
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aneta Otocka-Kmiecik | -- | 3296 | 2022-07-28 13:24:03 | | | |
| 2 | Catherine Yang | Meta information modification | 3296 | 2022-07-29 03:20:11 | | |
Video Upload Options
Paraoxonase 1 (PON1) is an antioxidant enzyme attached to HDL with an anti-atherogenic potential. It protects LDL and HDL from lipid peroxidation. The enzyme is sensitive to various modulating factors, such as genetic polymorphisms as well as pharmacological, dietary (including carotenoids), and lifestyle interventions. Carotenoids are nutritional pigments with antioxidant activity. Carotenoids administered as naturally occurring nutritional mixtures may present a synergistic beneficial effect on PON1 status. The effect of carotenoids on the enzyme depends on age, ethnicity, gender, diet, and PON1 genetic variation. Carotenoids, especially astaxanthin, β-carotene, and lycopene, increase PON1 activity. This effect may be explained by their ability to quench singlet oxygen and scavenge free radicals. β-carotene and lycopene were additionally shown to upregulate PON1 gene expression.
1. The Influence of Astaxanthin on PON1 Activity
1.1. The Influence of Astaxanthin on PON1 Activity in Animal Studies
1.2. The Influence of Astaxanthin on PON1 Activity in Clinical Studies
2. The Influence of β-Carotene on PON1 Activity and Gene Expression
The Influence of β-Carotene on PON1 Activity and Gene Expression in In Vitro Studies
3. The Influence of Lycopene on PON1 Activity and Gene Expression
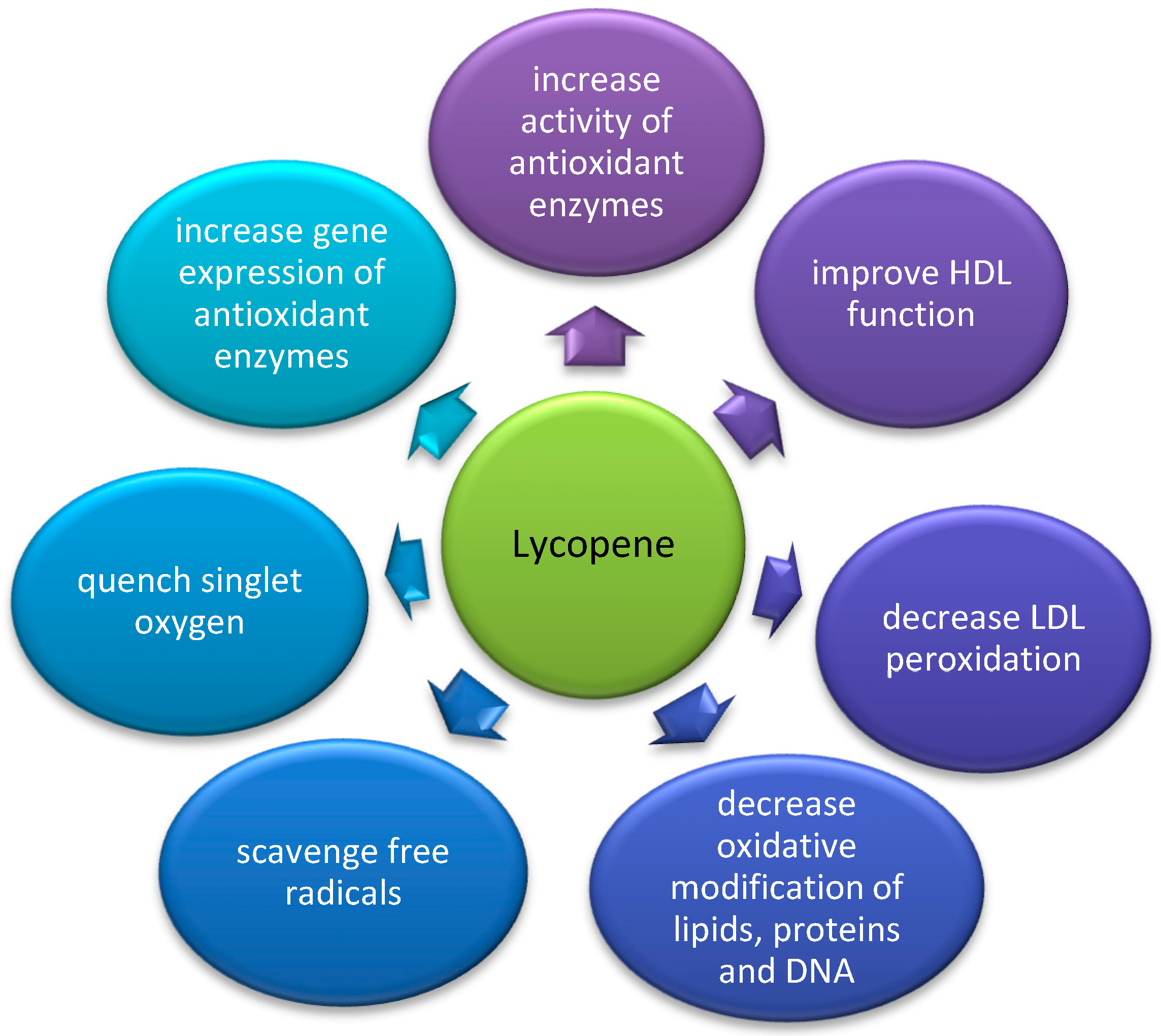
3.1. The Influence of Lycopene on PON1 Activity and Gene Expression in Animal Studies
3.2. The Influence of Lycopene on PON1 Activity and Gene Expression in Clinical Studies
4. The Effect of a Mixture of Carotenoids on PON1 Activity and LDL Oxidation
4.1. The Influence of a Mixture of Carotenoids on PON1 Activity in Animal Studies
4.2. The Influence of a Mixture of Carotenoids on PON1 Activity in Clinical Studies
4.3. Conclusion on the Effect of a Mixture of Carotenoids on PON1 Activity
References
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. In Bioactive Molecules in Food. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–35. ISBN 978-3-319-54528-8.
- Rajasingh, H.; Øyehaug, L.; Våge, D.I.; Omholt, S.W. Carotenoid Dynamics in Atlantic Salmon. BMC Biol. 2006, 4, 10.
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of Oxidative Injury of Biological Membranes by Astaxanthin. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38.
- McNulty, H.; Jacob, R.F.; Mason, R.P. Biologic Activity of Carotenoids Related to Distinct Membrane Physicochemical Interactions. Am. J. Cardiol. 2008, 101, S20–S29.
- Augusti, P.R.; Quatrin, A.; Somacal, S.; Conterato, G.M.M.; Sobieski, R.; Ruviaro, A.R.; Maurer, L.H.; Duarte, M.M.F.; Roehrs, M.; Emanuelli, T. Astaxanthin Prevents Changes in the Activities of Thioredoxin Reductase and Paraoxonase in Hypercholesterolemic Rabbits. J. Clin. Biochem. Nutr. 2012, 51, 42–49.
- Kükürt, A.; Karapehlivan, M. Protective Effect of Astaxanthin on Experimental Ovarian Damage in Rats. J. Biochem. Mol. Toxicol. 2022, 36, e22966.
- Baralic, I.; Djordjevic, B.; Dikic, N.; Kotur-Stevuljevic, J.; Spasic, S.; Jelic-Ivanovic, Z.; Radivojevic, N.; Andjelkovic, M.; Pejic, S. Effect of Astaxanthin Supplementation on Paraoxonase 1 Activities and Oxidative Stress Status in Young Soccer Players. Phyther. Res. 2013, 27, 1536–1542.
- Jaouad, L.; De Guise, C.; Berrougui, H.; Cloutier, M.; Isabelle, M.; Fulop, T.; Payette, H.; Khalil, A. Age-Related Decrease in High-Density Lipoproteins Antioxidant Activity Is Due to an Alteration in the PON1’s Free Sulfhydryl Groups. Atherosclerosis 2006, 185, 191–200.
- Sorenson, R.C.; Primo-Parmo, S.L.; Kuo, C.L.; Adkins, S.; Lockridge, O.; La Du, B.N. Reconsideration of the Catalytic Center and Mechanism of Mammalian Paraoxonase/Arylesterase. Proc. Natl. Acad. Sci. USA 1995, 92, 7187–7191.
- Yamagata, K.; Tanaka, N.; Matsufuji, H.; Chino, M. β-Carotene Reverses the IL-1β-Mediated Reduction in Paraoxonase-1 Expression via Induction of the CaMKKII Pathway in Human Endothelial Cells. Microvasc. Res. 2012, 84, 297–305.
- Loppnow, H.; Buerke, M.; Werdan, K.; Rose-John, S. Contribution of Vascular Cell-Derived Cytokines to Innate and Inflammatory Pathways in Atherogenesis. J. Cell. Mol. Med. 2011, 15, 484–500.
- Kumon, Y.; Suehiro, T.; Ikeda, Y.; Hashimoto, K. Human Paraoxonase-1 Gene Expression by HepG2 Cells Is Downregulated by Interleukin-1beta and Tumor Necrosis Factor-Alpha, but Is Upregulated by Interleukin-6. Life Sci. 2003, 73, 2807–2815.
- Tedgui, A.; Mallat, Z. Anti-Inflammatory Mechanisms in the Vascular Wall. Circ. Res. 2001, 88, 877–887.
- Libby, P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: The Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 2017, 70, 2278–2289.
- Martin, K.R.; Wu, D.; Meydani, M. The Effect of Carotenoids on the Expression of Cell Surface Adhesion Molecules and Binding of Monocytes to Human Aortic Endothelial Cells. Atherosclerosis 2000, 150, 265–274.
- Heber, D.; Lu, Q.Y. Overview of Mechanisms of Action of Lycopene. Exp. Biol. Med. 2002, 227, 920–923.
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the Most Efficient Biological Carotenoid Singlet Oxygen Quencher. Arch. Biochem. Biophys. 1989, 274, 532–538.
- Srinivasan, M.; Sudheer, A.R.; Pillai, K.R.; Kumar, P.R.; Sudhakaran, P.R.; Menon, V.P. Lycopene as a Natural Protector against Gamma-Radiation Induced DNA Damage, Lipid Peroxidation and Antioxidant Status in Primary Culture of Isolated Rat Hepatocytes in Vitro. Biochim. Biophys. Acta 2007, 1770, 659–665.
- Rao, A.V.; Agarwal, S. Role of Antioxidant Lycopene in Cancer and Heart Disease. J. Am. Coll. Nutr. 2000, 19, 563–569.
- Agarwal, S.; Rao, A.V. Tomato Lycopene and Low Density Lipoprotein Oxidation: A Human Dietary Intervention Study. Lipids 1998, 33, 981–984.
- Silaste, M.L.; Alfthan, G.; Aro, A.; Kesäniemi, Y.A.; Hörkkö, S. Tomato Juice Decreases LDL Cholesterol Levels and Increases LDL Resistance to Oxidation. Br. J. Nutr. 2007, 98, 1251–1258.
- Sultan Alvi, S.; Ansari, I.A.; Khan, I.; Iqbal, J.; Khan, M.S. Potential Role of Lycopene in Targeting Proprotein Convertase Subtilisin/Kexin Type-9 to Combat Hypercholesterolemia. Free Radic. Biol. Med. 2017, 108, 394–403.
- Deakin, S.; Leviev, I.; Guernier, S.; James, R.W. Simvastatin Modulates Expression of the PON1 Gene and Increases Serum Paraoxonase: A Role for Sterol Regulatory Element-Binding Protein-2. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2083–2089.
- Yegin, S.Ç.; Yur, F.; Ceylan, E. Effect of Lycopene Application in Rats with Experimental Diabetes Using Lipoprotein, Paraoxonase and Cytokines. J. Membr. Biol. 2013, 246, 621–626.
- Figueiredo, I.D.; Lima, T.F.O.; Inácio, M.D.; Costa, M.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Lycopene Improves the Metformin Effects on Glycemic Control and Decreases Biomarkers of Glycoxidative Stress in Diabetic Rats. Diabetes. Metab. Syndr. Obes. 2020, 13, 3117–3135.
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene Intervention Reduces Inflammation and Improves HDL Functionality in Moderately Overweight Middle-Aged Individuals. J. Nutr. Biochem. 2013, 24, 163–168.
- de la Iglesia, R.; Mansego, M.L.; Sánchez-Muniz, F.J.; Angeles Zulet, M.; Alfredo Martinez, J. Arylesterase Activity Is Associated with Antioxidant Intake and Paraoxonase-1 (PON1) Gene Methylation in Metabolic Syndrome Patients Following an Energy Restricted Diet. EXCLI J. 2014, 13, 416–426.
- Tsakiris, S.; Karikas, G.A.; Parthimos, T.; Tsakiris, T.; Bakogiannis, C.; Schulpis, K.H. Alpha-Tocopherol Supplementation Prevents the Exercise-Induced Reduction of Serum Paraoxonase 1/Arylesterase Activities in Healthy Individuals. Eur. J. Clin. Nutr. 2009, 63, 215–221.
- Malireddy, S.; Kotha, S.R.; Secor, J.D.; Gurney, T.O.; Abbott, J.L.; Maulik, G.; Maddipati, K.R.; Parinandi, N.L. Phytochemical Antioxidants Modulate Mammalian Cellular Epigenome: Implications in Health and Disease. Antioxid. Redox Signal. 2012, 17, 327–339.
- Vanderkraats, N.D.; Hiken, J.F.; Decker, K.F.; Edwards, J.R. Discovering High-Resolution Patterns of Differential DNA Methylation That Correlate with Gene Expression Changes. Nucleic Acids Res. 2013, 41, 6816–6827.
- Mackness, B.; Durrington, P.; McElduff, P.; Yarnell, J.; Azam, N.; Watt, M.; Mackness, M. Low Paraoxonase Activity Predicts Coronary Events in the Caerphilly Prospective Study. Circulation 2003, 107, 2775–2779.
- Poh, R.; Muniandy, S. Paraoxonase 1 Activity as a Predictor of Cardiovascular Disease in Type 2 Diabetes. Southestern Asian J. Trop. Med. Public Health 2010, 41, 1231–1246.
- Dugas, T.R.; Morel, D.W.; Harrison, E.H. Dietary Supplementation with Beta-Carotene, but Not with Lycopene, Inhibits Endothelial Cell-Mediated Oxidation of Low-Density Lipoprotein. Free Radic. Biol. Med. 1999, 26, 1238–1244.
- Fuhrman, B.; Volkova, N.; Rosenblat, M.; Aviram, M. Lycopene Synergistically Inhibits LDL Oxidation in Combination with Vitamin E, Glabridin, Rosmarinic Acid, Carnosic Acid, or Garlic. Antioxid. Redox Signal. 2000, 2, 491–506.
- Linseisen, J.; Hoffmann, J.; Riedl, J.; Wolfram, G. Effect of a Single Oral Dose of Antioxidant Mixture (Vitamin E, Carotenoids) on the Formation of Cholesterol Oxidation Products after Ex Vivo LDL Oxidation in Humans. Eur. J. Med. Res. 1998, 3, 5–12.
- Shokri, Y.; Variji, A.; Nosrati, M.; Khonakdar-Tarsi, A.; Kianmehr, A.; Kashi, Z.; Bahar, A.; Bagheri, A.; Mahrooz, A. Importance of Paraoxonase 1 (PON1) as an Antioxidant and Antiatherogenic Enzyme in the Cardiovascular Complications of Type 2 Diabetes: Genotypic and Phenotypic Evaluation. Diabetes Res. Clin. Pract. 2020, 161, 108067.
- Assis, R.; Arcaro, C.; Gutierres, V.; Oliveira, J.; Costa, P.; Baviera, A.; Brunetti, I. Combined Effects of Curcumin and Lycopene or Bixin in Yoghurt on Inhibition of LDL Oxidation and Increases in HDL and Paraoxonase Levels in Streptozotocin-Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 332.
- Somacal, S.; Figueiredo, C.G.; Quatrin, A.; Ruviaro, A.R.; Conte, L.; Augusti, P.R.; Roehrs, M.; Denardin, I.T.; Kasten, J.; da Veiga, M.L.; et al. The Antiatherogenic Effect of Bixin in Hypercholesterolemic Rabbits Is Associated to the Improvement of Lipid Profile and to Its Antioxidant and Anti-Inflammatory Effects. Mol. Cell. Biochem. 2015, 403, 243–253.
- Daniels, J.A.; Mulligan, C.; McCance, D.; Woodside, J.V.; Patterson, C.; Young, I.S.; McEneny, J. A Randomised Controlled Trial of Increasing Fruit and Vegetable Intake and How This Influences the Carotenoid Concentration and Activities of PON-1 and LCAT in HDL from Subjects with Type 2 Diabetes. Cardiovasc. Diabetol. 2014, 13, 16.
- Freese, R.; Alfthan, G.; Jauhiainen, M.; Basu, S.; Erlund, I.; Salminen, I.; Aro, A.; Mutanen, M. High Intakes of Vegetables, Berries, and Apples Combined with a High Intake of Linoleic or Oleic Acid Only Slightly Affect Markers of Lipid Peroxidation and Lipoprotein Metabolism in Healthy Subjects. Am. J. Clin. Nutr. 2002, 76, 950–960.
- Blum, S.; Aviram, M.; Ben-Amotz, A.; Levy, Y. Effect of a Mediterranean Meal on Postprandial Carotenoids, Paraoxonase Activity and C-Reactive Protein Levels. Ann. Nutr. Metab. 2006, 50, 20–24.
- DiMarco, D.M.; Norris, G.H.; Millar, C.L.; Blesso, C.N.; Fernandez, M.L. Intake of up to 3 Eggs per Day Is Associated with Changes in HDL Function and Increased Plasma Antioxidants in Healthy, Young Adults. J. Nutr. 2017, 147, 323–329.
- Chee, T.L.; Rowley, K.; Jenkins, A.J.; O’Dea, K.; Itsiopoulos, C.; Stoney, R.M.; Su, Q.; Giles, G.G.; Best, J.D. Paraoxonase Activity in Greek Migrants and Anglo-Celtic Persons in the Melbourne Collaborative Cohort Study: Relationship to Dietary Markers. Eur. J. Nutr. 2005, 44, 223–230.
- Fernández-Castillejo, S.; García-Heredia, A.I.; Solà, R.; Camps, J.; López de la Hazas, M.C.; Farràs, M.; Pedret, A.; Catalán, Ú.; Rubió, L.; Motilva, M.J.; et al. Phenol-Enriched Olive Oils Modify Paraoxonase-Related Variables: A Randomized, Crossover, Controlled Trial. Mol. Nutr. Food Res. 2017, 61, 1600932.
- Tomás, M.; Sentí, M.; Elosua, R.; Vila, J.; Sala, J.; Masià, R.; Marrugat, J. Interaction between the Gln-Arg 192 Variants of the Paraoxonase Gene and Oleic Acid Intake as a Determinant of High-Density Lipoprotein Cholesterol and Paraoxonase Activity. Eur. J. Pharmacol. 2001, 432, 121–128.
- Bub, A.; Barth, S.; Watzl, B.; Briviba, K.; Herbert, B.M.; Lührmann, P.M.; Neuhäuser-Berthold, M.; Rechkemmer, G. Paraoxonase 1 Q192R (PON1-192) Polymorphism Is Associated with Reduced Lipid Peroxidation in R-Allele-Carrier but Not in QQ Homozygous Elderly Subjects on a Tomato-Rich Diet. Eur. J. Nutr. 2002, 41, 237–243.
- Mackness, M.I.; Mackness, B.; Durrington, P.N.; Fogelman, A.M.; Berliner, J.; Lusis, A.J.; Navab, M.; Shih, D.; Fonarow, G.C. Paraoxonase and Coronary Heart Disease. Curr. Opin. Lipidol. 1998, 9, 319–324.
- Mendonça, M.I.; Dos Reis, R.P.; Freitas, A.I.; Sousa, A.C.; Pereira, A.; Faria, P.; Gomes, S.; Silva, B.; Santos, N.; Serrão, M.; et al. Human Paraoxonase Gene Polymorphisms and Coronary Artery Disease Risk. Rev. Port. Cardiol. 2008, 27, 1539–1555.
- Vaisi-Raygani, A.; Ghaneialvar, H.; Rahimi, Z.; Tavilani, H.; Pourmotabbed, T.; Shakiba, E.; Vaisi-Raygani, A.; Kiani, A.; Aminian, M.; Alibakhshi, R.; et al. Paraoxonase Arg 192 Allele Is an Independent Risk Factor for Three-Vessel Stenosis of Coronary Artery Disease. Mol. Biol. Rep. 2011, 38, 5421–5428.
- Gamboa, R.; Zamora, J.; Rodríguez-Pérez, J.M.; Fragoso, J.M.; Cardoso, G.; Posadas-Romero, C.; Vargas-Alarcón, G. Distribution of Paraoxonase PON1 Gene Polymorphisms in Mexican Populations. Its Role in the Lipid Profile. Exp. Mol. Pathol. 2006, 80, 85–90.
- Imai, Y.; Morita, H.; Kurihara, H.; Sugiyama, T.; Kato, N.; Ebihara, A.; Hamada, C.; Kurihara, Y.; Shindo, T.; Oh-Hashi, Y.; et al. Evidence for Association between Paraoxonase Gene Polymorphisms and Atherosclerotic Diseases. Atherosclerosis 2000, 149, 435–442.
- Wang, X.; Fan, Z.; Huang, J.; Su, S.; Yu, Q.; Zhao, J.; Hui, R.; Yao, Z.; Shen, Y.; Qiang, B.; et al. Extensive Association Analysis between Polymorphisms of PON Gene Cluster with Coronary Heart Disease in Chinese Han Population. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 328–334.
- Gardemann, A.; Philipp, M.; Heß, K.; Katz, N.; Tillmanns, H.; Haberbosch, W. The Paraoxonase Leu-Met54 and Gln-Arg191 Gene Polymorphisms Are Not Associated with the Risk of Coronary Heart Disease. Atherosclerosis 2000, 152, 421–431.
- Mackness, B.; Davies, G.K.; Turkie, W.; Lee, E.; Roberts, D.H.; Hill, E.; Roberts, C.; Durrington, P.N.; Mackness, M.I. Paraoxonase Status in Coronary Heart Disease: Are Activity and Concentration More Important than Genotype? Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1451–1457.
- Cohen, E.; Aviram, M.; Khatib, S.; Artoul, F.; Rabin, A.; Mannheim, D.; Karmeli, R.; Salamon, T.; Vaya, J. Human Carotid Plaque Phosphatidylcholine Specifically Interacts with Paraoxonase 1, Increases Its Activity, and Enhances Its Uptake by Macrophage at the Expense of Its Binding to HDL. Free Radic. Biol. Med. 2014, 76, 14–24.
- Helbecque, N.; Cottel, D.; Meirhaeghe, A.; Dallongeville, P.; Arveiler, D.; Wagner, A.; Ruidavets, J.B.; Ferrieres, J. Paraoxonase (Gln192-Arg) Polymorphism in French Type 2 Diabetics. Atherosclerosis 1999, 147, 415–416.
- MacKinnon, E.S.; El-Sohemy, A.; Rao, A.V.; Rao, L.G. Paraoxonase 1 Polymorphisms 172T→A and 584A→G Modify the Association between Serum Concentrations of the Antioxidant Lycopene and Bone Turnover Markers and Oxidative Stress Parameters in Women 25-70 Years of Age. J. Nutrigenet. Nutr. 2010, 3, 1–8.
- Cohen, J.; Jenkins, A.J.; Karschimkus, C.; Qing, S.; Lee, C.T.; O’Dea, K.; Best, J.D.; Rowley, K.G. Paraoxonase and Other Coronary Risk Factors in a Community-Based Cohort. Redox Rep. 2002, 7, 304–307.
- Ferrè, N.; Camps, J.; Fernández-Ballart, J.; Arija, V.; Murphy, M.M.; Ceruelo, S.; Biarnés, E.; Vilella, E.; Tous, M.; Joven, J. Regulation of Serum Paraoxonase Activity by Genetic, Nutritional, and Lifestyle Factors in the General Population. Clin. Chem. 2003, 49, 1491–1497.
- Kleemola, P.; Freese, R.; Jauhiainen, M.; Pahlman, R.; Alfthan, G.; Mutanen, M. Dietary Determinants of Serum Paraoxonase Activity in Healthy Humans. Atherosclerosis 2002, 160, 425–432.




