Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rodiah Rahmawaty Lubis | -- | 4061 | 2022-07-27 00:41:56 | | | |
| 2 | Sirius Huang | Meta information modification | 4061 | 2022-07-27 03:04:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Imelda, E.; Idroes, R.; Khairan, K.; Lubis, R.R.; Abas, A.H.; Nursalim, A.J.; Rafi, M.; Tallei, T.E. Natural Antioxidant Activities of Plants in Preventing Cataractogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/25549 (accessed on 07 February 2026).
Imelda E, Idroes R, Khairan K, Lubis RR, Abas AH, Nursalim AJ, et al. Natural Antioxidant Activities of Plants in Preventing Cataractogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/25549. Accessed February 07, 2026.
Imelda, Eva, Rinaldi Idroes, Khairan Khairan, Rodiah Rahmawaty Lubis, Abdul Hawil Abas, Ade John Nursalim, Mohamad Rafi, Trina Ekawati Tallei. "Natural Antioxidant Activities of Plants in Preventing Cataractogenesis" Encyclopedia, https://encyclopedia.pub/entry/25549 (accessed February 07, 2026).
Imelda, E., Idroes, R., Khairan, K., Lubis, R.R., Abas, A.H., Nursalim, A.J., Rafi, M., & Tallei, T.E. (2022, July 26). Natural Antioxidant Activities of Plants in Preventing Cataractogenesis. In Encyclopedia. https://encyclopedia.pub/entry/25549
Imelda, Eva, et al. "Natural Antioxidant Activities of Plants in Preventing Cataractogenesis." Encyclopedia. Web. 26 July, 2022.
Copy Citation
A cataract is a condition where the eye’s lens clouds and can lead to progressive loss of vision. The formation of cataracts is linked to both the production of reactive oxygen species (ROS) and the reduction of endogenous antioxidants. Numerous studies have demonstrated that plants contain numerous antioxidant compounds that can be used as cataract preventatives or inhibitors.
antioxidants t
cataract
reactive oxygen species
plants
1. Free Radicals Contribute to Cataract Formation
The electrons of an atom are arranged into orbitals, each of which can accommodate a different pair of electrons. Free radicals are molecules that have only one electron in their outermost orbital or an unpaired electron [1]. Free radicals will take electrons from each adjacent molecule to be stable, triggering cell damage. When each molecule gains or loses electrons, free radicals are produced. Free radicals can be created in the body in two ways: physiologically as part of normal metabolic processes, or pathologically due to illness [2][3][4].
The primary physiological source of free radicals is cellular respiration [5]. An electron transport chain carries electrons from complex to complex and ultimately to oxygen, providing a proton gradient that is utilized to make ATP. The process of generating ATP by donating electrons to the complex in the inner mitochondrial membrane is known as oxidative phosphorylation. In the last part of this process, a cytochrome c oxidase molecule transforms electrons into oxygen [5].
When oxygen accepts four electrons, it usually turns into water. If oxygen does not take all four electrons, it will have an unpaired electron in its orbital, which will cause free radicals to develop. Superoxide is produced when oxygen is supplied with only one electron (O2). It produces hydrogen peroxide (H2O2) with two electrons and hydroxyl radical with three electrons [6].
Free radicals can also be produced as a result of a pathogen. First, during inflammation, phagocytes such as macrophages can produce free radicals. Phagolysosomes are formed when infections enter the body and are consumed by phagocytes. NADPH oxidase, triggered by lysosomal enzymes and causing NADPH to be oxidized, losing two electrons, is also present in these phagocytes. These electrons can be captured by nearby oxygen molecules, forming O2 ions [7]. Superoxide dismutase (SOD), another enzyme, may combine these ions with hydrogen ions to create hydrogen peroxide. A respiratory burst (also known as an oxidative burst) is a process that results in the production of superoxide ions and hydrogen peroxide. Furthermore, phagocytes include a kind of nitric oxide synthase (eNOS), an enzyme that produces nitric oxide, which aids in the killing of infections [8]. On the other hand, nitric oxide reacts with superoxide ions to produce peroxynitrite free radicals (ONOO−). These ions and chemicals kill bacteria by rupturing cell membranes and disrupting protein synthesis [9].
Free radicals are also produced by exposure to ionizing radiation such as X-rays. Radiation steals electrons from water in tissues, converting them into hydroxyl radicals. When metals such as copper or iron accumulate in the body, free radicals are produced. Hemochromatosis, for example, is a condition in which the body absorbs too much iron. Excess iron is oxidized by hydrogen peroxide, yielding iron 3+, hydroxyl radicals, and hydroxide ions as byproducts; iron 3+ may then be reduced to iron 2+ by hydrogen peroxide, yielding peroxide radicals and protons, and the cycle can be repeated indefinitely. As a result, the Fenton reaction can break down H2O2 to OH- in the presence of transmission metals, such as Fe2+ or Cu2 +. Fenton reaction also produces free radicals, including numerous ROS such as superoxide anion radical (•O2−), H2O2, and hydroxyl free radical (•OH), and may lead to structural damage of the crystalline lens and contribute to cataract formation (Figure 1) [10]. This harms cells in numerous organs over time, resulting in cell death and tissue fibrosis [11].
Ischemia, or lack of blood flow to organs or tissues, also generates free radicals. Ischemic damage can cause mitochondria to produce ROS. Reperfusion occurs when blood flows back into is chemical tissue, carrying extra oxygen. When all this oxygen combines with pre-existing free radicals, it causes more cellular damage. Ischemia-reperfusion injury (IRI) is the medical term for this [15]. Free radicals are also produced when chemicals or drugs enter the body and are metabolized by the liver. Many free radicals are created when the liver metabolizes medicines such as acetaminophen or paracetamol (the primary active ingredient in TYLENOL® products), which may cause considerable liver damage [16].
Because the body creates free radicals under normal circumstances, defensive systems are in place to keep them in check. Antioxidants such as vitamin A, C, and E, for example, deliver electrons that neutralize free radicals and protect cells [17]. Glutathione, another chemical in our body, functions as an antioxidant and neutralizes H2O2. To work properly, the two glutathione must be in a reduced form, allowing them to donate electrons and protons to H2O2 and turn it into harmless water. Because this mechanism oxidizes glutathione, glutathione reductase needs reduced nicotinamide adenine dinucleotide phosphate (NADPH) as an electron donor to restore glutathione to its functional state before restarting its activity. NADPH forms nicotinamide adenine dinucleotide phosphate (NADP+) after losing electrons. To replenish the supply of NADPH, an enzyme called glucose-6-phosphate dehydrogenase (G6PD) oxidizes glucose-6-phosphate and converts NADP+ to NADPH. Since glucose-6-phosphate is a byproduct of glucose, humans usually have large amounts of this substance as long as they are not starving [18].
Metal-carrying proteins, which attach to metal ions and assist in transporting or storing them, are another protective mechanism. This mechanism fights free radicals as if the ions were hidden so they could not form free radicals. Transferrin, which binds to iron, and ceruloplasmin, which binds to copper, are two examples of proteins attaching to metals and transporting them through the bloodstream. On the other hand, free radical scavenging enzymes transform free radicals into non-toxic molecules such as water. The enzyme SOD converts superoxide to hydrogen peroxide. In peroxisomes, catalase (CAT) converts hydrogen peroxide to water, while glutathione peroxidase in the cytoplasm does the same. When the amount of free radicals created surpasses this defensive system, cell damage ensues [19][20].
2. Natural Ingredients’ Potential as an Alternative Cataract Treatment
There have been attempts to employ herbal medicines to prevent cataract advancement based on the model of cataract development and the mechanism of its production route. Natural antioxidant molecules have been reported to have an inevitable application in cataract prevention and control due to their easy availability and fewer complications [21]. These biomolecules are excellent at preventing other molecules from oxidizing and producing free radicals. These free radicals set off a chain reaction, causing all lens cells to be damaged. Most of these antioxidants are reducing agents, such as thiols or polyphenols, which inhibit free radical chain reactions. Flavonoids, phenolic acids, carotenoids, vitamins, and lactoferrin are natural antioxidant compounds with anticataract action [22].
In fact, many antioxidants derived from plants such as curcumin, vitamin C, and vitamin E have been well recognized as potential anticataractogenic therapeutics. Vitamin C has been shown to be effective against UV-induced cataracts and age-related cataracts. It also prevents nuclear cataract. Vitamin C also scavenges free radicals. Vitamin E has been shown to be effective against both UV-induced and age-related cataracts by postponing galactose and amino thiazole-induced cataract, inhibiting lipid peroxidation, and maintaining membrane integrity. Curcumin was discovered to be an effective free radical scavenger due to its cytoprotective effect on glutathione-S-transferase enzymes and its efficacy against hyperglycemia, galactose, and naphthalene-induced cataract. Curcumin can also inhibit NFκB [23].
3. Antioxidant Activities of Plants in Preventing Cataractogenesis
3.1. Antioxidant Activities of Plants
The function of oxidative stress in cataract formation has been well documented [24][25]. As a result, antioxidants and free radical scavengers might be used as therapeutic techniques to treat cataracts. The study of antioxidants is growing because of their protective role in food and pharmaceutical products against oxidative damage in the body and pathological processes mediated by oxidative stress [26]. To obtain good antioxidant activity, several things need to be considered, such as using the type of solvent, as [27] reported. The antioxidant activity of the methanol extract of Torilis leptophylla L. crude and its derivative fractions was found to be varied. In addition, screening plant antioxidant properties and their derivative compounds require appropriate methods [26].
This difference in antioxidant activity appears from the difference in the degree of polarity between the solvents used. The results of one-way analysis of variance (ANOVA) obtained in [28] showed that the extraction yield, phytochemical content, and antioxidant properties were significantly affected (p < 0.05) by the polarity of the extraction solvent. The results of other studies related to the different types of solvents on antioxidant activity were carried out by [29], who extracted Sargassum serratifolium leaves using various solvents such as ethyl acetate, ethanol, methanol, acetone, n-hexane, chloroform, and water. According to the study’s findings, ethanol is the most efficient extraction solvent and has the potential to operate as a natural antioxidant. Extraction in highly polar solvents yields high extracts but low phenolic and flavonoid content compared to non-polar ones [28]. The increase in total antioxidant activity and polarity-dependent reducing properties indicated the extraction of strong antioxidant compounds in polar solvents.
In addition to being influenced by the solvent used, antioxidant activity in several works of literature is also related to total phenolic and flavonoid levels. Research conducted by [30] has shown a strong association between antioxidant activity and total flavonoid content of many varieties of Nepalese vegetables.
Plant secondary metabolites with an aromatic ring containing at least one hydroxyl group are phenolic compounds and natural flavonoids [31]. Because their hydroxyl groups can directly contribute to antioxidant activity, phenolic substances are effective electron donors [32]. In addition, several of them promote the production of endogenous antioxidant molecules in cells [33]. According to various studies, free radical inhibition, peroxide decomposition, and metal inactivation are all properties of phenolic compounds [34]. The research conducted by [35] has shown a correlation between total phenolic content with total antioxidant capacity and lipid peroxidation inhibitory activity in in vitro studies.
Previous reports showed that Sargassum serratifolium extracted using several solvents exhibited different total phenolics and antioxidant activities [29]. In addition to differences in solvent types related to polarity, plant preparation methods were also reported to affect antioxidant activity, such as research on fresh leaves and dried leaves of Datura metel L., (Amethyst) plants extracted with several solvents. The tendency of the content is the same, but the antioxidant activity test shows a difference where the antioxidant activity of dry crude extract equivalent to DPPH is on the order of butanol > chloroform > ethyl acetate extract > methanol > hexane extract. However, the order of antioxidant activity of the fresh organic crude extract against DPPH was methanol > hexane > chloroform > ethyl acetate extract > butanol [36].
The strength of antioxidant activity in plants is affected by several factors such as polarity of the solvent extraction, growth location plant species, and mode of action of antioxidant compounds present in a sample. These factors need to be studied more deeply to understand the potency of plant species to obtain maximum antioxidant activity. The white horehound shows relatively high antioxidant activity. The highest EC50 is the MVA extract of Marrubium vulgare L. leaves, with an EC50 of 6.43 ± 0.0411 mg/mL. Ginger also showed promising results. The highest IC50 is the methanol extract of Nigerian Zingiber officinale with a FRAP assay result of 89.15 ± 0.29 μg/mL. Antioxidant activities of ginger extracts were also studied in acetone extract, which has a maximum IC50 value of 0.654 and 0.812 mg/mL.
3.2. Cataract Treatment with Herbal Plants
A cataract is a complex illness with several risk factors. Oxidative stress is a key factor in the onset and progression of cataracts [37][38]. An assessment of the contribution of this mechanism to cataract formation was carried out in a model of induced cataracts in experimental animals. A selenite-induced cataract is one of the good models of senile nuclear cataract and is very rapidly induced [39]. Degradation of calcium homeostasis increased ROS or free radical generation, calpain (calcium-activated protease) activation, insoluble protein, crystal precipitation, phase change, and cytoskeletal loss are the major causes of selenite-induced cataracts [40]. The eye lens possesses a robust antioxidant system as a defensive mechanism against harmful damage from ROS or free radicals. This system contains antioxidants such as reduced glutathione and antioxidant enzymes such as SOD, CAT, and glutathione reductase/peroxidase (GR/Gpx) [41].
Free radicals can cause gene mutations that lead to the formation of cataracts. Free radicals compete with electrons from intracellular molecules resulting in lipid peroxidation, protein modification, lesions on chromosomes, and mitochondrial DNA. This can result in impaired transmission and gene expression and react with DNA chains that also cause mitochondrial DNA (mtDNA or mDNA) damage. This DNA damage disrupts the gene regulatory system, interfering with protein regulation and expression. Mutations in the R48C gene impair A-crystallin stability, associated with lens opacities [42][43].
Meanwhile, free radicals also can cause autophagy, necrosis, and apoptosis of tissues. The regulation of the autophagic system in the body depends on the autophagic flux process, which is responsible for removing abnormal proteins. This results in impaired autophagosome binding to lysosomes, resulting in the accumulation of p62 (a classical receptor of autophagy). This accumulation activates caspases which then increase apoptosis due to the activation of factor-kappa B (NF-κB) [43][44].
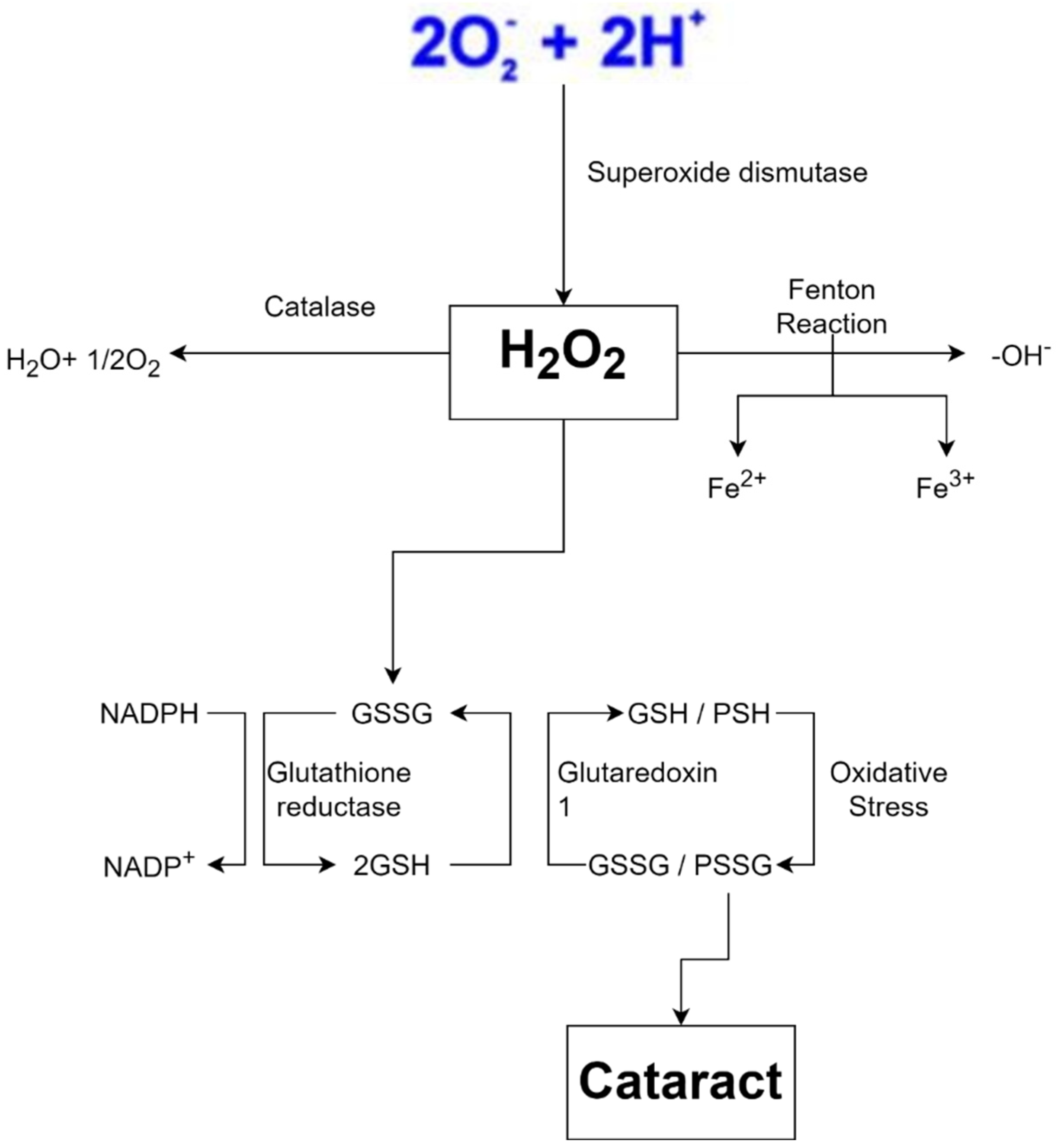
In biological systems, the balance between oxidants and antioxidants is of the utmost importance, which has both physiological relevance (beneficial) and pathological consequences (which usually lead to the formation of diseases, for example, cataracts). Several studies have shown a positive relationship between antioxidant intake and a reduction in the incidence or development of cataracts (Figure 2) [45].
In animal experiments with this condition, compounds of plant origin and herbal medicine have also been demonstrated to have anticataract potential. Quercetin, a flavonoid found in fruits and vegetables, is a potent antioxidant and free radical scavenger with various health advantages, including cardioprotective, anti-diabetic, anti-inflammatory, and anticancer properties [46]. In the study [47], in Sprague Dawley mice, quercetin reduced the onset and development of selenite-induced cataracts and maintained lens chaperone function. In another study, intraperitoneal injections of citrus flavonoids prevented selenite-induced lenticular opacities in Wistar rats, with a corresponding increase in antioxidant enzyme activity, CAT, SOD, glutathione peroxidase (GSH-Px), glutathione S-transferase (GST), and glutathione reductase (GSH-Rx), as well as a reduction in lipid peroxidation, when compared to lenses treated only with selenite [48].

Figure 2. Diagram of the role of antioxidants in inhibiting cataracts [49].
Curcumin is a brilliant yellow chemical with antioxidant qualities that is derived from the Zingiberaceae family’s Curcuma longa plant. Curcumin inhibits the formation of cataracts produced by galactose, oxidative stress, and streptozotocin by inhibiting lenticular antioxidants, lipid peroxidation, and the maintenance of soluble protein content. In Sprague Dawley mice, Nakazawa et al. (2017) found that both oil-soluble antioxidant compounds and water-soluble antioxidants may prevent the onset and progression of selenite-induced cataracts while still maintaining lens chaperone activity [50][51][52].
The report [53] stated that the ethanolic extract of the leaves and stems of Cineraria maritima showed promising results in treating cataracts in the eye lens of goats. According to the ethanol extract of the leaves of the binahong plant, the lens group of the goat lens induced with glucose and the addition of the binahong (Anredera cordifolia (Tenore) Steenis) extract exhibited more transparent results than the lens group induced with 55 mM glucose concentration. Binahong can suppress malondialdehyde generation at doses of 100 or 200 μg/mL [54]. In another study, it was stated that Lupeol, a pentacyclic triterpenoid isolated from Vernonia cinereal, was effective in the treatment of cataracts in the eye lens of Sprague Dawley rats induced by selenite from the results of testing biochemical parameters such as activity of SOD, CAT, GPx, GR, GST, Ca2+ ATPase, glutathione, ROS, and lipid peroxidation product (malondialdehyde) were found to be effective in the treatment of cataracts with lupeol [53][55].
Another study found that the root extract had more antioxidant activity than the leaf extract of the two extracts tested. This conclusion was corroborated by the presence of more apparent antioxidant components in the ethanolic extract of L. aspera root. The root extract of aspera root was tested in the lenses of cultured Wistar rats for probable anticataractogenic potential. The results showed that when the extract was combined with the extract aspera root ethanol in the lenses of selenite-induced Wistar rats, mean enzymatic antioxidant activity, mean levels of reduced glutathione, and mean malondialdehyde expression levels of genes encoding A- and B1-crystalline proteins were kept close to normal, and mean levels of crystalline proteins themselves were kept close to normal [56]. Kaemoferol, for example, is a natural flavonol, a secondary metabolite found in many plants, reveals effectiveness for anti-inflammatory and antioxidant properties. This compound also demonstrated therapeutic antiglaucoma efficacy through suppressing ocular hypertension, inflammation, and oxidative stress [57]. Table 1 shows the results of the analysis of several types of plants that are reported to be able to be used in cataracts management.
Table 1. A list of plants and parts of plants used to prevent cataractogenesis.
| Plants and Parts Used | Solvent | Test Animals | Results | Reference |
|---|---|---|---|---|
| Binahong (Anredera cordifolia (Tenore) Steenis) | Ethanol | Glucose-induced goat lens (ex vivo) | The lens group with added binahong extract had more transparent outcomes than the lens group induced with 55 mM glucose concentration). Binahong can suppress malondialdehyde generation at doses of 100 or 200. | [54] |
| Lupeol, a pentacyclic triterpenoid isolated from Vernonia cinerea | Ethyl acetate fraction of Vernonia cinerea methanol extract | Selenite-induced Sprague Dawley rat eye lens (in vivo) | Biochemical parameters such as the activity of SOD, CAT, GPx, GR, GST, Ca2+ ATPase, glutathione content, ROS, a lipid peroxidation product (malondialdehyde) was estimated and found to be effective in the treatment of cataracts with lupeol. | [55] |
| Heliotropium indicum | Water | 10-day-old Sprague Dawley rat pups of both sexes (in vivo) | Cataract scores showed that the extract significantly reduced selenite-induced cataracts at all dose levels (P 0.001). Lens transparency markers (aquaporin 0, alpha A and B crystallins) and total lens protein and lens glutathione levels were significantly preserved (P 0.01–0.001). The extract exhibited relevant activities for free radical scavenging and lipid peroxidation inhibition. The integrity of the lens epithelium and fibers in histopathological assessment was maintained with Heliotropium indicum extract treatment. | [58] |
| Foeniculum vulgare Mill. | Petroleum ether, chloroform, and dichloromethane | Streptozotocin induced mice (in vivo) | Trans-anethole can effectively exhibit anticataract activity by increasing soluble lens protein, decreasing glutathione, CAT, and SOD activity on in vitro incubation of ocular lens with 55 mM glucose. Trans-anethole showing non-competitiveness for mixed type lens aldose reductase inhibition using Lineweaver–Burk plots. | [59] |
| Cineraria maritime leaves and stems | Ethanol | Goat eye lens (ex vivo) | From the DPPH (2,2-diphenyl-1-picrylhydrazyl) method, the IC50 value of the standard compound was found to be 5.45 μg/mL and that of the ethanolic extract of the plant was 73.26 μg/mL. The hydrogen peroxide method was the second method which was used for the determination of antioxidant potential. In this method, ascorbic acid was used as a standard which showed an IC50 value of 0.89 mg/mL, while the IC50 value of the ethanolic extract of the plant was found to be 1.30 mg/mL. | [53] |
| Chromolaena odorata leaves | Ethanol extract Chromolaena odorata leaves (ACO) | Streptozotocin-induced diabetic mice (in vivo) | ACO treatment resulted in substantial improvements in glucose and insulin tolerance, glycogen content, glucose absorption by skeletal muscle, serum insulin, and HDL-c levels, and a reduction in HOMA and lipid profile. Furthermore, by boosting endogenous antioxidants, ACO decreases oxidative stress. Moreover, ACO therapy significantly reduced the incidence and extent of cataracts. | [60] |
| Leaves of Punica granatum | Methanolic extract of Punica granatum leaves (MPGL) | Goat eye lens (ex vivo) | Reduced glutathione and SOD levels were lower in the cataract lens, indicating opacity. MPGL and quercetin treatment reduced opacity and increased antioxidant activity. Punica granatum leaves reduced glucose-induced cataractogenesis by inhibiting AR, reducing oxidative stress, and enhancing antioxidant defense mechanisms. | [61] |
| Allium cepa (Onion) | Extraction of flavonoids from onion peel and its combination with silver particles showed its activity as nanoparticles. | - | From the observations, the anticataract activity of silver nanoparticles from the Allium cepa peel showed better results than the Allium cepa peel. | [62] |
| Grape Seed Proanthocyanidin Extract (GSPE) | Proanthocyanidin | Selenite-induced cataract in mice (in vivo) | Administration of GSPE was able to maintain this antioxidant enzyme activity and anti-OH independently-ability, accompanied by a significant decrease in malondialdehyde, NO, Ca2+ and iNOS levels, and calpain-2 protein and mRNA expression. | [63] |
| Tephrosia purpurea | Water | Streptozotocin-induced rats (in vivo) | The results showed that the aqueous extract of Tephrosia purpurea prevented streptozotocin-induced metabolic disorders and cardiovascular complications and reduced the risk of cataract development. | [64] |
| Tephrosia purpurea | 95% alcohol | Cataracts were induced by a single injection of sodium selenite (4 mg/kg, sc) into 9-day-old Sprague-Dawley rat pups (in vivo) | T. purpurea extract reduced core opacity in the lens while increasing insoluble protein, sulfhydryl protein, total nitrite, calcium levels, and Ca(2+)-ATPase activity. The extract reduces malondialdehyde levels while simultaneously preventing glutathione depletion. | [65] |
| P. densiflora pine bark | Extraction was performed using 60% EtOH in 50 °C for 3 h | Selenite-induced cataracts in the lens of Sprague Dawley rat pups (in vivo) | This study showed that the bark extract of P. densiflora independently could prevent cataract formation. Water-soluble protein, glutathione, SOD, glutathione peroxidase, and CAT activity levels were high. Conversely, water-insoluble protein, malondialdehyde and Ca2+-ATPase were low in the group treated with P. densiflora bark extract. | [66] |
Based on several references, as shown in Table 1, it can be seen that the use of plant extracts shows promising results in overcoming the problem of cataracts. The induction cataract model can show the effectiveness of the extracts given. In addition to plant extracts, nanoparticles synthesized from plants have also demonstrated effectiveness in treating cataracts, as reported by [62], where the nanoparticles synthesized from shallots showed good anticataract activity compared to shallot extracts that were not synthesized into nanoparticles. Another study investigated the antioxidant capacity and efficiency of silver nanoparticles (AgNPs) biosynthesized using an ethanolic extract of Tabernaemontana divaricata leaf in preventing selenite-induced opacification of the ocular lens in vitro (cataractogenesis). The activity of CAT, SOD, GPx, and GST, as well as levels of reduced glutathione and malondialdehyde, were measured in this investigation. The ethanolic extract of T. divaricata and AgNPs biosynthesized using T. divaricata extracts exhibit excellent in vitro antioxidant activity and the capacity to inhibit experimental selenite-induced opacification in Wistar mice’s lenses, according to the findings [67].
Several in-vivo studies have also proved the ability of plant products to have a positive effect on cataract [68]. Streptozotocin (STZ)-induced diabetic rats were used in the in vivo experiment by Chung et al. At 11 weeks following STZ injection, diabetic control rats acquired cataracts, but oral Aralia elata extract provided at 300 and 600 mg/kg body weight for 11 weeks decreased cataract formation by 15% and 12%, respectively [69].
The research looked at whether highbush blueberry leaf polyphenols could help prevent cataracts and the reasons behind it. HPLC-DAD was used to measure chlorogenic acid, quercetin, rutin, isoquercetin, and hyperoside in Vaccinium corymbosum leaf decoction (BBL). On postnatal days 11 and 12, Wistar rats were administered subcutaneously with 20 μmol selenite (Na2SeO3)/kg body weight or intraperitoneally with 100 mg dry BBL/kg body weight. Only normal saline was given to the control group. BBL considerably reduced lens opacification, according to a cataract examination. It also protected the lens from oxidative selenite assault, calpain activation, and protein loss and aggregation [70]. In model rats, rosmarinic acid, a polyphenol found in rosemary (Rosmarinus officinalis), was confirmed to delay cataract development and lower the degree of lens opacification [70].
Natural substances containing antioxidants or secondary anti-inflammatory metabolites may serve as anticataract agents in modern herbal medicine, which has played a significant role in treating oxidative stress and its consequences [71]. In most instances, free radicals cause lens opacity [23], and protein alteration by free radicals is also a result of extreme oxidative stress. Some plant-based substances can inhibit protein insolubilization, delaying lens opacification [23]. Natural chemicals that are antioxidants or secondary anti-inflammatory metabolites have the potential to be the most effective anticataract treatments. Antioxidant effects are one of the primary mechanisms for cataract prevention in most instances. However, not all plants with antioxidant potential can have anticataract properties. Plant polyphenols have been known to have an anticataractogenic effect has been thoroughly investigated in vitro and in animals [72][73].
As reported in the literature, the chemical structure of many antioxidants plays an important role in preventing ocular disease progression. The effect of aromatic ring number in phenolic compound-conjugated chitosan injectables was investigated with the purpose of developing a more sophisticated drug carrier with significant anti-inflammatory and antioxidant characteristics. Low and high numbers of aromatic rings might have negative effects on injectables’ pharmaceutical uses; however, a molecule with a moderate ring number has been shown to be the most effective agent for improving drug delivery and giving chitosan injectables medicinal qualities. The intracameral infusion of kaempferol-conjugated pilocarpine, which can treat progressive glaucoma by concurrently exerting various pharmacological actions to decrease ocular hypertension, inflammation, and oxidative stress, shows extraordinary efficacy [57].
References
- Staveness, D.; Bosque, I.; Stephenson, C.R.J. Free Radical Chemistry Enabled by Visible Light-Induced Electron Transfer. Acc. Chem. Res. 2016, 49, 2295–2306.
- Cadenas, E. Mitochondrial Free Radical Production and Cell Signaling. Mol. Asp. Med. 2004, 25, 17–26.
- Verhaar, M.C.; Westerweel, P.E.; van Zonneveld, A.J.; Rabelink, T.J. Free Radical Production by Dysfunctional ENOS. Heart 2004, 90, 494–495.
- Maeda, H.; Akaike, T. Oxygen Free Radicals as Pathogenic Molecules in Viral Diseases. Proc. Soc. Exp. Biol. Med. 1991, 198, 721–727.
- Ziegler, D.V.; Wiley, C.D.; Velarde, M.C. Mitochondrial Effectors of Cellular Senescence: Beyond the Free Radical Theory of Aging. Aging Cell 2015, 14, 1–7.
- Lambert, A.J.; Brand, M.D. Reactive Oxygen Species Production by Mitochondria. Mitochondrial DNA 2009, 554, 165–181.
- Supinski, G.S.; Callahan, L.A. Free Radical-Mediated Skeletal Muscle Dysfunction in Inflammatory Conditions. J. Appl. Physiol. 2007, 102, 2056–2063.
- Thomas, D.C. The Phagocyte Respiratory Burst: Historical Perspectives and Recent Advances. Immunol. Lett. 2017, 192, 88–96.
- Radi, R.; Peluffo, G.; Alvarez, M.N.; Naviliat, M.; Cayota, A. Unraveling Peroxynitrite Formation in Biological Systems. Free Radic. Biol. Med. 2001, 30, 463–488.
- Ho, M.-C.; Peng, Y.-J.; Chen, S.-J.; Chiou, S.-H. Senile Cataracts and Oxidative Stress. J. Clin. Gerontol. Geriatr. 2010, 1, 17–21.
- Salgado, P.; Melin, V.; Contreras, D.; Moreno, Y.; Mansilla, H.D. Fenton Reaction Driven by Iron Ligands. J. Chil. Chem. Soc. 2013, 58, 2096–2101.
- Al-Dalaen, S.M.; Al-Qtaitat, A.I. Review Article: Oxidative Stress Versus Antioxidants. Am. J. Biosci. Bioeng. 2014, 2, 60.
- Granger, D.N. Role of Xanthine Oxidase and Granulocytes in Ischemia-Reperfusion Injury. Am. J. Physiol. Circ. Physiol. 1988, 255, H1269–H1275.
- Fenton, H.J.H. LXXIII.—Oxidation of Tartaric Acid in Presence of Iron. J. Chem. Soc. Trans. 1894, 65, 899–910.
- Sun, M.-S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.-L.; Guo, Z.-N.; Yang, Y. Free Radical Damage in Ischemia-Reperfusion Injury: An Obstacle in Acute Ischemic Stroke after Revascularization Therapy. Oxid. Med. Cell. Longev. 2018, 2018, 3804979.
- Dou, X.; Li, J.; Danelisen, I.; Trush, M.A.; Misra, H.P.; Zhu, H.; Jia, Z.; Li, Y. Acetaminophen, the Active Ingredient of Tylenol, Protects against Peroxynitrite-Induced DNA Damage: A Chemiluminometric and Electron Paramagnetic Resonance Spectrometric Study. React. Oxyg. Species 2017, 3, 127–134.
- Rutkowski, M.; Matuszewski, T.; Kedziora, J.; Paradowski, M.; Kłos, K.; Zakrzewski, A. Vitamins E, A and C as Antioxidatives. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2010, 29, 377–381.
- Iskusnykh, I.Y.; Popova, T.N.; Agarkov, A.A.; Pinheiro de Carvalho, M.Â.A.; Rjevskiy, S.G. Expression of Glutathione Peroxidase and Glutathione Reductase and Level of Free Radical Processes under Toxic Hepatitis in Rats. J. Toxicol. 2013, 2013, 870628.
- Ganini, D.; Canistro, D.; Jang, J.; Stadler, K.; Mason, R.P.; Kadiiska, M.B. Ceruloplasmin (Ferroxidase) Oxidizes Hydroxylamine Probes: Deceptive Implications for Free Radical Detection. Free Radic. Biol. Med. 2012, 53, 1514–1521.
- Takami, T.; Sakaida, I. Iron Regulation by Hepatocytes and Free Radicals. J. Clin. Biochem. Nutr. 2011, 48, 103–106.
- Moure, A.; Cruz, J.M.; Franco, D.; Dominguez, J.M.; Sineiro, J.; Dominguez, H.; Nunez, M.J.; Parajo, J.C. Natural Antioxidants from Residual Sources. Food Chem. 2001, 72, 145–171.
- Sunkireddy, P.; Jha, S.N.; Kanwar, J.R.; Yadav, S.C. Natural Antioxidant Biomolecules Promises Future Nanomedicine Based Therapy for Cataract. Colloids Surf. B Biointerfaces 2013, 112, 554–562.
- Thiagarajan, R.; Manikandan, R. Antioxidants and Cataract. Free Radic. Res. 2013, 47, 337–345.
- Spector, A. Oxidative Stress-Induced Cataract: Mechanism of Action. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1995, 9, 1173–1182.
- Truscott, R.J.W. Age-Related Nuclear Cataract-Oxidation Is the Key. Exp. Eye Res. 2005, 80, 709–725.
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715.
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant Activity, Total Phenolic and Total Flavonoid Contents of Whole Plant Extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221.
- Nawaz, H.; Shad, M.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of Solvent Polarity on Extraction Yield and Antioxidant Properties of Phytochemicals from Bean (Phaseolus Vulgaris) Seeds. Braz. J. Pharm. Sci. 2020, 56, e17129.
- Lim, S.; Choi, A.-H.; Kwon, M.; Joung, E.-J.; Shin, T.; Lee, S.-G.; Kim, N.-G.; Kim, H.-R. Evaluation of Antioxidant Activities of Various Solvent Extract from Sargassum Serratifolium and Its Major Antioxidant Components. Food Chem. 2019, 278, 178–184.
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96.
- Lee, Y.H.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. An Appraisal of Eighteen Commonly Consumed Edible Plants as Functional Food Based on Their Antioxidant and Starch Hydrolase Inhibitory Activities. J. Sci. Food Agric. 2015, 95, 2956–2964.
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and Structure—Activity Relationships (SARs) of Some Phenolic and Anilines Compounds. Ann. Agric. Sci. 2013, 58, 173–181.
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.-F.; Lacroix, M. Bioactive Compounds in Cranberries and Their Biological Properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679.
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K. Therapeutic and Nutraceutical Potential of Bioactive Compounds Extracted from Fruit Residues. Crit. Rev. Food Sci. Nutr. 2015, 55, 319–337.
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of Antioxidant Activity and Total Phenol in Different Varieties of Lantana Camara Leaves. BMC Res. Notes 2014, 7, 560.
- Alabri, T.H.A.; Al Musalami, A.H.S.; Hossain, M.A.; Weli, A.M.; Al-Riyami, Q. Comparative Study of Phytochemical Screening, Antioxidant and Antimicrobial Capacities of Fresh and Dry Leaves Crude Plant Extracts of Datura metel L. J. King Saud Univ.-Sci. 2014, 26, 237–243.
- Beebe, D.C.; Holekamp, N.M.; Shui, Y.-B. Oxidative Damage and the Prevention of Age-Related Cataracts. Ophthalmic Res. 2010, 44, 155–165.
- Berthoud, V.M.; Beyer, E.C. Oxidative Stress, Lens Gap Junctions, and Cataracts. Antioxid. Redox Signal. 2009, 11, 339–353.
- Sakthivel, M.; Elanchezhian, R.; Ramesh, E.; Isai, M.; Jesudasan, C.N.; Thomas, P.A.; Geraldine, P. Prevention of Selenite-Induced Cataractogenesis in Wistar Rats by the Polyphenol, Ellagic Acid. Exp. Eye Res. 2008, 86, 251–259.
- Biju, P.G.; Rooban, B.N.; Lija, Y.; Devi, V.G.; Sahasranamam, V.; Abraham, A. Drevogenin D Prevents Selenite-Induced Oxidative Stress and Calpain Activation in Cultured Rat Lens. Mol. Vis. 2007, 13, 1121–1129.
- Wojcik, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A. A Review of Natural and Synthetic Antioxidants Important for Health and Longevity. Curr. Med. Chem. 2010, 17, 3262–3288.
- Wu, J.; Xu, W.; Wu, W.; Xu, J.; Zheng, S.; Shentu, X.; Chen, X. Cataract-Causing Mutation R48C Increases ΓA-Crystallin Susceptibility to Oxidative Stress and Ultraviolet Radiation. Int. J. Biol. Macromol. 2022, 194, 688–694.
- Hsueh, Y.-J.; Chen, Y.-N.; Tsao, Y.-T.; Cheng, C.-M.; Wu, W.-C.; Chen, H.-C. The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases. Int. J. Mol. Sci. 2022, 23, 1255.
- Huang, J.; Yu, W.; He, Q.; He, X.; Yang, M.; Chen, W.; Han, W. Autophagy Facilitates Age-Related Cell Apoptosis—A New Insight from Senile Cataract. Cell Death Dis. 2022, 13, 37.
- Kyselova, Z. Different Experimental Approaches in Modelling Cataractogenesis: An Overview of Selenite-Induced Nuclear Cataract in Rats. Interdiscip. Toxicol. 2010, 3, 3–14.
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89.
- Shetty, L.; Harikiran, H.; Sharma, A. In Vitro Prophylactic Cataract Prevention Study on Glucose Induced Cataract by Quercetin and Alpha Tocopherol. Int. J. Pharm. Sci. Res. 2010, 1, 41–45.
- Isai, M.; Sakthivel, M.; Ramesh, E.; Thomas, P.A.; Geraldine, P. Prevention of Selenite-Induced Cataractogenesis by Rutin in Wistar Rats. Mol. Vis. 2009, 15, 2570–2577.
- Nakazawa, Y.; Nagai, N.; Ishimori, N.; Oguchi, J.; Tamura, H. Administration of Antioxidant Compounds Affects the Lens Chaperone Activity and Prevents the Onset of Cataracts. Biomed. Pharmacother. 2017, 95, 137–143.
- Padmaja, S.; Raju, T.N. Antioxidant Effect of Curcumin in Selenium Induced Cataract of Wistar Rats. Indian J. Exp. Biol. 2004, 42, 601–603.
- Suryanarayana, P.; Krishnaswamy, K.; Reddy, G.B. Effect of Curcumin on Galactose-Induced Cataractogenesis in Rats. Mol. Vis. 2003, 9, 223–230.
- Manikandan, R.; Thiagarajan, R.; Beulaja, S.; Sudhandiran, G.; Arumugam, M. Curcumin Prevents Free Radical-Mediated Cataractogenesis through Modulations in Lens Calcium. Free Radic. Biol. Med. 2010, 48, 483–492.
- Durgapal, S.; Juyal, V.; Verma, A. In Vitro Antioxidant and Ex Vivo Anti-Cataract Activity of Ethanolic Extract of Cineraria Maritima: A Traditional Plant from Nilgiri Hills. Futur. J. Pharm. Sci. 2021, 7, 105.
- Feriyani, F.; Maulanza, H.; Lubis, R.R.; Balqis, U.; Darmawi, D. Effects of Binahong (Anredera Cordifolia (Tenore) Steenis) Extracts on the Levels of Malondialdehyde (MDA) in Cataract Goat Lenses. Sci. World J. 2021, 2021, 6617292.
- Asha, R.; Gayathri Devi, V.; Abraham, A. Lupeol, a Pentacyclic Triterpenoid Isolated from Vernonia Cinerea Attenuate Selenite Induced Cataract Formation in Sprague Dawley Rat Pups. Chem. Biol. Interact. 2016, 245, 20–29.
- Sundararajan, M.; Thomas, P.A.; Babyshalini, K.; Geraldine, P. Identification of Phytoconstituents and In-Vitro Evaluation of the Putative Anticataractogenic Effect of an Ethanolic Root Extract of Leucas Aspera. Biomed. Pharmacother. 2017, 85, 87–101.
- Nguyen, D.D.; Luo, L.-J.; Lue, S.J.; Lai, J.-Y. The Role of Aromatic Ring Number in Phenolic Compound-Conjugated Chitosan Injectables for Sustained Therapeutic Antiglaucoma Efficacy. Carbohydr. Polym. 2020, 231, 115770.
- Kyei, S.; Koffuor, G.A.; Ramkissoon, P.; Afari, C.; Asiamah, E.A. The Claim of Anti-Cataract Potential of Heliotropium Indicum: A Myth or Reality? Ophthalmol. Ther. 2015, 4, 115–128.
- Dongare, V.; Kulkarni, C.; Kondawar, M.; Magdum, C.; Haldavnekar, V.; Arvindekar, A. Inhibition of Aldose Reductase and Anti-Cataract Action of Trans-Anethole Isolated from Foeniculum Vulgare Mill. Fruits. Food Chem. 2012, 132, 385–390.
- Onkaramurthy, M.; Veerapur, V.P.; Thippeswamy, B.S.; Madhusudana Reddy, T.N.; Rayappa, H.; Badami, S. Anti-Diabetic and Anti-Cataract Effects of Chromolaena Odorata Linn., in Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2013, 145, 363–372.
- Mestry, S.N.; Juvekar, A.R. Aldose Reductase Inhibitory Potential and Anti-Cataract Activity of Punica Granatum Linn. Leaves against Glucose-Induced Cataractogenesis in Goat Eye Lens. Orient. Pharm. Exp. Med. 2017, 17, 277–284.
- Sruthi, T.; Sasikala, V.; Vangalapati, M. Anti Cataract Activity of Synthesized Silver Nano Particles from Skin Of Allium Cepa Species. In Proceedings of the Journal of Physics, Xi’an, China, 18–19 October 2020; Volume 1455, p. 12017.
- Zhang, X.; Hu, Y. Inhibitory Effects of Grape Seed Proanthocyanidin Extract on Selenite-Induced Cataract Formation and Possible Mechanism. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012, 32, 613–619.
- Bhadada, S.V.; Goyal, R.K. Effect of Aqueous Extract of Tephrosia Purpurea on Cardiovascular Complications and Cataract Associated with Streptozotocin-Induced Diabetes in Rats. Indian J. Pharm. Sci. 2015, 77, 522–529.
- Bhadada, S.V.; Bhadada, V.J.; Goyal, R.K. Preventive Effect of Tephrosia Purpurea on Selenite-Induced Experimental Cataract. Curr. Eye Res. 2016, 41, 222–231.
- Kim, J.; Choung, S.-Y. Pinus Densiflora Bark Extract Prevents Selenite-Induced Cataract Formation in the Lens of Sprague Dawley Rat Pups. Mol. Vis. 2017, 23, 638–648.
- Anbukkarasi, M.; Thomas, P.A.; Sheu, J.-R.; Geraldine, P. In Vitro Antioxidant and Anticataractogenic Potential of Silver Nanoparticles Biosynthesized Using an Ethanolic Extract of Tabernaemontana Divaricata Leaves. Biomed. Pharmacother. 2017, 91, 467–475.
- Tewari, D.; Samoilă, O.; Gocan, D.; Mocan, A.; Moldovan, C.; Devkota, H.P.; Atanasov, A.G.; Zengin, G.; Echeverría, J.; Vodnar, D.; et al. Medicinal Plants and Natural Products Used in Cataract Management. Front. Pharmacol. 2019, 10, 466.
- Chung, Y.-S.; Choi, Y.-H.; Lee, S.-J.; Choi, S.; Lee, J.; Kim, H.; Hong, E.-K. Water Extract of Aralia Elata Prevents Cataractogenesis in Vitro and in Vivo. J. Ethnopharmacol. 2005, 101, 49–54.
- Ferlemi, A.-V.; Makri, O.E.; Mermigki, P.G.; Lamari, F.N.; Georgakopoulos, C.D. Quercetin Glycosides and Chlorogenic Acid in Highbush Blueberry Leaf Decoction Prevent Cataractogenesis in Vivo and in Vitro: Investigation of the Effect on Calpains, Antioxidant and Metal Chelating Properties. Exp. Eye Res. 2016, 145, 258–268.
- Heruye, S.H.; Maffofou Nkenyi, L.N.; Singh, N.U.; Yalzadeh, D.; Ngele, K.K.; Njie-Mbye, Y.-F.; Ohia, S.E.; Opere, C.A. Current Trends in the Pharmacotherapy of Cataracts. Pharmaceuticals 2020, 13, 15.
- Rooban, B.N.; Sasikala, V.; Sahasranamam, V.; Abraham, A. Analysis on the Alterations of Lens Proteins by Vitex Negundo in Selenite Cataract Models. Mol. Vis. 2011, 17, 1239–1248.
- Sasikala, V.; Rooban, B.N.; Sahasranamam, V.; Abraham, A. Rutin Ameliorates Free Radical Mediated Cataract by Enhancing the Chaperone Activity of α-Crystallin. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albr. von Graefes Arch. fur Klin. Exp. Ophthalmol. 2013, 251, 1747–1755.
More
Information
Subjects:
Ophthalmology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
02 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No