Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jorma Olavi Ryhänen | -- | 1909 | 2022-07-27 08:37:25 | | | |
| 2 | Rita Xu | Meta information modification | 1909 | 2022-07-27 08:49:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Löppönen, P.; Hulkkonen, S.; Ryhänen, J. Differential Diagnosis of Carpal Tunnel Syndrome. Encyclopedia. Available online: https://encyclopedia.pub/entry/25566 (accessed on 08 February 2026).
Löppönen P, Hulkkonen S, Ryhänen J. Differential Diagnosis of Carpal Tunnel Syndrome. Encyclopedia. Available at: https://encyclopedia.pub/entry/25566. Accessed February 08, 2026.
Löppönen, Pekka, Sina Hulkkonen, Jorma Ryhänen. "Differential Diagnosis of Carpal Tunnel Syndrome" Encyclopedia, https://encyclopedia.pub/entry/25566 (accessed February 08, 2026).
Löppönen, P., Hulkkonen, S., & Ryhänen, J. (2022, July 27). Differential Diagnosis of Carpal Tunnel Syndrome. In Encyclopedia. https://encyclopedia.pub/entry/25566
Löppönen, Pekka, et al. "Differential Diagnosis of Carpal Tunnel Syndrome." Encyclopedia. Web. 27 July, 2022.
Copy Citation
Carpal tunnel syndrome (CTS) is the most common median nerve compression neuropathy. Its symptoms and clinical presentation are well known. However, symptoms at median nerve distribution can also be caused by a proximal problem. Pronator syndrome (PS) and anterior interosseous nerve syndrome (AINS) with their typical characteristics have been thought to explain proximal median nerve problems.
carpal tunnel syndrome
median neuropathy
pronator syndrome
Lacertus syndrome
anterior interosseus nerve syndrome
AIN syndrome
Martin-Gruber anastomosis
double crush syndrome
nerve compression
nerve entrapment
1. Introduction
Carpal tunnel syndrome (CTS) is the most common median nerve compression neuropathy. As its clinical symptoms and presentation are well known, the correct diagnosis and treatment are evident. However, symptoms at median nerve distribution can also be caused by nerve compression proximal to the carpal tunnel. This should be remembered when symptoms are atypical to CTS or persist after carpal tunnel release (CTR).
Proximal median nerve compression (PMNC) is more uncommon than CTS and probably underdiagnosed. Diagnosis can be difficult due to overlapping symptoms with CTS; moreover, both can coexist in the same patient [1][2][3][4][5][6]. In addition, multiple anatomical features can cause entrapment of the median nerve, presenting various symptoms. Therefore, successful treatment of PMNC requires a thorough understanding of the median nerve’s anatomical aspects, comprehension of its pathology and recovery, and experience interpreting the varying clinical presentations.
Only limited good-quality clinical research exists on PMNC. Most studies are small hospital-based series of surgically treated patients. No comparative trials have been published on treatment of PMNC. The largest problem with PMNC is the difficulty in differentiating whether or not it is a compressive neuropathy.
2. Anatomy and Sites of Median Nerve Compression
The literature is filled with different terminology representing a simplified understanding of the condition. The same diagnoses with different criteria have been accepted and used by many clinicians and authors. However, there are reasons why this traditional division should be reconsidered.
Median nerve fibers can be traced back to cervical roots of C5-C8 and thoracic root Th1. C5-C7 form the lateral cord, and C8 and Th1 form a smaller medial cord. Lateral and medial cords form the median nerve. The nerve then travels across the axilla and the medial side of the upper arm with the brachial artery between the biceps brachii and brachialis muscles. It further continues to travel to the forearm giving several motor branches to muscles and then distal to the hand, giving critical sensation to the thumb, index, middle finger, and medial half of the ring finger.
2.1. Supracondylar Process, Ligament of Struthers, and Supracondylar Process Syndrome
Figure 1 shows the anatomy of the elbow. A supracondylar process or a bony spur about 3–6 cm proximal to the medial epicondyle is present in 1–2% of individuals. The ligament of Struthers is a fibrous arch between this process and the medial epicondyle [7], and the median nerve and brachial artery travel underneath it if it exists [8][9][10]. The median nerve can become compressed under this bony process or the ligament [9][11][12]. This neuropathy is known as supracondylar process syndrome [8][9][12][13]. The ligament of Struthers has been described to occur and cause compression of the median nerve even without the presence of a clinical supracondylar process [13][14][15].
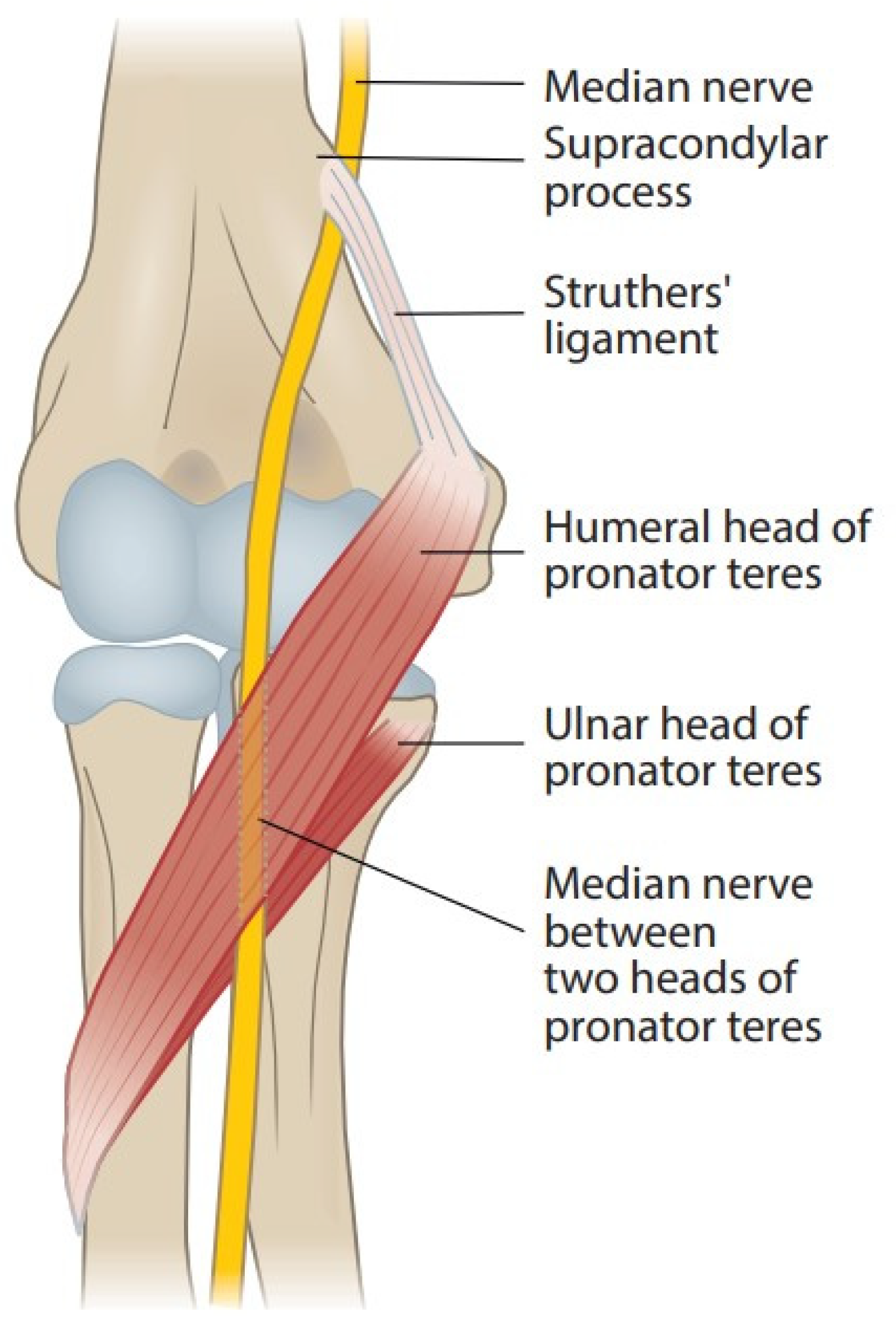
Figure 1. Course of the median nerve at the elbow.
2.2. Lacertus Fibrosus and Lacertus Syndrome
Bicipital aponeurosis or lacertus fibrosus originates from the biceps brachii muscle and joins the fascia of the pronator-flexor mass (Figure 2). The median nerve and brachial artery pass underneath and are prone to compression in forearm pronosupination [2][6][11][14][15][16][17][18][19][20]. Persisting median artery has also been described to cause median nerve compression [6].
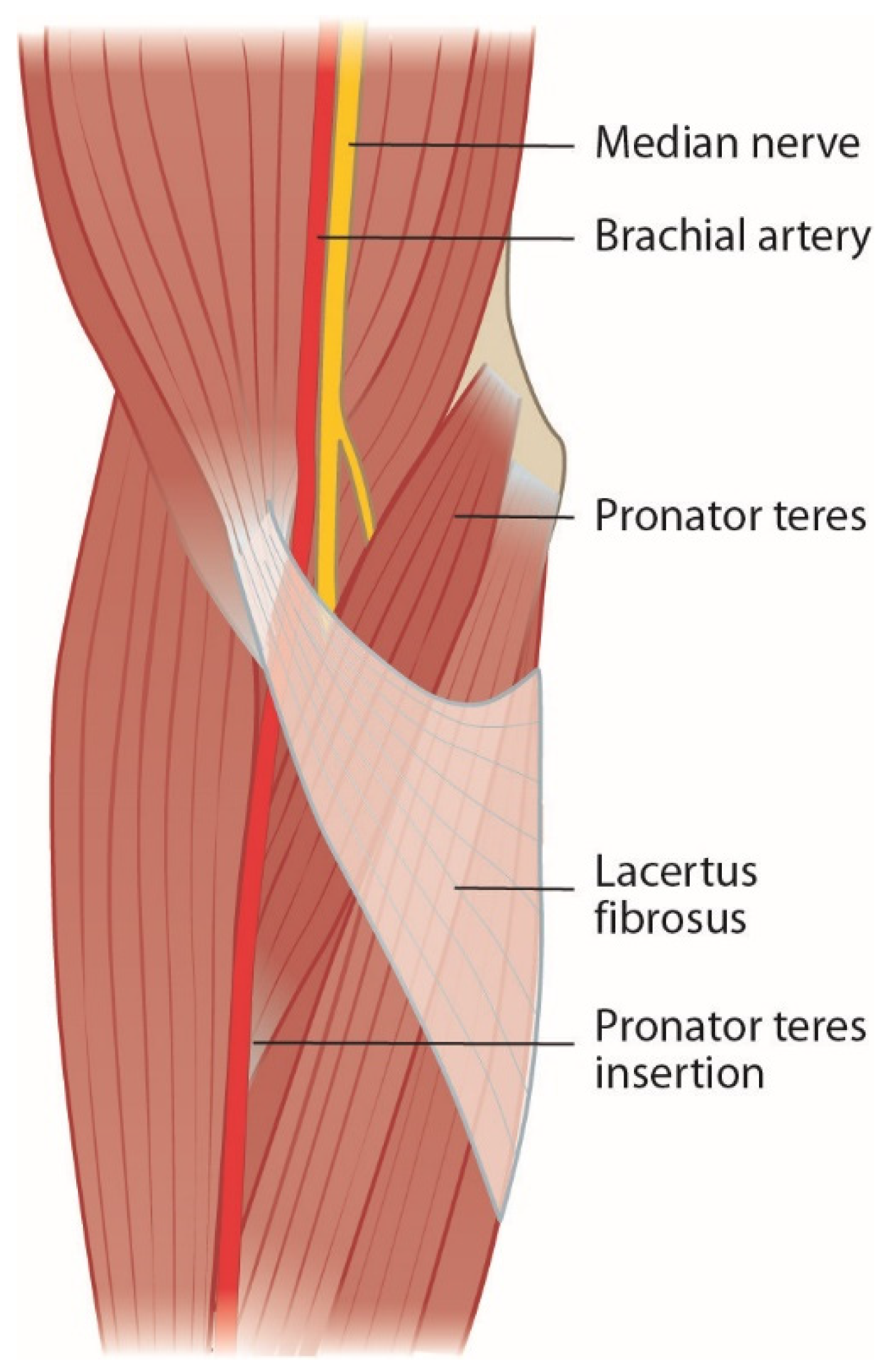
Figure 2. Lacertus fibrosus covers the pronator-flexor mass and the median nerve.
Lacertus syndrome (LS) and its symptoms in baseball pitchers were first described by Bennett in 1959 [21]. It has more recently been popularized by Hagert and Lalonde [22]. Symptoms of LS are described as a loss of key and pinch strength, loss of fine motor skills, and sense of clumsiness. Hagert states that patients with LS have (1) weakness in median nerve innervated muscles distal to lacertus fibrosus, (2) pain when compressing the nerve at the level of lacertus fibrosus, and (3) a positive scratch collapse test. These patients rarely have paresthesia in the median nerve innervated hand [23]. More simple anatomical compression tends to lead to more accessible operative treatment.
2.3. Pronator Teres Muscle and Pronator Syndrome
Branches of the median nerve pass the pronator teres (PT), flexor digitorum superficialis (FDS), flexor carpi radialis (FCR), and palmaris longus (PL) before passing through the PT muscle (Figure 1) [24]. In most people, the median nerve passes between the humeral (superficial) and ulnar (deep) head of PT, the prevailing location of the PMNC; in some people the latter is missing, and the nerve passes only under the humeral head of the muscle. Rare variations of the nerve passing behind the ulnar head or through the humeral head of PT have been described [25]. Thickened tendinous bands, fibrous arches, and intramuscular bands can arise from the muscles [2][6][11][15][16][17][18][20][24][25][26][27]. Hypertrophy of the PT muscle might impact the compression [17][25].
Median nerve compression by the PT muscle was first reported by Seyffarth in 1951 [28]. He suggested using the term pronator syndrome (PS), which remains the most common term to describe PMNCs. By PS, most authors mean various combinations of symptoms that usually include proximal volar forearm pain at the region of the PT muscle, with varied median neuropathy such as weakness and sensory changes. Symptoms are typically provoked by strenuous repetitive activity such as forearm pronosupination.
2.4. Flexor Digitorum Superficialis Arch and Superficialis/Pronator Syndrome
After PT, the nerve courses between the muscles flexor digitorum profundus (FDP) and FDS. In up to 75% of forearms, the FDS muscle has a fibrous leading edge that can be tight while covering the median nerve and anterior interosseous nerve (AIN) (Figure 3) [2][11][15][16][17][18][20][24][29]. Even elbow extension alone can cause median nerve and AIN compression at the FDS arch in certain forearms [29]. To further distinguish and specify the compressive point over the median nerve and the very close relation between FDS and PT, the term superficialis/pronator syndrome was recently proposed by Tang [30].
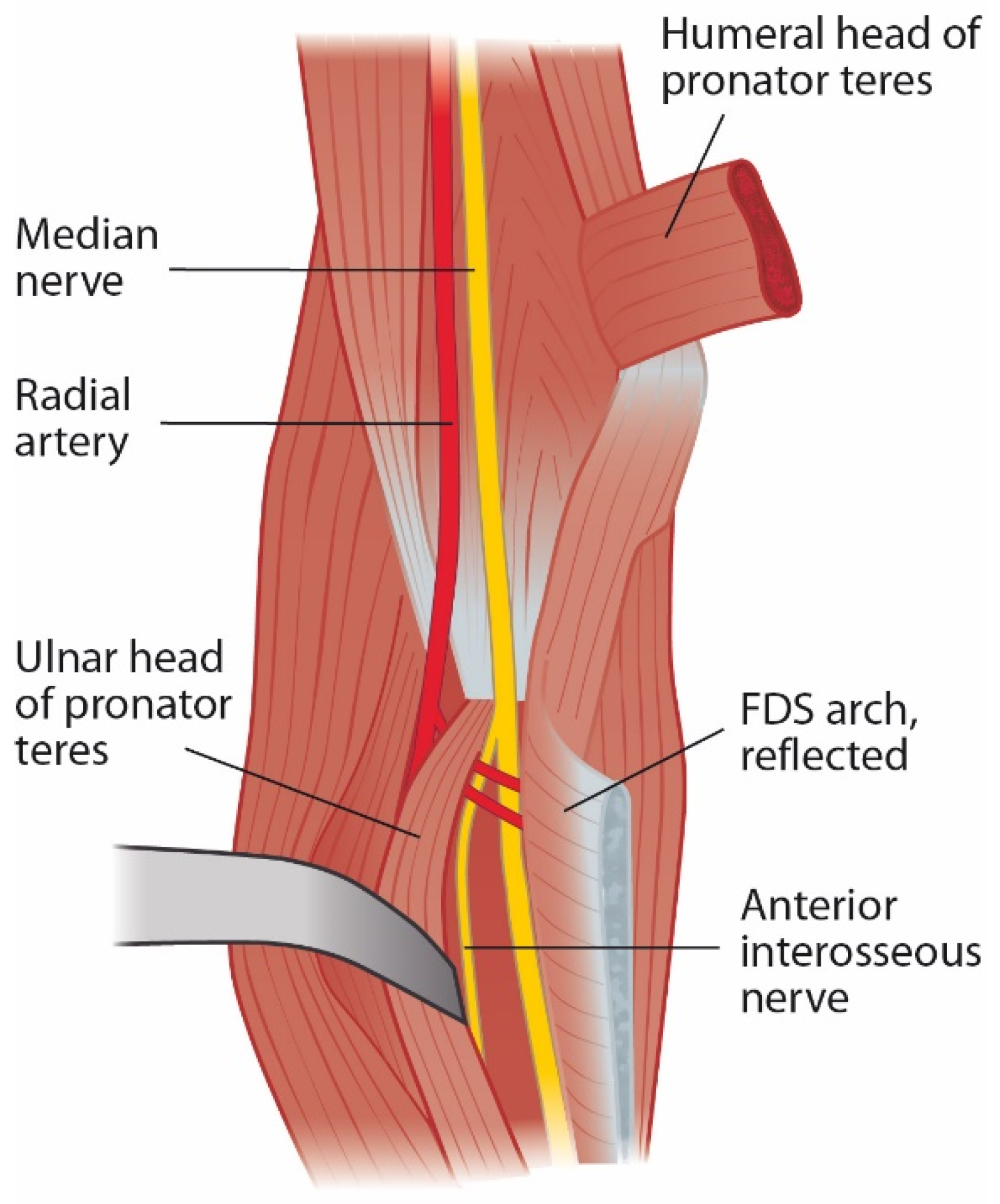
Figure 3. Leading tendinous edge of the flexor digitorum superficialis arch can cause compression of the median nerve and anterior interosseous nerve.
2.5. Anterior Interosseus Nerve and AIN Syndrome
AIN branches from the main median nerve at about the level of PT and innervates FDP2, flexor pollicis longus (FPL), and pronator quadratus (PQ). FDP3 is usually innervated by AIN but can be partially innervated by the ulnar nerve [31]. There are variations on the origin of AIN, however. When branching from the radial side of the median nerve, it is more susceptible to compression of musculoaponeurotic arches than when it originates from the posterior side [16]. A cadaveric study showed that AIN can easily be intraneurally separated from the main median nerve well above medial epicondyle level, even though the actual branching happens much more distally. The branch to the FCR arose from the AIN when dissected proximally [32]. In addition, in cases of trauma, oedema, or traction, small and less mobile nerves, such as AIN, have been shown to be at greater risk of injury and avulsions than bigger and more mobile nerves such as the main median nerve trunk [33][34][35].
Clearly differentiated from PS, anterior interosseous nerve syndrome (AINS) has been presented. Duchenne [36] reported one case of isolated palsy of the FPL already in 1872, but Kiloh and Nevin first described AINS in 1952 [37]. It is characterized by weakness or paralysis of FPL, FDP2, and PQ without sensory changes. Many authors discriminate an incomplete AIN palsy from a complete AIN palsy, and the term pseudo-AINS is also used for incomplete cases. It is debated whether AINS is a compression neuropathy or not.
2.6. Other Compressive Structures
On rare occasions, patients have been described to have other compression points, including vascular structures, thrombosis of crossing vessels, enlarged bicipital bursa, scar, hypertrophic brachialis muscle, and anomalous muscles surrounding the median nerve [11][15][16][17][20][38][39].
2.7. Distal Course of the Median Nerve
The palmar cutaneous branch of the median nerve (PCBMN) emerges about 5 cm proximal to the wrist crease. It usually runs between the tendons of FCR, and PL. PCBMN gives sensory branches to the skin at the palm and thenar area. Anatomical variations exist. The main median nerve then continues inside the carpal tunnel dividing into a motor branch to the thenar and a sensory branch to the thumb, index, long, and radial side of the ring finger.
2.8. Other Conflicting Factors
2.8.1. Martin–Gruber Anastomosis
Martin–Gruber anastomosis is an anomalous nerve connection from the median nerve to the ulnar nerve. It is prevalent in about 20% of the population [40]. Multiple branching variations have been described, but the two most common are from the main median nerve trunk before the pronator teres branch and from the AIN branch. Its significance appears in high ulnar nerve injuries when the normal median nerve can preserve a better distal sensory and motor function in the ulnar nerve distribution. Conversely, neuropathy in the median nerve can sometimes lead to symptoms in the ulnar part of the hand. Therefore, proximal median nerve compression can result in more diverse symptoms that would normally be expected [31][41].
2.8.2. Nerve Lesions and Renervation
Classifications for nerve injury have been described by Seddon [42] and Sunderland [43]. However, nerve injury can also be a mixture of the different grades and has a varying potential for recovery. Symptoms of momentary ischemia by external compression resolve quickly, but scarring by prolonged compression or axonal damage requires surgical decompression to enable nerve recovery. More difficult injury takes longer to heal and reinnervation speed is limited. Chronic denervation leads to permanent muscle fibrosis. Within these physiological boundaries, decisions must be made regarding the need for surgical intervention.
2.8.3. Double Crush Syndrome
Double crush syndrome was first hypothesized in 1973 by Upton and McComas [44]. Their clinical and electrodiagnostic observations led to the suspicion that one compression site of a nerve makes it more susceptible to other compressions. This clinical hypothesis was later confirmed in animal models and humans, as nerve compression leads to changes in axoplasmic flow and decreased transport of neurotrophic substances [45].
Distal median nerve compression, CTS, is more common than PMNC. Some patients with CTS experience pain also in the proximal arm and shoulder. In 1996, Lundborg suggested the term reverse double crush to reflect that a distal compression of a nerve predisposes it to proximal compressions and symptoms [46].
With the understanding of double or multiple crush syndromes, it has also been proposed that because there are many smaller potential sites of compression of the median nerve, all of which are asymptomatic by themselves, the cumulation of these compressions may eventually result in clinical problems for the patient. In addition, more general factors impairing the function of nerves, such as smoking, alcoholism, diabetes, and thyroid disease, are independent crushes themselves that need to be addressed [45].
2.8.4. Neuralgic Amyotrophy
In 1948, Parsonage and Turner reported an unusual syndrome of pain and paralysis around the shoulder in soldiers during the war years [47]. The name neuralgic amyotrophy (NA) was proposed, but named after the physicians, Parsonage–Turner syndrome has also been used for this condition. NA can be presented in multiple ways, but classically sudden pain at the top of the shoulder blade develops, lasting for a few days or weeks, eventually leading to paralysis of the shoulder girdle. Most patients with NA have difficulties in scapulothoracic movements, resulting in scapular winging, typically affecting the long thoracic and suprascapular nerves. Some patients develop median nerve sensory changes, and, like AINS, some patients also develop weakness of the AIN innervated muscles. The pathophysiology of NA remains unknown and likely includes genetic, autoimmune, and mechanical factors [48].
References
- Asheghan, M.; Hollisaz, M.T.; Aghdam, A.S.; Khatibiaghda, A. The Prevalence of Pronator Teres among Patients with Carpal Tunnel Syndrome: Cross-Sectional Study. Int. J. Biomed. Sci. 2016, 12, 89–94.
- Hsiao, C.-W.; Shih, J.-T.; Hung, S.-T. Concurrent Carpal Tunnel Syndrome and Pronator Syndrome: A Retrospective Study of 21 Cases. Orthop. Traumatol. Surg. Res. 2017, 103, 101–103.
- Mujadzic, M.; Papanicolaou, G.; Young, H.; Tsai, T.-M. Simultaneous Surgical Release of Ipsilateral Pronator Teres and Carpal Tunnel Syndromes. Plast. Reconstr. Surg. 2007, 119, 2141–2147.
- Olehnik, W.K.; Manske, P.R.; Szerzinski, J. Median Nerve Compression in the Proximal Forearm. J. Hand Surg. Am. 1994, 19, 121–126.
- Luangjarmekorn, P.; Tsai, T.M.; Honsawek, S.; Kitidumrongsook, P. Role of Pronator Release in Revision Carpal Tunnel Surgery. SICOT J. 2016, 2, 9.
- Gainor, B.J. The Pronator Compression Test Revisited. A Forgotten Physical Sign. Orthop. Rev. 1990, 19, 888–892.
- Struthers, J. On a Peculiarity of the Humerus and Humeral Artery. Mon. J. Med. Sci. 1848, 28, 264–267.
- Ivins, G.K. Supracondylar Process Syndrome: A Case Report. J. Hand Surg. Am. 1996, 21, 279–281.
- Kessel, L.; Rang, M. Supracondylar Spur of the Humerus. J. Bone Jt. Surg. Br. 1966, 48, 765–769.
- Barnard, L.B.; McCoy, S.M. The Supra Condyloid Process of the Humerus. J. Bone Jt. Surg. Am. 1946, 28, 845–850.
- Bilecenoglu, B.; Uz, A.; Karalezli, N. Possible Anatomic Structures Causing Entrapment Neuropathies of the Median Nerve: An Anatomic Study. Acta Orthop. Belg. 2005, 71, 169–176.
- Shon, H.-C.; Park, J.-K.; Kim, D.-S.; Kang, S.-W.; Kim, K.-J.; Hong, S.-H. Supracondylar Process Syndrome: Two Cases of Median Nerve Neuropathy Due to Compression by the Ligament of Struthers. J. Pain Res. 2018, 11, 803–807.
- Opanova, M.I.; Atkinson, R.E. Supracondylar Process Syndrome: Case Report and Literature Review. J. Hand Surg. Am. 2014, 39, 1130–1135.
- Gessini, L.; Jandolo, B.; Pietrangeli, A. Entrapment Neuropathies of the Median Nerve at and above the Elbow. Surg. Neurol. 1983, 19, 112–116.
- Sos, C.; Roulet, S.; Lafon, L.; Corcia, P.; Laulan, J.; Bacle, G. Median Nerve Entrapment Syndrome in the Elbow and Proximal Forearm. Anatomic Causes and Results for a 55-Case Surgical Series at a Mean 7years’ Follow-Up. Orthop. Traumatol. Surg. Res. 2021, 107, 102825.
- Dellon, A.L.; Mackinnon, S.E. Musculoaponeurotic Variations along the Course of the Median Nerve in the Proximal Forearm. J. Hand Surg. Br. 1987, 12, 359–363.
- Hartz, C.R.; Linscheid, R.L.; Gramse, R.R.; Daube, J.R. The Pronator Teres Syndrome: Compressive Neuropathy of the Median Nerve. J. Bone Jt. Surg. Am. 1981, 63, 885–890.
- Hill, N.A.; Howard, F.M.; Huffer, B.R. The Incomplete Anterior Interosseous Nerve Syndrome. J. Hand Surg. Am. 1985, 10, 4–16.
- Seitz, W.H.; Matsuoka, H.; McAdoo, J.; Sherman, G.; Stickney, D.P. Acute Compression of the Median Nerve at the Elbow by the Lacertus Fibrosus. J. Shoulder Elb. Surg. 2007, 16, 91–94.
- Matsuzaki, A. Operative Treatment of Pronator Syndrome. Orthop. Traumatol. 1999, 7, 34–43.
- Bennett, G.E. Injuries Characteristic of Particular Sports Elbow and Shoulder Lesions of Baseball Players. Am. J. Surg. 1959, 98, 484–492.
- Hagert, E.; Lalonde, D.H. Lacertus Syndrome: Median Nerve Release at the Elbow. In Wide Awake Hand Surgery; Lalonde, D.H., Ed.; Thieme: New York, NY, USA, 2016; pp. 141–145.
- Hagert, E. Clinical Diagnosis and Wide-Awake Surgical Treatment of Proximal Median Nerve Entrapment at the Elbow: A Prospective Study. HAND 2013, 8, 41–46.
- Fuss, F.K.; Wurzl, G.H. Median Nerve Entrapment. Pronator Teres Syndrome. Surgical Anatomy and Correlation with Symptom Patterns. Surg. Radiol. Anat. 1990, 12, 267–271.
- Beaton, L.E.; Anson, B.J. The Relation of the Median Nerve to the Pronator Teres Muscle. Contribution no. 289 from the Anatomical Laboratories of Northwestern University Medical School. Anat. Rec. 1939, 75, 23–26.
- Werner, C.O.; Rosén, I.; Thorngren, K.G. Clinical and Neurophysiologic Characteristics of the Pronator Syndrome. Clin. Orthop. Relat. Res. 1985, 197, 231–236.
- Zancolli, E.R.; Zancolli, E.P.; Perrotto, C.J. New Mini-Invasive Decompression for Pronator Teres Syndrome. J. Hand Surg. Am. 2012, 37, 1706–1710.
- Seyffarth, H. Primary Myoses in the M. Pronator Teres as Cause of Lesion of the N. Medianus (the Pronator Syndrome). Acta Psychiatr. Neurol. Scand. Suppl. 1951, 74, 251–254.
- Tubbs, R.S.; Marshall, T.; Loukas, M.; Shoja, M.M.; Cohen-Gadol, A.A. The Sublime Bridge: Anatomy and Implications in Median Nerve Entrapment. J. Neurosurg. 2010, 113, 110–112.
- Tang, J.B. Median Nerve Compression: Lacertus Syndrome versus Superficialis-Pronator Syndrome. J. Hand. Surg. Eur. Vol. 2021, 46, 1017–1022.
- Spinner, M.; Spencer, P.S. Nerve Compression Lesions of the Upper Extremity. A Clinical and Experimental Review. Clin. Orthop. Relat. Res. 1974, 104, 46–67.
- Jabaley, M.E.; Wallace, W.H.; Heckler, F.R. Internal Topography of Major Nerves of the Forearm and Hand: A Current View. J. Hand Surg. Am. 1980, 5, 1–18.
- Collins, D.N.; Weber, E.R. Anterior Interosseous Nerve Avulsion. Clin. Orthop. Relat. Res. 1983, 181, 175–178.
- Howard, F.M. Compression Neuropathies in the Anterior Forearm. Hand Clin. 1986, 2, 737–745.
- Vincelet, Y.; Journeau, P.; Popkov, D.; Haumont, T.; Lascombes, P. The Anatomical Basis for Anterior Interosseous Nerve Palsy Secondary to Supracondylar Humerus Fractures in Children. Orthop. Traumatol. Surg. Res. 2013, 99, 543–547.
- Duchenne de Boulogne, G.-B.-A. De L’électrisation Localisée et de Son Application À La Pathologie et À La Thérapeutique, 3rd ed.; Baillière, J.B., Ed.; J.-B. Baillière et Fils: Paris, France, 1872.
- Kiloh, L.G.; Nevin, S. Isolated Neuritis of the Anterior Interosseous Nerve. Br. Med. J. 1952, 1, 850–851.
- Al-Qattan, M.M. Gantzer’s Muscle. An Anatomical Study of the Accessory Head of the Flexor Pollicis Longus Muscle. J. Hand Surg. Br. 1996, 21, 269–270.
- Caetano, E.B.; Sabongi, J.J.; Vieira, L.Â.; Caetano, M.F.; Moraes, D.V. Gantzer Muscle. An Anatomical Study. Acta Ortop. Bras. 2015, 23, 72–75.
- Roy, J.; Henry, B.M.; PĘkala, P.A.; Vikse, J.; Saganiak, K.; Walocha, J.A.; Tomaszewski, K.A. Median and Ulnar Nerve Anastomoses in the Upper Limb: A Meta-Analysis. Muscle Nerve 2016, 54, 36–47.
- Haussmann, P.; Patel, M.R. Intraepineurial Constriction of Nerve Fascicles in Pronator Syndrome and Anterior Interosseous Nerve Syndrome. Orthop. Clin. N. Am. 1996, 27, 339–344.
- Seddon, H.J. A Classification of Nerve Injuries. Br. Med. J. 1942, 2, 237–239.
- Sunderland, S. A Classification of Peripheral Nerve Injuries Producing Loss of Function. Brain 1951, 74, 491–516.
- Upton, A.R.; McComas, A.J. The Double Crush in Nerve Entrapment Syndromes. Lancet 1973, 2, 359–362.
- Mackinnon, S.E. Double and Multiple “Crush” Syndromes. Double and Multiple Entrapment Neuropathies. Hand Clin. 1992, 8, 369–390.
- Lundborg, G.; Dahlin, L.B. Anatomy, Function, and Pathophysiology of Peripheral Nerves and Nerve Compression. Hand Clin. 1996, 12, 185–193.
- Parsonege, M.J.; Turner, J.W.A. Neuralgic Amyotrophy; the Shoulder-Girdle Syndrome. Lancet 1948, 1, 973–978.
- van Eijk, J.J.J.; Groothuis, J.T.; van Alfen, N. Neuralgic Amyotrophy: An Update on Diagnosis, Pathophysiology, and Treatment. Muscle Nerve 2016, 53, 337–350.
More
Information
Subjects:
Surgery; Clinical Neurology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
27 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




