and the di-reduced (neutral) form. The electrochromic properties of the viologen systems can be modulated by varying the molecular structure by changing the substituents on the nitrogen atom and by changing their environment (electrolytic media). Electrochromic (EC) gels based on single asymmetric viologens provide a more neutral-colored state than their corresponding symmetric viologens while maintaining a satisfactory colorless, bleached state and optical contrasts. The researchers of such findings worked with two asymmetrically 1-alkyl-1′-aryl-substituted viologens, specifically 1-ethyl-1′-(p-cyanophenyl)-4,4′-bipyridinium dibromide (Et-pCNVio) and 1-benzyl-1′-(p-cyanophenyl)-4,4′-bipyridinium dibromide (Bn-pCNVio). Electrochromic devices (ECDs) based on alkyl substituents show violet coloration, while viologens with aryl groups demonstrate green coloration for their first reduced form [31]. The synthetic route successfully followed the reactions shown in Scheme 1.
Several studies regarding viologens have revealed their vulnerability and instability in the presence of oxygen and other radical scavengers. This capacity has been successfully exploited to develop strategies of paramount importance to stabilize the photogenerated radicals. Due to their inherent porosity, MOFs can also be loaded with photoactive materials such as the viologen cations that undergo some electron transfer upon irradiation in a similar way to molecules that are known to change color due to a cis–trans isomerization process. The success of this method depends entirely on the size of the pores within the MOFs [32].
Devices using water-soluble, chromogenic thiazolo(5,4-d) thiazole (TTz) dyes were developed by a research group headed by Michael Walter [33]. They synthesized viologens from TTz and these were later studied in PVA/borax hydrogel devices from which the ease-to-assemble and high oxidative stability in the air were highlighted. These multifunctional chromogenic devices (CGDs) were efficiently reversible and stable, with valuable fast-switching steps. They also exhibited stable multichromic properties.
The MOF material with the methyl viologen (MV) [Zn3(IPA)4 MV] (H2IPA = isophthalic acid, MV⋅2NO3 = 1,1′-dimethyl-1,1′-diium dinitrate), constructed in coordination with Zn and isophthalic acid, was developed by Liu et al. with the purpose of being used in inkless and erasable printing. This compound showed fast-responsive reversible photochromism under UV-Vis radiation due to the electron transfer between the host skeleton and guest MV2+ [34].
A particular and interesting photoswitching mechanism that resulted in the modulation of the MOF’s properties without changing its structure was reported. In this context, UV-triggered inter-linker electron transfer was favored by the pseudo-rotaxane assembly of an Eu-containing MOF. Viologen radicals resulted from this process, which conferred a blue color to the material and quenched the fluorescence of the Eu(III) cations [35].
The study of the redox processes in electrochromic materials is fascinating. These materials can reversibly change their color due to an electrochemical reaction and are an essential component in all kinds of ECDs. To develop and produce tailored ECDs, it is important to understand the mechanisms of the electron transfer reactions in such materials and to identify the species responsible for the coloration.
Considerable research has been recently conducted to study the integration and synchronization of controlled molecular motions, as described by the research team of Giuseppone [36]. One of their proposals implies classifying the molecular structures according to their hierarchical dynamics, potential functions, and applications at micro- and macro-scopic scales. Typically, molecular switches are defined as chemical species possessing at least two thermodynamically (meta)stable states and with the characteristic of reversible interconversion to external stimuli. Such switches are typically activated by various chemical, molecular electrochemical, or photochemical stimuli. One of the most relevant structural factors is that their isomers should be stable enough to avoid spontaneous thermal relaxation [36].
3. Photoswitchable Control of Supramolecular Organic Systems
Considering the superiority of photoresponsive supramolecular switches, researchers will summarize recent progress to provide a comprehensive overview of these host–guest systems with the peculiarity of reversibly shifted and present different structural states by light stimuli. Such photoswitchable systems have been applied both in tissue engineering and drug delivery and to produce smart materials. In addition, researchers approach a range of applications of self-assembly materials such as metal–organic cages (MOCs), metal–organic frameworks (MOFs), and hydrogen-bonded organic frameworks (HOFs) and their opportunity schemes as the next generation of materials that respond to external stimuli and that are part of the new technologies that inspire new research ideas.
3.1. Metal–Organic Polyhedra (MOP)
Several research groups around the world have dedicated a large space in their agendas to synthesizing and establishing broad interrelated characterization routes on metal–organic polygons and polyhedra (MOPs), namely squares, cubes, tetrahedra, and hexahedra. A large number of studies revealed the possibility to achieve desired structures constructed from nodes of either single metal ions or metal carboxylate clusters connected by organic links [37]. In this sense, the construction of self-organizing systems concerns the reticular chemistry that links molecular building units through strong bonds forming predefined structures. The research on reticular synthesis mainly focuses on 2D or 3D extended frameworks; according to these properties, each molecular unit in reticulation is built with the functionality demanded to form specific linkages chemically and geometrically to shape the framework [38][39].
The great structural design of the MOP and their ability to form several topological structures has been exhaustively covered by Lee et al.; they extensively explain common designs, such as regular convex polyhedra or “Platonic solids”, and which consequently have one kind of vertex, one kind of edge, and one kind of face. There are five different regular polyhedra with transitivity values of (1, 1, 1): the tetrahedron (tet), cube (cub), octahedron (oct), dodecahedron (dod), and icosahedron (ico). The second essential polyhedra are “quasiregular” polyhedra, which have one kind of vertex and edge but two kinds of face, and therefore a transitivity of (1, 1, 2), such as the cuboctahedron (cuo) and icosidodecahedron (ido). In addition, there are two more edge-transitive polyhedra with a transitivity (2, 1, 1), which present one kind of edge and one kind of face, such as the triacontahedron (trc) and rhombic dodecahedron (rdo) [40]. Only fourteen types of polyhedra have been discovered in MOP assembly compared to innumerable topologies of MOFs known. Moreover, the captivating symmetric topologies and the discrete structural features limit the applications of MOPs, unlike MOFs.
Specifically speaking, microporous metal–organic polyhedra are similar to supramolecular cages in such a way that they can be accurately defined as a subset of them. In this regard, pioneering works on the syntheses of tetranuclear magnesium-based tetrahedron and palladium are systems broadly representative of the class of coordination cages [41]. Following the same line of research, the work carried out by Jiang et al. is remarkable [42]. They emphasize that the confinement strategy of a PMOP in nano-scaled spaces of MS endows photoresponsive MOPs with real photoresponsiveness and, consequently, they have control over their photoresponsive efficiency.
There are characteristics linking chemical structures that have resulted in the recent development of orthogonal surface chemistry and the assembly of more stable cages, and for the same reason, it has been established that the MOPs are a subclass of coordination cages [43][44].
Cage-like molecules, built by the coordination of organic links and metal ions, are opening new horizons in material science due to their design flexibility. Metal–organic cages (MOCs) are systems with a high degree of symmetry, which is promising for several applications such as separation, adsorption, catalysis, molecular recognition, chirality sensing, and reactive species stabilization [45][46][47]. MOCs are often produced using self-assembly mechanisms, in which components undergo a reversible process that leads to thermodynamic equilibrium. Chemists have largely avoided some of the complications of MOC formation by concentrating on rigid and symmetrical building blocks, metals with well-defined geometries, and the design principles that result from those choices. However, there has been a trend to develop more sophisticated and functional MOCs by self-sorting diverse ligands into heteroleptic cages, unsymmetrical ligands into asymmetrical MOCs, or ligands with secondary functionalities into functional materials. The extremely large availability of MOC precursors allows researchers to tailor them for specific properties [48][49]. Moreover, their discrete, rather than extended, structures with well-defined shapes, specific cavities, nano-scale sizes, and symmetrical geometries allow them to be used as self-sufficient materials in a wide range of applications but also to tune soft materials used in medicine, robotics, and battery research [50].
3.2. Metal–Organic Framework (MOF)
MOFs are inorganic–organic hybrid materials with varied structural geometries and, consequently, due to their enormous surface area and functionality, these materials have highly targeted applications.
MOFs, also known as porous coordination polymers (PCPs), are assembled from metal ions or clusters and organic linkers via metal−ligand coordination bonds and have captivated significant scientific interest on account of their high crystallinity, exceptional porosity and tunable pore size, high modularity, and diverse functionality. However, to date, the potential of chiral MOF (CMOF) materials for specific applications include chiral recognition, separation technologies, and catalysis [51]. Regarding the above, the definition that has been best carried out for MOFs is the one that Barton and collaborators very accurately expose. They correctly establish the topologic descriptors to enhance crystal structures of MOFs and 3D coordination polymers [52].
According to Ma et al., MOFs and covalent organic frameworks (COFs) are two classes of crystalline polymeric and porous materials. On the one hand, the MOFs are assembled by metal ions/clusters and organic linkers; on the other hand, the COFs are constructed by pure organic building blocks [53]. Using the technologies of design MOFs and COFs, they perform a great job of reviewing the anchoring aggregation-induced emission (AIE) and AIE luminogens (AIEgens) in those structures.
AIEgens and the corresponding supramolecular materials expand the detailed information beyond the fundamental insights and into the self-assembly of nonplanar molecules and the frontiers of supramolecular chemistry. AIEgens are likely to undergo photoexcitation due to their restricted intramolecular movements in the presence of neighboring molecules [54]. The design and synthesis specific to AIEgens with photocontrollable emissions covering violet, blue, green, yellow, orange, red, deep red, and NIR regions have been associated with the HOMO-LUMO energy level by donor (D)–acceptor (A), exemplified by the blocks formed by phenylamine (TPA)–thiophene [55]. Cytotoxicity studies in HeLa cells were evaluated using pyridinium-substituted tetraphenylethylene salts-based compounds (identified as TPEPy-I and TPEPy-PF6 by the researchers), whose efficacy was stipulated by Lingyun Wang and Wei Chen. The researchers made it clear that significant cytotoxicity of AIGens occurs when they are excited by microwave (MW) towards HeLa cells, even at low concentrations. Under these assertions, the average HeLa cell viabilities turned out to be 58.4% vs. 62.5%, 31.1% vs. 36.7%, and 9.3% vs. 14.2% at 2.5, 5, and 10 μM of the iodine compound TPEPy-I and TPEPy-PF6 with hexafluorophosphate in its structure, respectively, under 10 W (2450 MHz) of MW irradiation for 1.5 min [56][57].
The synthetic route to obtain the products TPEPy-I and TPEPy-PF6 has been reported in detail, where it predominantly involves the Suzuki reaction using 5-formyl-2-thiopheneboronic acid and 4,4′,4″-(2-(4 bromophenyl)ethene-1,1,2-triyl)tris(methoxybenzene) through acceptor structural intermediates such as tetraphenylethene, thiophene, and aldehyde, and thus obtains a robust starting compound harboring donor-π-acceptor units [58][59].
Undoubtedly, the combination of materials, organic and inorganic, is the key element in COFs, and the emphasis devoted by the scientific community to making optimal topologies of two dimensions (2D) is greatly determined by the connectivity and symmetries of the building blocks involved. Therefore, the morphology and symmetries of building blocks of a 2D COF have relevant implications on its topologies and structural networks. Going into detail, Peng et al. show a procedure for preparing flexible conformation 2D COFs that can provide additional degrees of freedom for their topologies. Specifically, the products were synthetized from tetrakis(4-aminophenyl)ethene [60] with 2,3-dihydroxyterephthalaldehyde (2,3-DHTA) and with 2,3-dimethoxyterephthalaldehyde (2,3-DMTA) to obtain TPE-COF-OH and TPE-COF-OMe, respectively, with different topologies and porosities. This was achieved through the activation or passivation of intramolecular hydrogen bonding, which requires looking deeper into the conformation of molecular linkages in the networks, leading to the topology regulation of COF networks [61].
In various aspects of the nature of energy-emitting centers, electron transfer is inherent in π-conjugated systems; that is, the combination of interactions of chemical structures where some are donors and others are acceptors facilitates intramolecular charge transfers (ICT), which systematically result in low electronic band gaps, broad absorption, and long emission wavelengths [56].
The use of AIEgens to structure MOFs and COFs through non-covalent interactions and well-established interactions has been described, and not in an unpredictable structural way as presented by the AIEgens [53]. The well-established strategies for the design of materials such as connecting AIEgens based on reticular chemistry can be understood by the thermodynamics and the kinetics of the reaction via altering the reaction conditions, and crystalline AIEgen-MOFs or AIEgen-COFs with distinct topologies can even be made using the same organic building units and metal sources.
Increasing productivity in material synthesis and the characterization for evaluating the photoisomerization and physical behaviors of an imine-linked photoresponsive COF have given way to significantly planning the reaction between dithienylethene-dialdehyde and 5,10,15,20-tetra(4-aminophenyl)-porphyrin (H2TAPP) [62].
References
- Tao, W.; Wang, J.; Parak, W.J.; Farokhzad, O.C.; Shi, J. Nanobuffering of PH-Responsive Polymers: A Known but Sometimes Overlooked Phenomenon and Its Biological Applications. ACS Nano 2019, 13, 4876–4882.
- Cui, J.; Li, Y.; Chen, D.; Zhan, T.G.; Zhang, K.-D. Ionic Liquid-Based Stimuli-Responsive Functional Materials. Adv. Funct. Mater. 2020, 30, 2005522.
- Brighenti, R.; Cosma, M.P. Swelling Mechanism in Smart Polymers Responsive to Mechano-Chemical Stimuli. J. Mech. Phys. Solids 2020, 143, 104011.
- Wani, O.M.; Schenning, A.P.H.J.; Priimagi, A. A Bifacial Colour-Tunable System: Via Combination of a Cholesteric Liquid Crystal Network and Hydrogel. J. Mater. Chem. C 2020, 8, 10191–10196.
- Kauscher, U.; Holme, M.N.; Björnmalm, M.; Stevens, M.M. Physical Stimuli-Responsive Vesicles in Drug Delivery: Beyond Liposomes and Polymersomes. Adv. Drug Deliv. Rev. 2019, 138, 259–275.
- Dong, Y.Z.; Choi, H.J. Synthesis of Smart Poly(Diphenylamine)/Magnetic Particle Composites and Their Electric/Magnetic Stimuli-Response. Macromol. Res. 2018, 26, 667–670.
- Zhang, Z.Z.; Chen, Y.R.; Wang, S.J.; Zhao, F.; Wang, X.G.; Yang, F.; Shi, J.J.; Ge, Z.G.; Ding, W.Y.; Yang, Y.C.; et al. Orchestrated Biomechanical, Structural, and Biochemical Stimuli for Engineering Anisotropic Meniscus. Sci. Transl. Med. 2019, 11, eaao0750.
- Ru, Y.; Shi, Z.; Zhang, J.; Wang, J.; Chen, B.; Huang, R.; Liu, G.; Yu, T. Recent Progress of Photochromic Materials towards Photocontrollable Devices. Mater. Chem. Front. 2021, 5, 7737–7758.
- Göstl, R.; Senf, A.; Hecht, S. Remote-Controlling Chemical Reactions by Light: Towards Chemistry with High Spatio-Temporal Resolution. Chem. Soc. Rev. 2014, 43, 1982–1996.
- Lugger, S.J.D.; Houben, S.J.A.; Foelen, Y.; Debije, M.G.; Schenning, A.P.H.J.; Mulder, D.J. Hydrogen-Bonded Supramolecular Liquid Crystal Polymers: Smart Materials with Stimuli-Responsive, Self-Healing, and Recyclable Properties. Chem. Rev. 2022, 122, 4946–4975.
- Pilz Da Cunha, M.; Van Thoor, E.A.J.; Debije, M.G.; Broer, D.J.; Schenning, A.P.H.J. Unravelling the Photothermal and Photomechanical Contributions to Actuation of Azobenzene-Doped Liquid Crystal Polymers in Air and Water. J. Mater. Chem. C 2019, 7, 13502–13509.
- Gelebart, A.H.; Vantomme, G.; Meijer, E.W.; Broer, D.J. Mastering the Photothermal Effect in Liquid Crystal Networks: A General Approach for Self-Sustained Mechanical Oscillators. Adv. Mater. 2017, 29, 1606712.
- Lu, X.; Zhang, H.; Fei, G.; Yu, B.; Tong, X.; Xia, H.; Zhao, Y. Liquid-Crystalline Dynamic Networks Doped with Gold Nanorods Showing Enhanced Photocontrol of Actuation. Adv. Mater. 2018, 30, e1706597.
- Neilson, B.M.; Bielawski, C.W. Illuminating Photoswitchable Catalysis. ACS Catal. 2013, 3, 1874–1885.
- Jiang, Y.; Heinke, L. Photoswitchable Metal-Organic Framework Thin Films: From Spectroscopy to Remote-Controllable Membrane Separation and Switchable Conduction. Langmuir 2021, 37, 2–15.
- Adelizzi, B.; Gielen, V.; Le Saux, T.; Dedecker, P.; Jullien, L. Quantitative Model for Reversibly Photoswitchable Sensors. ACS Sensors 2021, 6, 1157–1165.
- Cheng, H.B.; Cui, Y.; Wang, R.; Kwon, N.; Yoon, J. The Development of Light-Responsive, Organic Dye Based, Supramolecular Nanosystems for Enhanced Anticancer Therapy. Coord. Chem. Rev. 2019, 392, 237–254.
- Shen, X.; Song, J.; Sevencan, C.; Leong, D.T.; Ariga, K. Bio-Interactive Nanoarchitectonics with Two-Dimensional Materials and Environments. Sci. Technol. Adv. Mater. 2022, 23, 199–224.
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional Nanoparticles through π-Conjugated Polymer Self-Assembly. Nat. Rev. Mater. 2021, 6, 7–26.
- Li, G.; Wang, H.; Zhu, Z.; Fan, J.B.; Tian, Y.; Meng, J.; Wang, S. Photo-Irresponsive Molecule-Amplified Cell Release on Photoresponsive Nanostructured Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 29681–29688.
- Sun, F.; Wang, Y.; Wang, Q.; Wang, X.; Yao, P.; Feng, W.; Yuan, Q.; Qi, X.; Chen, S.; Pu, W.; et al. Self-Illuminating Triggered Release of Therapeutics from Photocleavable Nanoprodrug for the Targeted Treatment of Breast Cancer. ACS Appl. Mater. Interfaces 2022, 14, 8766–8781.
- Ikeda, T.; Iijima, T.; Sekiya, R.; Takahashi, O.; Haino, T. Cooperative Self-Assembly of Carbazole Derivatives Driven by Multiple Dipole-Dipole Interactions. J. Org. Chem. 2016, 81, 6832–6837.
- Chen, C.H.; Wang, Y.; Michinobu, T.; Chang, S.W.; Chiu, Y.C.; Ke, C.Y.; Liou, G.S. Donor-Acceptor Effect of Carbazole-Based Conjugated Polymer Electrets on Photoresponsive Flash Organic Field-Effect Transistor Memories. ACS Appl. Mater. Interfaces 2020, 12, 6144–6150.
- Ono, Y.; Hirao, T.; Ikeda, T.; Haino, T. Self-Assembling Behavior and Chiroptical Properties of Carbazole-Cored Phenyl Isoxazolyl Benzenes. J. Org. Chem. 2021, 86, 5499–5505.
- Piwoński, H.; Michinobu, T.; Habuchi, S. Controlling Photophysical Properties of Ultrasmall Conjugated Polymer Nanoparticles through Polymer Chain Packing. Nat. Commun. 2017, 8, 15256.
- Bhattacharyya, A.; De Sarkar, S.; Das, A. Supramolecular Engineering and Self-Assembly Strategies in Photoredox Catalysis. ACS Catal. 2021, 11, 710–733.
- Banerjee, T.; Podjaski, F.; Kröger, J.; Biswal, B.P.; Lotsch, B.V. Polymer Photocatalysts for Solar-to-Chemical Energy Conversion. Nat. Rev. Mater. 2021, 6, 168–190.
- Sánchez-Fernández, J.A.; Cué-Sampedro, R. Biopolymers and Their Composites for Drug Delivery. In Green Biocomposites for Biomedical Engineering: Design, Properties, and Applications; Hoque, E., Sharif, A., Jawaid, M., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; pp. 363–387.
- Walunj, M.B.; Dutta, S.; Srivatsan, S.G. Architectures of Nucleolipid Assemblies and Their Applications. In Molecular Architectonics and Nanoarchitectonics; Govindaraju, T., Ariga, K., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2022; pp. 307–334.
- Begley, M.R.; Gianola, D.S.; Ray, T.R. Bridging Functional Nanocomposites to Robust Macroscale Devices. Science 2019, 364, eaav4299.
- Alesanco, Y.; Viñuales, A.; Cabañero, G.; Rodriguez, J.; Tena-Zaera, R. Colorless to Neutral Color Electrochromic Devices Based on Asymmetric Viologens. ACS Appl. Mater. Interfaces 2016, 8, 29619–29627.
- Mehlana, G.; Bourne, S.A. Unravelling Chromism in Metal-Organic Frameworks. CrystEngComm 2017, 19, 4238–4259.
- Adams, T.J.; Brotherton, A.R.; Molai, J.A.; Parmar, N.; Palmer, J.R.; Sandor, K.A.; Walter, M.G. Obtaining Reversible, High Contrast Electrochromism, Electrofluorochromism, and Photochromism in an Aqueous Hydrogel Device Using Chromogenic Thiazolothiazoles. Adv. Funct. Mater. 2021, 31, 2103408.
- Liu, J.J.; Fu, J.J.; He, C.X.; Liu, T.; Cheng, F.X. A Viologen-Derived Host-Guest MOF Material: Photochromism, Photoswitchable Luminescence, and Inkless and Erasable Printing. J. Solid State Chem. 2022, 306, 122812.
- Castellanos, S.; Kapteijn, F.; Gascon, J. Photoswitchable Metal Organic Frameworks: Turn on the Lights and Close the Windows. CrystEngComm 2016, 18, 4006–4012.
- Dattler, D.; Fuks, G.; Heiser, J.; Moulin, E.; Perrot, A.; Yao, X.; Giuseppone, N. Design of Collective Motions from Synthetic Molecular Switches, Rotors, and Motors. Chem. Rev. 2020, 120, 310–433.
- Koh, K.; Wong-Foy, A.G.; Matzger, A.J. Coordination Copolymerization Mediated by Zn4O(CO 2R)6 Metal Clusters: A Balancing Act between Statistics and Geometry. J. Am. Chem. Soc. 2010, 132, 15005–15010.
- Diercks, C.S.; Yaghi, O.M. The Atom, the Molecule, and the Covalent Organic Framework. Science 2017, 355, eaal1585.
- Lisensky, G.C.; Yaghi, O.M. Visualizing Pore Packing and Topology in MOFs. J. Chem. Educ. 2022, 99, 1998–2004.
- Lee, S.; Jeong, H.; Nam, D.; Lah, M.S.; Choe, W. The Rise of Metal-Organic Polyhedra. Chem. Soc. Rev. 2021, 50, 528–555.
- Gosselin, A.J.; Rowland, C.A.; Bloch, E.D. Permanently Microporous Metal-Organic Polyhedra. Chem. Rev. 2020, 120, 8987–9014.
- Jiang, Y.; Park, J.; Tan, P.; Feng, L.; Liu, X.Q.; Sun, L.B.; Zhou, H.C. Maximizing Photoresponsive Efficiency by Isolating Metal−organic Polyhedra into Confined Nanoscaled Spaces. J. Am. Chem. Soc. 2020, 141, 8221–8227.
- Carné-Sánchez, A.; Craig, G.A.; Larpent, P.; Guillerm, V.; Urayama, K.; Maspoch, D.; Furukawa, S. A Coordinative Solubilizer Method to Fabricate Soft Porous Materials from Insoluble Metal–Organic Polyhedra. Angew. Chem. 2019, 131, 6413–6416.
- Khobotov-Bakishev, A.; Hernández-López, L.; von Baeckmann, C.; Albalad, J.; Carné-Sánchez, A.; Maspoch, D. Metal–Organic Polyhedra as Building Blocks for Porous Extended Networks. Adv. Sci. 2022, 9, 2104753.
- Zhang, D.; Ronson, T.K.; Zou, Y.Q.; Nitschke, J.R. Metal–Organic Cages for Molecular Separations. Nat. Rev. Chem. 2021, 5, 168–182.
- Zhu, C.Y.; Pan, M.; Su, C.Y. Metal-Organic Cages for Biomedical Applications. Isr. J. Chem. 2019, 59, 209–219.
- Wezenberg, S.J. Light-Switchable Metal-Organic Cages. Chem. Lett. 2020, 49, 609–615.
- Tarzia, A.; Jelfs, K.E. Unlocking the Computational Design of Metal–Organic Cages. Chem. Commun. 2022, 58, 3717–3730.
- Sánchez-González, E.; Tsang, M.Y.; Troyano, J.; Craig, G.A.; Furukawa, S. Assembling Metal–Organic Cages as Porous Materials. Chem. Soc. Rev. 2022, 51, 4876–4889.
- Jahović, I.; Zou, Y.Q.; Adorinni, S.; Nitschke, J.R.; Marchesan, S. Cages Meet Gels: Smart Materials with Dual Porosity. Matter 2021, 4, 2123–2140.
- Gong, W.; Chen, Z.; Dong, J.; Liu, Y.; Cui, Y. Chiral Metal-Organic Frameworks. Chem. Rev. 2022, 122, 9078–9144.
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724.
- Ma, L.; Feng, X.; Wang, S.; Wang, B. Recent Advances in AIEgen-Based Luminescent Metal-Organic Frameworks and Covalent Organic Frameworks. Mater. Chem. Front. 2017, 1, 2474–2486.
- Li, J.; Wang, J.; Li, H.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular Materials Based on AIE Luminogens (AIEgens): Construction and Applications. Chem. Soc. Rev. 2020, 49, 1144–1172.
- Xu, W.; Lee, M.M.S.; Zhang, Z.; Sung, H.H.Y.; Williams, I.D.; Kwok, R.T.K.; Lam, J.W.Y.; Wang, D.; Tang, B.Z. Facile Synthesis of AIEgens with Wide Color Tunability for Cellular Imaging and Therapy. Chem. Sci. 2019, 10, 3494–3501.
- Xiong, W.; Wang, L.; Chen, X.; Tang, H.; Cao, D.; Zhang, G.; Chen, W. Pyridinium-Substituted Tetraphenylethylene Salt-Based Photosensitizers by Varying Counter Anions: A Highly Efficient Photodynamic Therapy for Cancer Cell Ablation and Bacterial Inactivation. J. Mater. Chem. B 2020, 8, 5234–5244.
- Pandey, N.K.; Xiong, W.; Wang, L.; Chen, W.; Bui, B.; Yang, J.; Amador, E.; Chen, M.; Xing, C.; Athavale, A.A.; et al. Aggregation-Induced Emission Luminogens for Highly Effective Microwave Dynamic Therapy. Bioact. Mater. 2022, 7, 112–125.
- Li, Y.; Li, Z.; Wang, Y.; Compaan, A.; Ren, T.; Dong, W.J. Increasing the Power Output of a CdTe Solar Cell via Luminescent down Shifting Molecules with Intramolecular Charge Transfer and Aggregation-Induced Emission Characteristics. Energy Environ. Sci. 2013, 6, 2907–2911.
- Wang, L.; Chen, X.; Xiong, W.; Ran, X.; Tang, H.; Cao, D. Design and Synthesis of an AIEgen with Multiple Functions: Solvatochromism, Chromism, Lipid Droplet Imaging. Dye. Pigments 2020, 181, 108537.
- Cui, Y.; Liu, Y.; Liu, J.; Du, J.; Yu, Y.; Wang, S.; Liang, Z.; Yu, J. Multifunctional Porous Tröger’s Base Polymers with Tetraphenylethene Units: CO2 Adsorption, Luminescence and Sensing Properties. Polym. Chem. 2017, 8, 4842–4848.
- Peng, Y.; Li, L.; Zhu, C.; Chen, B.; Zhao, M.; Zhang, Z.; Lai, Z.; Zhang, X.; Tan, C.; Han, Y.; et al. Intramolecular Hydrogen Bonding-Based Topology Regulation of Two-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 13162–13169.
- Sun, N.; Jin, Y.; Wang, H.; Yu, B.; Wang, R.; Wu, H.; Zhou, W.; Jiang, J. Photoresponsive Covalent Organic Frameworks with Diarylethene Switch for Tunable Singlet Oxygen Generation. Chem. Mater. 2022, 34, 1956–1964.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Chemical Bond
Revisions:
2 times
(View History)
Update Date:
01 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No


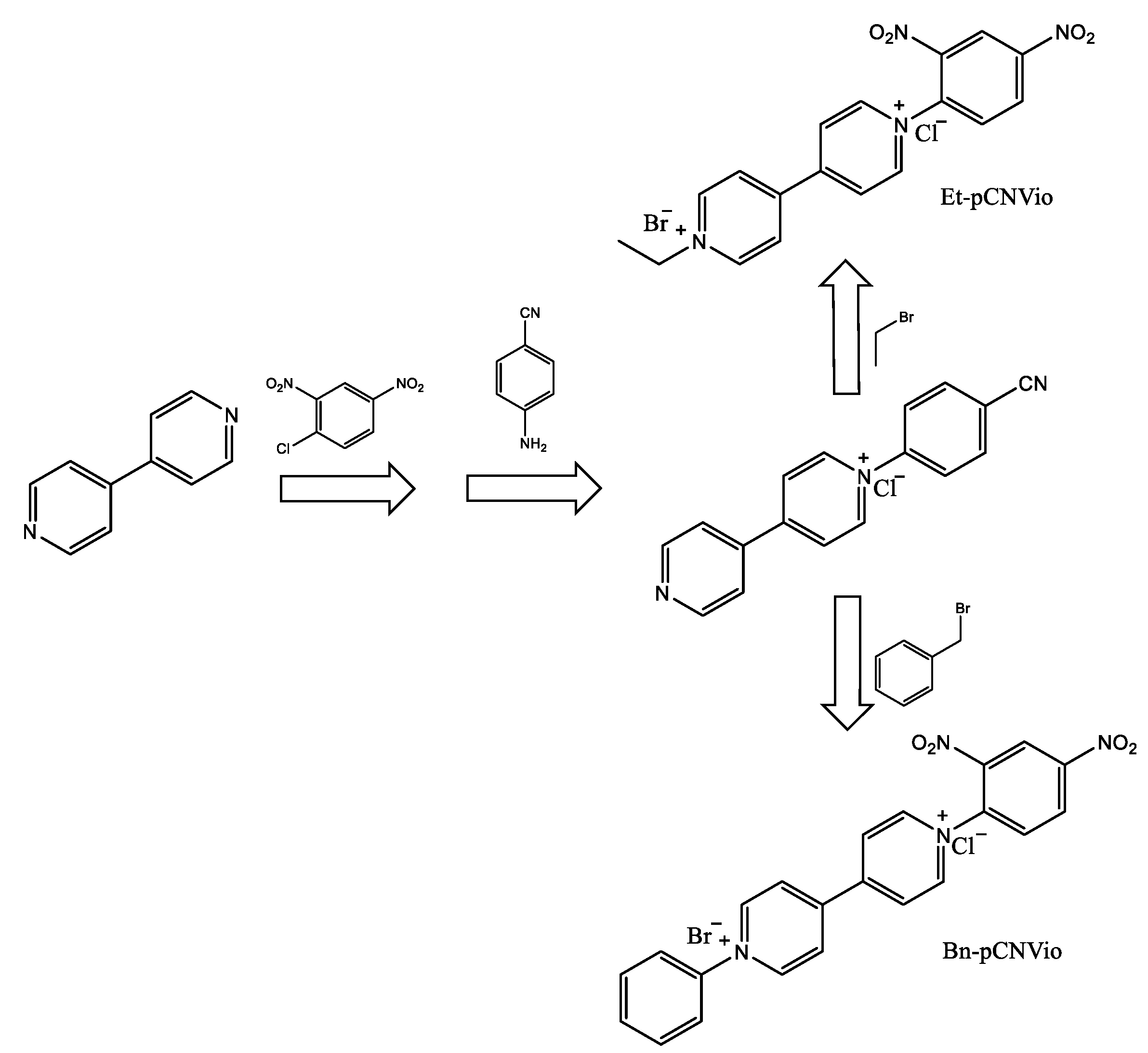 Scheme 1. Synthesis route to obtain the asymmetric viologens Et
Scheme 1. Synthesis route to obtain the asymmetric viologens Et

