Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carlos Perez-Ramirez | -- | 2065 | 2022-07-20 09:21:50 | | | |
| 2 | Rita Xu | Meta information modification | 2065 | 2022-07-27 03:31:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Basurto-Hurtado, J.A.; Cruz-Albarran, I.A.; Toledano-Ayala, M.; Ibarra-Manzano, M.A.; Morales-Hernandez, L.A.; Perez-Ramirez, C.A. Breast Cancer Detection. Encyclopedia. Available online: https://encyclopedia.pub/entry/25546 (accessed on 08 February 2026).
Basurto-Hurtado JA, Cruz-Albarran IA, Toledano-Ayala M, Ibarra-Manzano MA, Morales-Hernandez LA, Perez-Ramirez CA. Breast Cancer Detection. Encyclopedia. Available at: https://encyclopedia.pub/entry/25546. Accessed February 08, 2026.
Basurto-Hurtado, Jesus A., Irving A. Cruz-Albarran, Manuel Toledano-Ayala, Mario Alberto Ibarra-Manzano, Luis A. Morales-Hernandez, Carlos A. Perez-Ramirez. "Breast Cancer Detection" Encyclopedia, https://encyclopedia.pub/entry/25546 (accessed February 08, 2026).
Basurto-Hurtado, J.A., Cruz-Albarran, I.A., Toledano-Ayala, M., Ibarra-Manzano, M.A., Morales-Hernandez, L.A., & Perez-Ramirez, C.A. (2022, July 26). Breast Cancer Detection. In Encyclopedia. https://encyclopedia.pub/entry/25546
Basurto-Hurtado, Jesus A., et al. "Breast Cancer Detection." Encyclopedia. Web. 26 July, 2022.
Copy Citation
Breast cancer is one the main death causes for women worldwide, as 16% of the diagnosed malignant lesions worldwide are its consequence. In this sense, it is of paramount importance to diagnose these lesions in the earliest stage possible, in order to have the highest chances of survival. While there are several works that present selected topics in this area, none of them present a complete panorama, that is, from the image generation to its interpretation.
breast cancer
mammography
magnetic resonance
1. Introduction
According to the World Health Organization, Breast Cancer (BC) represents around 16% of the malignant tumors diagnosed worldwide [1]. In Mexico, BC is the leading death cause for cancer in the female population [2]. BC develops when any lump begins an angiogenesis process, that is, the process that causes the development of new blood vessels and capillaries from the existent vasculature [3]. Unfortunately, BC has a mortality rate of 69% in emergent countries, which is greater than the one in developed countries [1]. This increase is explained as the cancer is detected in a later stage, making the treatment a financial obstacle as its price increases, especially if the disease is detected in an advanced stage [4]. Hence, the development of strategies that can perform an early detection of BC is a priority topic for governments, as an early detection increases the survival chances and lowers the financial burden the disease imposes to families and health systems [4].
A methodology for the BC detection can be composed of 4 steps: (1) image acquisition, (2) Segmentation and preprocessing, (3) feature extraction, and (4) classification. An illustration of the abovementioned concepts is described in Figure 1.
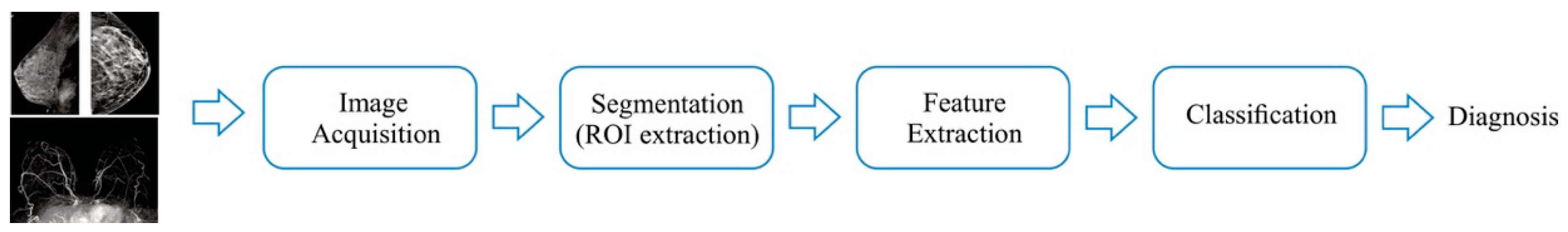
Figure 1. BC detection using image processing strategies.
From this figure, it can be seen that the first step uses the different technologies available to acquire the internal tissue dynamics of the breast, so they can be expressed in an image; the second step is used to execute algorithms that perform basic tasks on the images (for instance, correcting the color scale), so the segmentation, which is the detection of Region-of-interest (ROI), can be done; then, the third step quantifies the differences between images that have abnormalities from the ones that do not have; finally, once the differences are quantified, it is necessary to classify them to provide a diagnosis. With the rapid development of novel technologies that can capture more accurately the dynamics of the breast tissues, numerous advances have been done in all the aforementioned fields; in this sense, the goal of detecting all the abnormalities without generating false alarms is still a highly desirable feature for all the proposals [5][6].
2. Technologies Used to Obtain Breast Tissue Images
One of the steps require to develop a diagnose system is the representation of the breast tissue dynamics. In this sense, there are several technologies that are commonly used to represent the tissue by means of images. This section presents the most used ones.
2.1. Mammography
Mammography is a study used to screen the breast tissue in order to detect abnormalities that could indicate the prescience of cancer or other breast diseases [7]. This technique has a sensibility of up to 85% in the recommended population. Essentially, mammography uses low doses of X-ray to form a picture of the breast internal tissues [8]. To form the picture, the breasts are compressed by two plates with the aim of mitigating the dispersion of the rays, allowing to obtain a better picture without using an X-ray high-dose [8], where the tissue changes might appear as white zones on a grey contrast [8]. On average, the total radiation dose for a typical mammogram with 2 views for each breast is about 0.4 [8]. Figure 2 illustrates the mammography procedure.
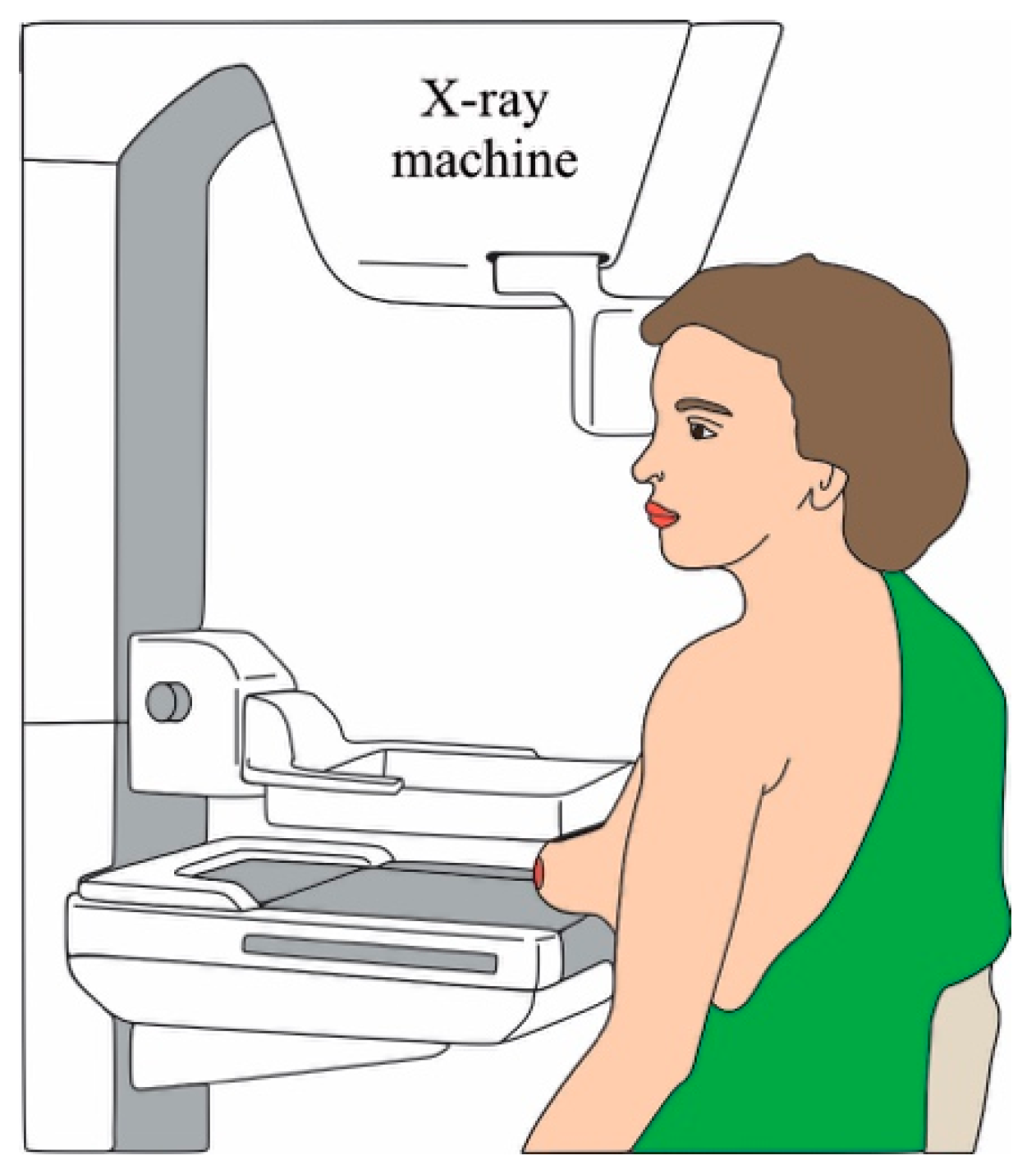
Figure 2. Mammography procedure.
Several works have focused on the processing of the digital mammographies to detect the most common symptoms that could indicate the presence of cancer: calcifications or masses [9]. Traditionally, the specialist looks for zones that have a different appearance (size, shape, contrast, edges, or bright spots) than the normal tissue. With the employment of segmentation algorithms [10][11][12], the automatization of this task has been proposed, where some attempts using neural networks have done [9][13][14], delivering encouraging results.
Recently, the utilization of the Breast tomosynthesis (BT) and the Contrast-Enhanced Mammography (CEM) [7] have been proposed as improvements to the traditional digital mammography. The former is a 3D breast reconstruction that allows to further improve the image resolution whereas the latter improves the image resolution injecting a contrast agent; in this way, the anatomic and vascularity definition of the abnormalities is exposed. In this sense, some improvements when dealing with breast-dense tissue patients are obtained; yet, the detection of clustered micro calcifications is still an issue [7]; on the other hand, additional screening tests are required to determine if the abnormality detected by CEM is cancer or not, besides of requiring more expensive equipment.
2.2. Ultrasound
Ultrasound is a non-invasive and non-irradiating technique that uses sound waves to create images from organs, in this case the breasts, to detect changes in their form. To create the images, a transducer sends high-frequency sound waves (>20 kHz) and measures the reflected ones [7]. The image is formed using the wave sound reflected from the internal tissues. This procedure is depicted in Figure 3.
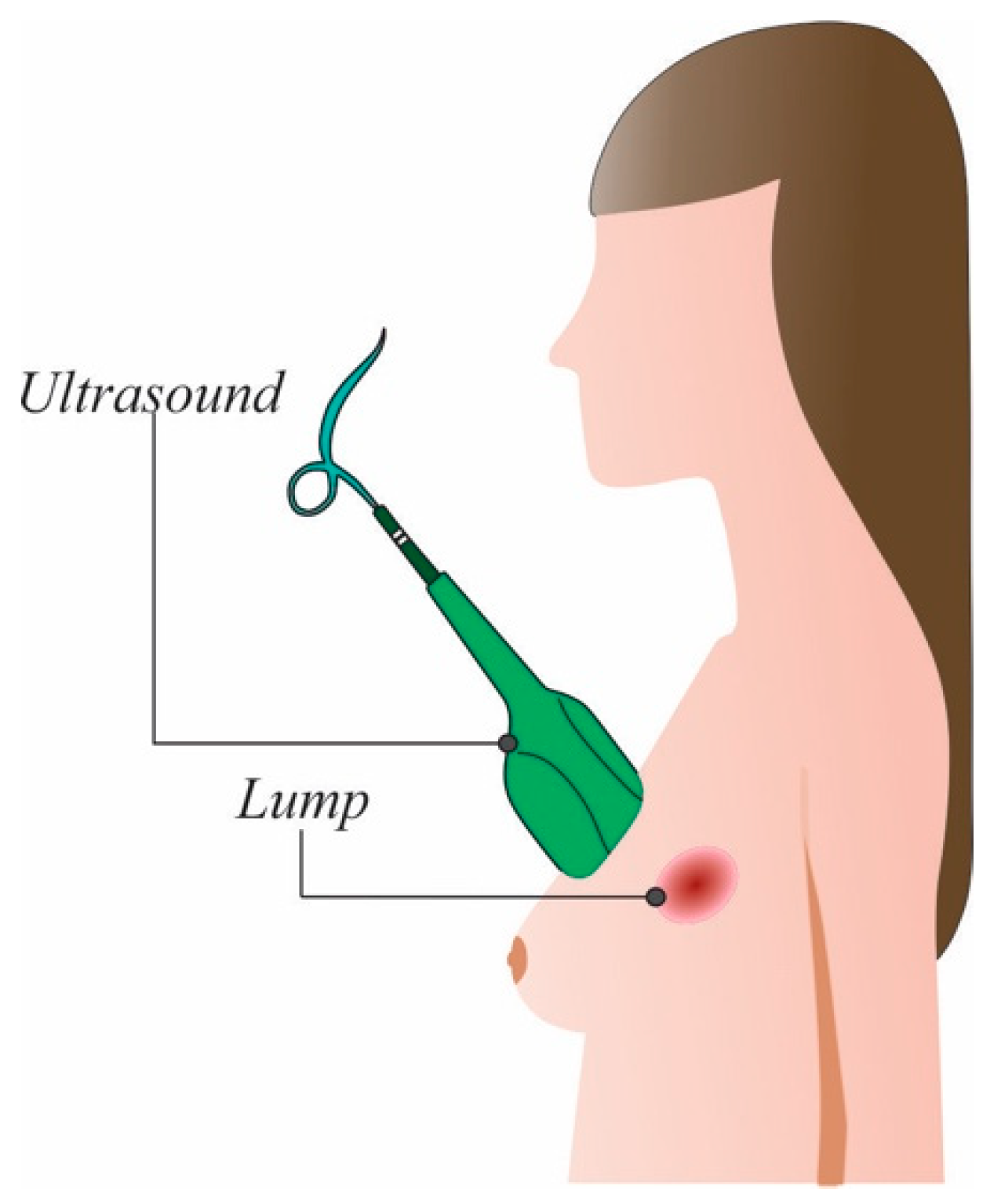
Figure 3. Ultrasound procedure.
Ultrasound is used for three purposes: (1) assessing and determining the abnormality condition, that is, to help doctors if the abnormal mass is solid, which might require further examination, is fluid-filled, or has both features; (2) as an auxiliary screen tool, which is used when the patient has dense breasts and the mammography is not the reliable enough, (3) or as a guide to develop a biopsy in the suspected abnormality [7]. Several computer-aided diagnose (CAD) systems that analyze ultrasound images have been proposed [15]. One of the points they note it is necessary to improve is the resolution of the images [16] using specific-designed filters. Another modification proposed is the utilization of micro-bubbles that are injected into the abnormalities detected at first sight [17].
It should be noticed that the mass tends to stay in its position when compressed, i.e., they do not displace. Elastography is the technique that is employed to measure the tumor displacement when compressed using a special transducer [18]. These developments have led to discover masses that usually require performing a biopsy to determine the mass nature, which delay the diagnosis confirmation [7][18]; moreover, the image interpretation requires a well-trained specialist, which is not always available to perform all the studies.
2.3. Magnetic Resonance Imagining (MRI)
Breast MRI (BMRI) uses a magnetic field and radio waves to create a detailed image from the breast. Usually, a 1.5 T magnet is used along with a contrast, usually gadolinium, to generate the images of both breasts [19]. To acquire the images, the patient is located in a prone position, in order to minimize the respiration movement and to allow the expansion of the breast tissue [7][19]. When the magnet is turned on, the magnetic field temporary realigns the water molecules; thus, when radio waves are applied, the emitted radiation is captured using specific-designed coils, located at the breast positions, which transforms the captured radiation in electrical signals. The coils position must ensure an appropriate field-of-vision from the clavicle to the infra-mammary fold, including axilla [7]. An illustration of the patient position is depicted in Figure 4.
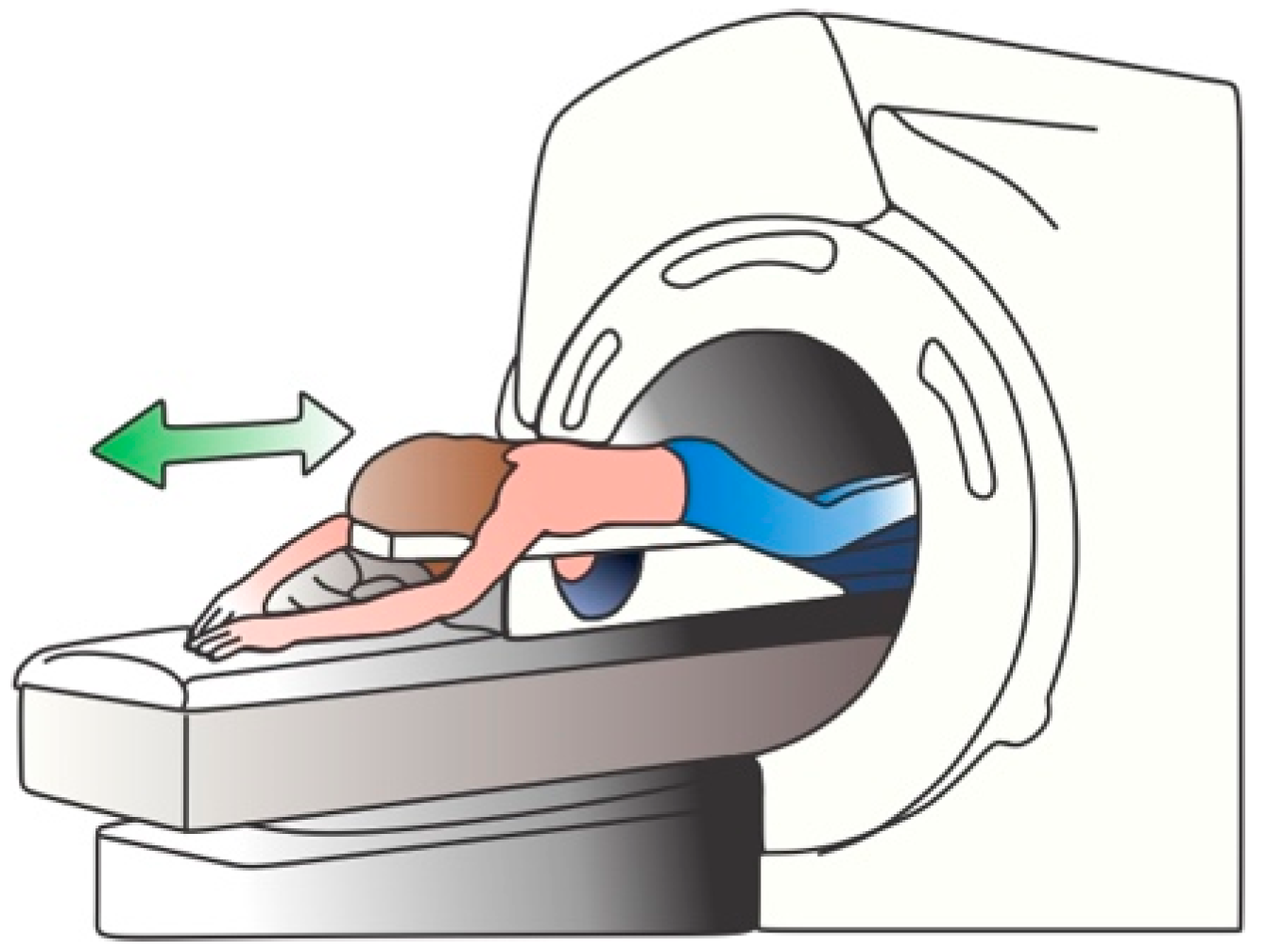
Figure 4. BMRI procedure.
The main objective of getting the images is to assess for the breast symmetry and the possible changes in the parenchymal tissue, since those changes might indicate the presence of lesions that can be malignant. In general, malignant lesions have irregular margins (or asymmetry), whereas the benign ones usually have a round or oval geometrical shape with well-defined margins (symmetry). To deliver the best possible result, it is necessary to remove the homogenous fat around the breast and parenchyma since fat can render images that can be uninterpretable, specially to detect subtle lesions [7][19].
On the other hand, one of the problems that BMRI has is the false-positive (specificity) rate, as the technique can detect low-size masses (lesions whose size is less than 5 mm) that are benign [7][19]. To mitigate the aforementioned issue, nanomaterials have been developed, so they stick to the cancer masses but not to the benign ones [20] as well as contrast agents [21]. Recently, it has been proposed that a multiparametric approach has been suggested as a strategy to improve the specificity rate [7].
2.4. Other Approaches
Recently, microwave radiation has been employed as an alternative to obtain information about the breast tissue. The microwaves, whose frequency range varies from 1 to 20 GHz, are applied to the breast and the reflected waves are measured using specific-designed antennas. To have the best possible results, some works propose that the tissue must be immersed in a liquid [22]. In this sense, some works have proposed acquisition systems that deal with this issue [23][24][25][26].
When it is necessary to perform a biopsy to confirm, images from the cells that form the abnormalities are obtained using among other techniques, the fine needle aspiration citology (FNAC), core or excisional biopsy. Once the cell images are captured, an image processing technique is applied in order to detect the differences between normal and malignant cells, which are classified using modern strategies [27][28][29] such as neural networks, probabilistic-based algorithms and association rules coupled with neural networks.
It should be pointed out that other alternatives for imaging are employed such as Computed Tomography (CT) or Positron Emission Tomography (PET). The former employ X-rays to form images from the chest using different angles; using image processing and reconstruction algorithms, a 3D image of the chest (including the breasts) is obtained [30][31]; on the other hand, the latter uses a small amount of tracer, that is a specific-designed sugar with radioactive properties known as fluorodeoxyglucose-18. The main idea of using this type of sugar is that cancer cells have an increased consume of glucose compared with the normal cells; in this sense, the tracer sticks in the zones where there is an increased glucose consume [32][33]. It is worth noticing that these techniques are recommended to determine the cancer stage rather than first-line diagnosis scheme [7][34]. In this way, they complement the three main techniques to provide more information from the tissues surrounding the breasts [34]. Table 1 presents a table that summarizes the abovementioned methods.
Table 1. Summary of the used breast image generation technologies.
| Imagining Technique | Advantages | Disadvantages | Recommended Population | Some Types of Cancer Detected | Sensitivity and/or Specificity |
|---|---|---|---|---|---|
| Mammography | 1. Equipment is widely available worldwide. 2. Methods, such as tomosynthesis, can improve the specificity and sensibility of the technique with patients that have dense breasts [7] |
1. The rate of both false positive and false negatives increases since there is no possibility to determine if the masses are benign 2. The procedure used to obtain the images could be bothersome. 3. Dense breasts or young patients are not indicated to use this imaging technique. |
Women whose age is greater than 40 years, have low-dense breast and an average risk of contracting the disease. | 1. Ductal Carcinoma in Situ 2. Invasive Breast Cancer. |
Sensitivity up to 85%. |
| Ultrasound | 1. Can be used in young patients or have dense breast. 2. The equipment used is available in most of the hospitals |
1. Calcifications could not be detected. 2. Sensitivity depends on the operator ability to interpret the images 3. False-positivity rate is an issue. |
Women with heterogeneously or extremely dense breast tissue [35][36]. Women that are pregnant or lactating [37]. |
1. Ductal Carcinoma in Situ. 2. Invasive ductal carcinoma |
Sensitivity ranging between 40–75% in younger high-risk women [37]. |
| Magnetic Resonance Imaging | 1. Effective for detecting suspicious masses in high-risk population [7]. 2. The breast tissue density is no longer an issue [35][36][37]. 3. Multifocal lesions can be detected [7][38] |
1. Equipment is only available in specialized hospitals. 2. Expensive 3. False positive findings are an important concern [38] |
1. Women that may carry mutation in ATM, BRCA1, BRCA2, CHEK2, PALB2, PTEN, TP53 genes. 2. Women that had radiation therapy in the chest zone during the childhood. |
1. Ductal in situ carcinomas 2. Invasive ductal carcinomas. 3. Invasive lobular carcinomas 4. Invasive mammary carcinomas with mixed ductal and lobular features [21] |
Sensitivity ranging from 83 to 100% [39][40][41]. |
As it is seen in Table 1, numerous advances for imagining techniques have been achieved in the last years; still, there is a necessity of developing strategies that can allow obtaining sharp images, even for dense breast tissues. In this sense, the obtained images can be used to perform a focused surveillance on the patients that have a higher risk for developing the disease, allowing to achieve the cancer detection in the earliest possible stage. On the other hand, these novel imagining techniques should be able to operate without requiring additional requirements, such as specific electrical or mechanical conditions, so they can be easily adopted in hospitals, or in an ambulatory area.
References
- World Health Organization (WHO). Cáncer de Mama: Prevención y Control. Available online: https://www.who.int/topics/cancer/breastcancer/es/index1.html (accessed on 3 May 2022).
- Villa-Guillen, D.E.; Avila-Monteverde, E.; Gonzalez-Zepeda, J.H. Breast cancer risk and residential exposure to envi-ronmental hazards in Hermosillo, Sonora, Mexico . In Proceedings of the 2019 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 10–14 December 2019; AACR: Philadelphia, PA, USA.
- Keith, B.; Simon, M.C. Tumor Angiogenesis. The Molecular Basis of Cancer, 4th ed.; Mendelsohn, J., Gray, J.W., Howley, P.M., Israel, M.A., Thompson, C.B., Eds.; Elsevier: Philadelphia, PA, USA, 2015; pp. 257–268.
- Semin, J.N.; Palm, D.; Smith, L.M.; Ruttle, S. Understanding breast cancer survivors’ financial burden and distress after financial assistance. Support. Care Cancer 2020, 28, 4241–4248.
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536.
- Vobugari, N.; Raja, V.; Sethi, U.; Gandhi, K.; Raja, K.; Surani, S.R. Advancements in Oncology with Artificial Intelligence—A Review Article. Cancers 2022, 14, 1349.
- Jochelson, M. Advanced Imaging Techniques for the Detection of Breast Cancer; American Society of Clinical Oncology Educational Book: Alexandria, VA, USA, 2012; pp. 65–69.
- Yaffe, M.J. AAPM tutorial. Physics of mammography: Image recording process. RadioGraphics 1990, 10, 341–363.
- Pak, F.; Kanan, H.R.; Alikhassi, A. Breast cancer detection and classification in digital mammography based on Non-Subsampled Contourlet Transform (NSCT) and Super Resolution. Comput. Methods Programs Biomed. 2015, 122, 89–107.
- Geweid, G.G.N.; Abdallah, M.A. A Novel Approach for Breast Cancer Investigation and Recognition Using M-Level Set-Based Optimization Functions. IEEE Access 2019, 7, 136343–136357.
- Guzmán-Cabrera, R.; Guzmán-Sepúlveda, J.R.; Torres-Cisneros, M.; May-Arrioja, D.A.; Ruiz-Pinales, J.; Ibarra-Manzano, O.G.; Aviña-Cervantes, G.; Parada, A.G. Digital Image Processing Technique for Breast Cancer Detection. Int. J. Thermophys. 2012, 34, 1519–1531.
- Avuti, S.K.; Bajaj, V.; Kumar, A.; Singh, G.K. A novel pectoral muscle segmentation from scanned mammograms using EMO algorithm. Biomed. Eng. Lett. 2019, 9, 481–496.
- Vijayarajeswari, R.; Parthasarathy, P.; Vivekanandan, S.; Basha, A.A. Classification of mammogram for early detection of breast cancer using SVM classifier and Hough transform. Measurement 2019, 146, 800–805.
- Rodríguez-Álvarez, M.X.; Tahoces, P.G.; Cadarso-Suárez, C.; Lado, M.J. Comparative study of ROC regression techniques—Applications for the computer-aided diagnostic system in breast cancer detection. Comput. Stat. Data Anal. 2011, 55, 888–902.
- Cheng, H.D.; Shan, J.; Ju, W.; Guo, Y.; Zhang, L. Automated breast cancer detection and classification using ultrasound images: A survey. Pattern Recognit. 2010, 43, 299–317.
- Ouyang, Y.; Tsui, P.-H.; Wu, S.; Wu, W.; Zhou, Z. Classification of Benign and Malignant Breast Tumors Using H-Scan Ultrasound Imaging. Diagnostics 2019, 9, 182.
- Ouyang, Y.; Tsui, P.-H.; Wu, S.; Wu, W.; Zhou, Z. Breast cancer detection by B7-H3–targeted ultrasound molecular imaging. Cancer Res. 2015, 75, 2501–2509.
- Athanasiou, A.; Tardivon, A.; Ollivier, L.; Thibault, F.; El Khoury, C.; Neuenschwander, S. How to optimize breast ultrasound. Eur. J. Radiol. 2009, 69, 6–13.
- Mumin MRad, N.A.; Hamid MRad, M.T.R.; Ding Wong, J.H.; Rahmat MRad, K.; Hoong Ng, K. Magnetic Resonance Imaging Phenotypes of Breast Cancer Molecular Subtypes: A Systematic Review. Acad. Radiol. 2022, 29, S89–S106.
- Han, C.; Zhang, A.; Kong, Y.; Yu, N.; Xie, T.; Dou, B.; Li, K.; Wang, Y.; Li, J.; Xu, K. Multifunctional iron oxide-carbon hybrid nanoparticles for targeted fluorescent/MR dual-modal imaging and detection of breast cancer cells. Anal. Chim. Acta 2019, 1067, 115–128.
- Mango, V.L.; Morris, E.A.; Dershaw, D.D.; Abramson, A.; Fry, C.; Moskowitz, C.S.; Hughes, M.; Kaplan, J.; Jochelson, M.S. Abbreviated protocol for breast MRI: Are multiple sequences needed for cancer detection? Eur. J. Radiol. 2015, 84, 65–70.
- Nikolova, N.K. Microwave Imaging for Breast Cancer. IEEE Microw. Mag. 2011, 12, 78–94.
- Xu, M.; Thulasiraman, P.; Noghanian, S. Microwave tomography for breast cancer detection on Cell broadband engine processors. J. Parallel Distrib. Comput. 2021, 72, 1106–1116.
- Grzegorczyk, T.M.; Meaney, P.M.; Kaufman, P.A.; di Florio-Alexander, R.M.; Paulsen, K.D. Fast 3-D Tomographic Microwave Imaging for Breast Cancer Detection. IEEE Trans. Med. Imaging 2012, 31, 1584–1592.
- AlSawaftah, N.; El-Abed, S.; Dhou, S.; Zakaria, A. Microwave Imaging for Early Breast Cancer Detection: Current State, Challenges, and Future Directions. J. Imaging 2022, 8, 123.
- Zerrad, F.-E.; Taouzari, M.; Makroum, E.M.; El Aoufi, J.; Islam, M.T.; Özkaner, V.; Abdulkarim, Y.I.; Karaaslan, M. Multilayered metamaterials array antenna based on artificial magnetic conductor’s structure for the application diagnostic breast cancer detection with microwave imaging. Med. Eng. Phys. 2022, 99, 103737.
- Karabatak, M.; Ince, M.C. An expert system for detection of breast cancer based on association rules and neural network. Expert Syst. Appl. 2009, 36, 3465–3469.
- Wang, P.; Hu, X.; Li, Y.; Liu, Q.; Zhu, X. Automatic cell nuclei segmentation and classification of breast cancer histopathology images. Signal Process. 2016, 122, 1–13.
- Wahab, N.; Khan, A.; Lee, Y.S. Two-phase deep convolutional neural network for reducing class skewness in histopathological images based breast cancer detection. Comput. Biol. Med. 2017, 85, 86–97.
- Fan, Y.; Wang, H.; Gemmeke, H.; Hopp, T.; Hesser, J. Model-data-driven image reconstruction with neural networks for ultrasound computed tomography breast imaging. Neurocomputing 2022, 467, 10–21.
- Koh, J.; Yoon, Y.; Kim, S.; Han, K.; Kim, E.-K. Deep Learning for the Detection of Breast Cancers on Chest Computed Tomography. Clin. Breast Cancer 2021, 22, 26–31.
- Zangheri, B.; Messa, C.; Picchio, M.; Gianolli, L.; Landoni, C.; Fazio, F. PET/CT and breast cancer. Euro. J. Nuclear Med. Mol. Imaging. 2004, 31, S135–S142.
- Sollini, M.; Cozzi, L.; Ninatti, G.; Antunovic, L.; Cavinato, L.; Chiti, A.; Kirienko, M. PET/CT radiomics in breast cancer: Mind the step. Methods 2020, 188, 122–132.
- Salaün, P.-Y.; Abgral, R.; Malard, O.; Querellou-Lefranc, S.; Quere, G.; Wartski, M.; Coriat, R.; Hindie, E.; Taieb, D.; Tabarin, A.; et al. Good clinical practice recommendations for the use of PET/CT in oncology. Eur. J. Nuclear Med. Mol. Imaging 2020, 47, 28–50.
- Yi, A.; Jang, M.-J.; Yim, D.; Kwon, B.R.; Shin, S.U.; Chang, J.M. Addition of Screening Breast US to Digital Mammography and Digital Breast Tomosynthesis for Breast Cancer Screening in Women at Average Risk. Radiology 2021, 298, 568–575.
- Spak, D.A.; Le-Petross, H.T. Screening Modalities for Women at Intermediate and High Risk for Breast Cancer. Curr. Breast Cancer Rep. 2019, 11, 111–116.
- Lee, T.C.; Reyna, C.; Shaughnessy, E.; Lewis, J.D. Screening of populations at high risk for breast cancer. J. Surg. Oncol. 2019, 120, 820–830.
- Shah, T.A.; Guraya, S.S. Breast cancer screening programs: Review of merits, demerits, and recent recommendations practiced across the world. J. Microsc. Ultrastruct. 2017, 5, 59–69.
- Nguyen, D.L.; Myers, K.S.; Oluyemi, E.; Mullen, L.A.; Panigrahi, B.; Rossi, J.; Ambinder, E.B. BI-RADS 3 Assessment on MRI: A Lesion-Based Review for Breast Radiologists. J. Breast Imaging 2022, wbac032.
- Daimiel Naranjo, I.; Gibbs, P.; Reiner, J.S.; Lo Gullo, R.; Thakur, S.B.; Jochelson, M.S.; Thakur, N.; Baltzer, P.A.T.; Helbich, T.H.; Pinker, K. Breast Lesion Classification with Multiparametric Breast MRI Using Radiomics and Machine Learning: A Comparison with Radiologists’ Performance. Cancers 2022, 14, 1743.
- Shimauchi, A.; Jansen, S.A.; Abe, H.; Jaskowiak, N.; Schmidt, R.A.; Newstead, G.M. Breast Cancers Not Detected at MRI: Review of False-Negative Lesions. Am. J. Roentgenol. 2010, 194, 1674–1679.
More
Information
Subjects:
Engineering, Biomedical; Primary Health Care
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
27 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




