Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Peiwen Zhang | -- | 2850 | 2022-07-25 10:31:37 | | | |
| 2 | Jessie Wu | -3 word(s) | 2847 | 2022-07-26 05:46:05 | | | | |
| 3 | Jessie Wu | Meta information modification | 2847 | 2022-07-26 05:47:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, P.; He, Y.; Wu, S.; Li, X.; Lin, X.; Gan, M.; Chen, L.; Zhao, Y.; Niu, L.; Zhang, S.; et al. White Fat Browning Associate with Obesity. Encyclopedia. Available online: https://encyclopedia.pub/entry/25486 (accessed on 08 February 2026).
Zhang P, He Y, Wu S, Li X, Lin X, Gan M, et al. White Fat Browning Associate with Obesity. Encyclopedia. Available at: https://encyclopedia.pub/entry/25486. Accessed February 08, 2026.
Zhang, Peiwen, Yuxu He, Shuang Wu, Xinrong Li, Xutao Lin, Mailin Gan, Lei Chen, Ye Zhao, Lili Niu, Shunhua Zhang, et al. "White Fat Browning Associate with Obesity" Encyclopedia, https://encyclopedia.pub/entry/25486 (accessed February 08, 2026).
Zhang, P., He, Y., Wu, S., Li, X., Lin, X., Gan, M., Chen, L., Zhao, Y., Niu, L., Zhang, S., Li, X., Zhu, L., & Shen, L. (2022, July 25). White Fat Browning Associate with Obesity. In Encyclopedia. https://encyclopedia.pub/entry/25486
Zhang, Peiwen, et al. "White Fat Browning Associate with Obesity." Encyclopedia. Web. 25 July, 2022.
Copy Citation
Obesity is negatively associated with the increase in social productivity and endangers people’s health. It is caused by the excessive energy consumption as well as the collection and an excessive level of triglycerides and lipids, which are stored in adipocytes. Studying the signal transduction pathways of the white fat browning might provide novel ideas for the treatment of obesity and alleviation of obesity-related glucose and lipid metabolism disorders.
obesity
brown fat
adipocytes
1. Types and Functions of Fat
Adipose tissues are the body’s most important metabolic and endocrine sites as well as the primary energy storage sites. Therefore, researchers are deeply concerned about fat formation and metabolism [1].
Fat is a tissue and an organ with secretory functions and has been roughly divided into white fat, brown fat, and ectopic deposited fat [2]. Hormones can be secreted by adipose tissue [3], regulatory factors [4], and exosomes [5], acting on other related organs or adipose tissues to regulate their metabolism and development [6]. Adipose tissue is derived from the mesoderm [7], which is divided into paraxial mesoderm and lateral mesoderm, producing white adipocytes and brown adipocytes; the latter is produced only by the paraxial mesoderm [8][9][10]. Both WAT and BAT have similar origins, but different developmental modes and morphologies (Figure 1). The progenitors of white, brown, and beige fat are mesenchymal stem cells with different molecular markers, e.g., the precursor cells of white fat are Myf5+ progenitors while brown fat progenitors are the opposite. In the presence of external stimuli, white adipose beige adipose can be interconverted under certain conditions. In contrast, brown fat is darker and more mitochondria-rich, beige fat is second to brown fat and white fat has the least mitochondria and is not thermogenic, as shown in the Figure 1.
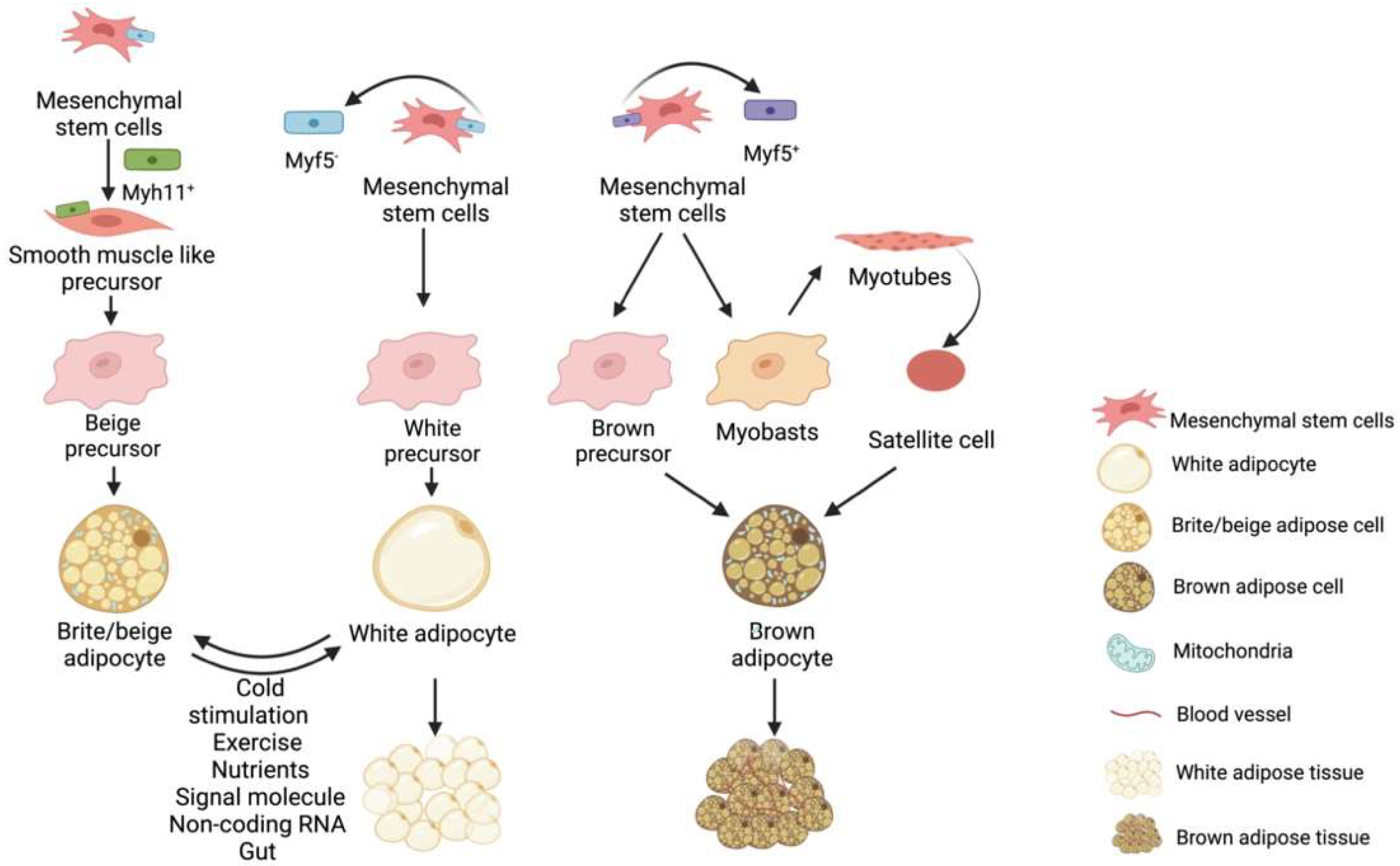
Figure 1. Brown fat, beige fat and white fat precursor cells are derived from different mesenchymal stem cells. Beige adipose precursor cells are derived from Myf5− derived mesenchymal stem cells differentiated into Myh11+ positive smooth muscle stem cells. White adipose precursor cells are derived from Myf5− mesenchymal stem cells, while brown adipose precursor cells are derived from Myf5+ mesenchymal stem cells. In addition, muscle satellite cells can also differentiate into brown adipocytes under certain conditions. Under certain conditions, white fat and beige fat can be converted into each other.
White adipose tissue appears and develops later than BAT. White adipocytes are differentiated after birth, while the brown adipocytes are differentiated during the embryonic stage [11]. Unlike rodents, human infants maintain their body temperature after birth mainly through heat production in the perinephric region and in the brown adipose tissue on the sides of the spine [12]. Moreover, the morphologies and biological functions performed by the WAT and BAT also differ [9]. White adipose tissue has a single large lipid droplet and fewer blood vessels than the brown adipose tissue. The WATs are stored in the animal body under the skin and around the organs and store excess energy in the body as triglycerides. Brown adipocytes have many small lipid droplets and small cell areas with dense mitochondrial distribution. The UCP-1 (uncoupling protein 1) mediates the heat-generating respiration [13]. The BATs are formed at the embryonic stage and are matured at birth. After birth, the WATs increase in size and number. The body temperature is maintained primarily in a non-shivering thermogenesis after birth [14]. The BATs are widely distributed throughout the body [15], including the interscapular area, mediastinum, kidney, adrenal fat areas, para-arterial area, and neck areas. The number of brown adipocytes gradually decreases with age and are first disappeared in the interscapular area (Figure 2). The BATs exist in the deep parts of the body for a long time before disappearing into the surface area. The BAT and WAT develop from the distinct differentiation precursors.
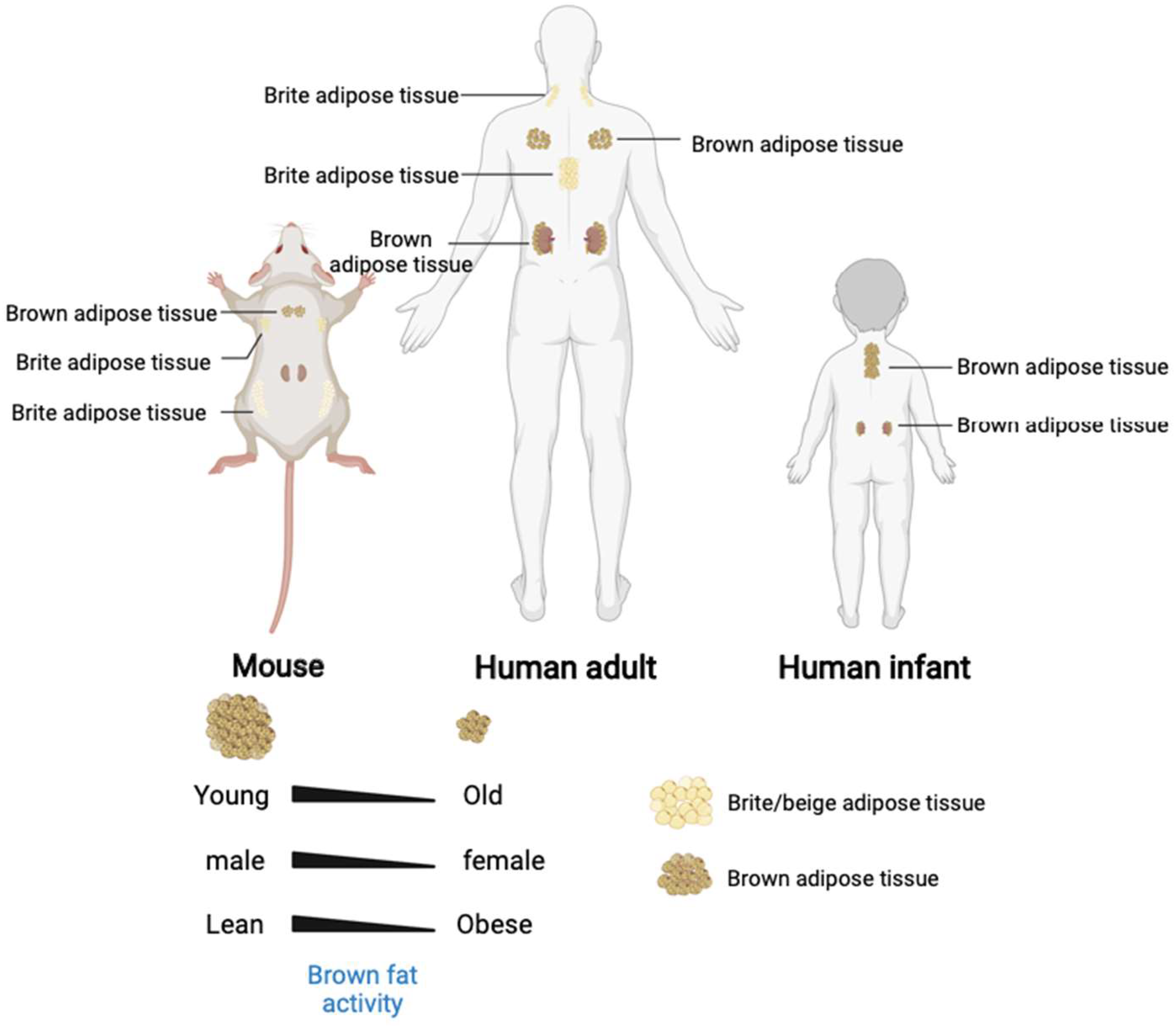
Figure 2. The brown and beige adipose tissue distribution in human and mouse. In addition, the brown adipose tissue activity and quantity decreased with age and associated with gender and stature.
Brown adipose tissue differentiation is closely linked to myogenic factor 5 (Myf5). The Myf5+ progenitor cells can be induced to differentiate into skeletal muscle cells, central rawhide sarcomere, and classic brown adipocytes [16]. White adipocytes are formed by the differentiation of the vascular and stromal layer by Myf5− [17]. Therefore, the metabolic properties of BAT are more like those of the skeletal muscle cells and are primarily reflected in its structure and mitochondrial abundance in two ways. It is worth mentioning that rodents have BATs throughout their lives [18] and have become the ideal research models. In recent years, researchers have focused on the thermogenesis mechanism of BATs for anti-obesity research. Numerous studies have shown that, under certain conditions, WATs and BATs are interconvertible [19]. Nuria et al. indicated that inhibiting the expression of MKK6 (mitogen-activated protein kinase kinase 6) in WAT could increase the level of oxidative phosphorylation of UCP-1 to promote the WATB [20].
Besides the traditional WATs and BATs, there is another type of fat known as a beige fat. Cold stimulation causes the brown-like fat cells to appear in some WATs in mammals. This process is called “white fat browning (WFB)” [21] (Figure 1). These brown-like fat cells in the mammals, which were induced by specific factors, were termed “beige fats” by researchers. The origins of classic BAT and beige adipose tissue are clearly different. The BATs are derived from the Myf5+ cells, while the beige adipocytes are formed by the trans-differentiation of WATs [22] or derived from Pdgfra+ fat precursor cells [23]. Furthermore, the gene expression markers, such as TbxI, Tmem26, and CD137, are reported in the beige fat [24]. In contrast to brown fat, beige fat cells are close in colour to white adipose tissue and beige fat only expresses thermogenic genes upon stimulation [25]. When beige fat is stimulated by external stimuli and starts to produce heat due to the activation of thermogenic genes such as UCP-1 by cAMP signalling, the morphology of lipid droplets of beige fat changes and gradually forms multi-lamellar adipocytes, which resemble brown adipocytes in morphology [26]. The functions of beige adipose tissue are like those of the BATs, which consume energy, improve metabolism, glucose tolerance, and insulin sensitivity, and alleviate metabolic diseases caused by the excessive WAT deposition [27][28]. As a result, encouraging the WFB might be treatment option for obesity and other obesity-related metabolic diseases.
2. Regulators of WFB
White fat browning is a relatively a complicated process. In addition to the environmental factors, nutrients, and body metabolites, other regulatory factors are also involved in the deposition and thermogenesis of brown fat, such as PRDM16, Zfp516, PGC-1α, PPARr, C/EBP-β family members, HSF1 and IRF4 regulators [29]. The injection of PPARγ agonists in mice could significantly promote the brown fat marker genes, such as UCP-1 and PGC1a (Table 1).
Table 1. Characteristics of brown, beige, and white fat cells.
| Brown | Beige | White | |
|---|---|---|---|
| Morphological |  |
 |
 |
| Origin cells | Myf5+ cells | Myf5− cells | Myf5− cells |
| Transcription factors | C/EBPβ; EBF2; PRDM16; UCP-2; PPARγ; PGC1α | C/EBPβ; EBF2; PRDM16; UCP-2; PPARγ; PGC1α | ZFP423; PPARγ |
| Marker genes | UCP-1; PGC1α; Dio2; Cidea; PPARα; Cox8b; Ppargc1a | UCP-1; PGC1α; Dio2; Cidea; PPARα; Cox8b; Ppargc2a; CD137; TMEM26 | Leptin; Fabp4; PPARγ; C/EBPβ |
| Activators | Cold; β3-AR; Exercise; NP; TH; FGF21; Bmp7; Bmp8bIrisin | Cold; β3-AR; Exercise; NP; TH; FGF21; Irisin |
The PPARγ is expressed in the mammalian adipose tissues, vascular smooth muscle tissues, and cardiac muscle tissues. The PPARγ is more abundantly expressed in WAT than the UCP-1 and is involved in regulating the proliferation and differentiation of adipose tissues [30][31]. In addition, PPARγ, in the activated state, could also lower blood glucose levels, increase insulin sensitivity, and reduce inflammatory response, thereby maintaining the normal metabolism in the body. Subsequently, studies reported that PPARγ had a positive regulatory role in inducing WFB based on its key role in regulating the proliferation and differentiation of adipose tissues and enhancing the insulin sensitivity. Both the SIRT1 and SIRT3 of the SIRT family of histone deacetylases could activate PPARγ by deacetylation; a process, which is also prone to β-adrenergic effects of norepinephrine. The factors PPARγ, PGC-1α, and PRDM16 could also play synergistic roles, acting as mutual coactivators, to promote the WFB-associated processes. Therefore, PPARγ has been recognized as a powerful regulator and agonist in WFB and its abnormal expression often leads to the different degrees of obesity syndrome. The PPARγ agonists, such as rosiglitazone, have been identified as potential browning agents and are widely used in clinical trials. Rosiglitazone belongs to the thiazolidinedione group of hypoglycaemic agents and is one of the insulin sensitizers [32], mainly activating a specific receptor in the body, which increases the sensitivity of the body to insulin, like muscle, liver and fat, in order to lower blood sugar and improve lipid metabolism [33]. Rosiglitazone also promotes the redistribution of fat in the body, causing visceral fat to shift under the skin, which may be related to the effect of increasing insulin sensitivity, as well as improving the beta-cell function of the pancreatic islets and treating fatty liver and obesity in combination with diabetes [34].
Uncoupling proteins (UCPs) are a family of transporter proteins, which are present in the inner mitochondrial membrane. Among them, UCP-1 is expressed only in the BAT and beige fat cells, and UCP-2 is expressed in many organs, while UCP-3 is expressed in the skeletal muscle cells. Because the β3-AR are found mainly in adipose tissues, their selective agonists stimulate the adipose thermogenesis as well as lipid mobilization in the WAT. Therefore, the role of UCP-1 in thermogenesis has been extensively studied. Its role is under the direct sympathetic control, exerting an uncoupled oxidative phosphorylation while increasing the ATP production in the mitochondria, which is mediated mainly through the β-adrenergic action of norepinephrine. In addition, UCP-1 also plays an important role in WFB, activating the regulation of mitochondrial function under cold stimulation or other external stimuli. The UCP-1 was significantly positively correlated with the mitochondrial content and respiration and upregulated the expression of Parkin-dependent and independent mitochondrial autophagy-associated marker genes. It could also regulate the expression levels of endolipin, lipocalin Cidea, and Nrg4, thereby showing a guiding and central role in the overall WFB and thermogenesis. Given these reasons, UCP-1 has been widely studied as a marker of BAT and WAT browning (As shown in Figure 3).
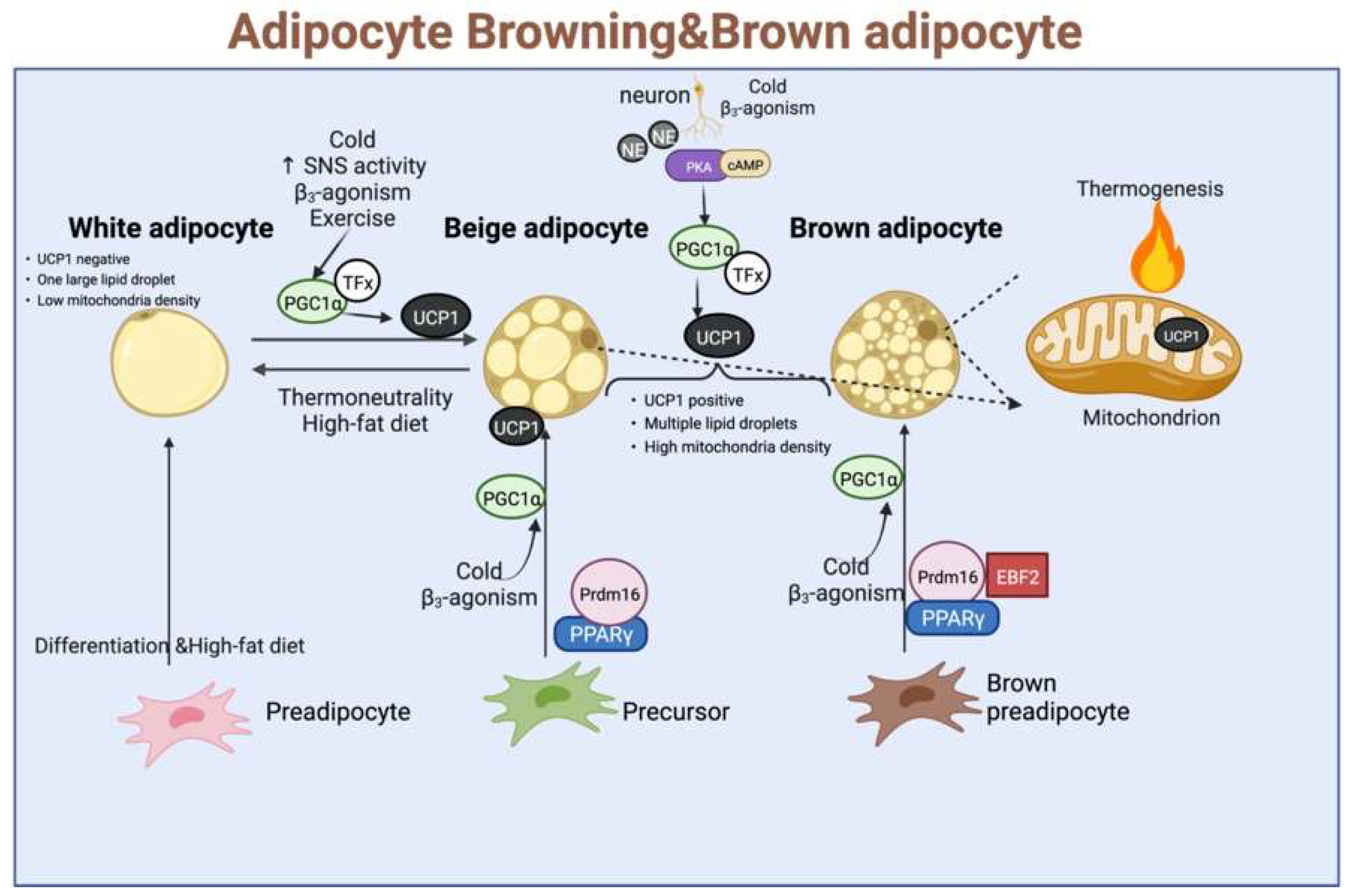
Figure 3. The classic regulatory model for the development of brown fat, beige fat, and white fat, as well as thermogenesis, involves stimulation by external conditions such as cold stimuli, catecholamines, norepinephrine, and exercise. TFx: Trans factors X, where UCP-1 is activated and enters the inner mitochondrial membrane to expend energy in the form of heat dissipation.
The SIRT1 protein, a member of the SIRT family, mainly regulates the post-transcriptional modifications and belongs to a family of histone deacetylases. In bacteria, yeasts, and mammalian cells, the SIRT family of proteins have a highly conserved similar catalytic core region. The SIRT1 is mainly localized in the nucleus. However, it can be induced by UCP-1 to migrate into the cytoplasm under cold stimulation, where it plays a regulatory role mainly through the lys268 and lys293 pathways. It can also deacetylate PPARγ to enhance the WFB activity and inhibit lipogenesis in beige adipose tissue as well as stimulate lipolysis. In addition, SIRT1 can also regulate the deacetylation of various proteins through metabolic pathways, such as AMPK, FOXO, and mTOR, which have indirect regulatory effects. Therefore, researchers refer to the correlations among SIRT1, PPARγ, PGC-1α, and AMPK as the SIRT1/PPARγ/PGC-1α and AMPK/SIRT1/PGC-1α pathways.
The PGC-1α is a transcriptional co-activator, mediating many biological responses related to energy metabolism [19]. In the past, PGC-1α was considered a powerful transcriptional co-activator of PPARγ and was also named PPARγ coactivator 1α due to its initial role as a PPARγ- interacting protein. However, subsequent studies revealed that PGC-1α could bind to a variety of transcription factors [35], including PPARγ, NRF, and MEF2C, thereby participating in the mitochondrial biosynthesis, adaptive thermogenesis, skeletal muscle type switching, fatty acid oxidation, oxidative stress, gluconeogenesis, and other metabolic processes. In general, the PGC-1α expression is low in WAT. However, in response to external stimuli, such as cold, the activation of sympathetic nerves and β-adrenaline, as well as the ectopic expression of PGC-1α, can significantly increase the expression of UCP-1 and other brown-specific genes in WAT, thereby directly or indirectly regulating the mitochondrial respiration and biosynthetic capacity as well as WFB. The extensive binding capacity of PGC-1α is indispensable for the promoting the WFB and its thermogenesis. Therefore, it has a high potential for clinical studies to be used as therapeutic target for drugs and agonists.
The regulatory factor PRDM16 is abundantly present in BAT and browning WAT [36][37], regulating the function of classical brown adipocytes and beige adipocytes. Numerous previous studies have reported the importance of PRDM16 in converting the WAT to beige adipocytes. The deletion of PRDM16 could induce muscle differentiation by decreasing the thermal gene expression and increasing the muscle-specific gene expression in BATs. Furthermore, PRDM16 could induce an almost complete brown fat genetic program, including the increased mitochondrial biogenesis, cellular respiration, and selective expression of brown fat-associated genes. The highly expressed PRDM16 can form a complex with zinc finger proteins and bind to the promoter region of UCP-1, thereby upregulating the UCP-1 transcription. The activated UCP-1 can block the phosphorylation process during ATP production. Then, the potential energy of the proton gradient no longer flows back down the ATP synthase but follows the UCP-1 uncoupling channel back to the mitochondrial matrix, releasing potential energy as heat energy [37][38]. In addition, PRDM16 can also interact with transcription factors, such as PPARα, PPARγ, and C/EBP family members, and cofactors, such as PGC-1α, and enhance their transcriptional activity, thereby playing an indirect role in the WFB.
White fat browning is a complex process, which is regulated by multiple nutrients and metabolites and affects the related signalling pathways. White fat browning is regulated by numerous regulatory factors, such as UCP-1, PPARγ, PRDM-16, and PGC-1α [39]. The classical theory of WFB is the excitation of sympathetic nervous system and activation of the β3-ARs signalling pathway induced by PRDM-16 and SIRT1, which exert deacetylation effects on PPARγ [40]. The latter process is dependent on the induction and translocation of the transcriptional co-activator PGC1α into the nucleus and its association with the gene promoter region. In addition to sympathetic nerves, cellular energy-sensing is a driving force in regulating browning by regulatory transcriptional networks, such as AMPK, which inhibits fat deposition and induces WFB by downregulating the ACC expression and inhibiting the gluconeogenesis, among other pathways [41]. Therefore, it is theoretically possible to treat a variety of obesity and other metabolic disorders by inducing and activating the production of beige fat to regulate the expression of thermogenesis-related genes.
3. White Fat Browning and Resistance to Obesity
Obesity has become a major public health problem worldwide [42], caused by genetic, environmental and social factors, with over two billion people worldwide currently obese. The excessive accumulation of visceral fat caused by obesity can lead to many chronic diseases, such as type 2 diabetes, atherosclerosis and polycystic ovary syndrome [43]. Obesity is caused by a chronic excess of energy intake, which is converted into triglycerides and stored in the adipose tissue [44]. Therefore, reversing obesity is about reversing the energy metabolic balance of the obese body. Most of the previous research in the treatment of obesity has focused on reducing the energy intake of obese individuals. However, as most obese people have insulin resistance which promotes appetite, restricting energy intake to achieve weight loss often leads to weight loss failure. In recent years, many studies have turned to investigating how to enhance the body’s energy expenditure for the purpose of weight loss [45][46]. Brown adipose tissue, an adipose tissue that oxidises fatty acids and thus burns energy, has emerged as a hot topic of research in this area [47]. In addition, beige adipocytes, which are of a completely different origin to brown adipose progenitor cells, have a similar function (Figure 1). Studies have shown that obese patients have significantly lower levels of brown fat and are less active than people with a normal BMI [28]. Therefore, the promotion of browning of white fat and the activation of brown fat activity in the body has become a focus of research in the field of resistance to obesity [46]. Brown adipose tissue is one of the most insulin-sensitive tissues and therefore enhances the body’s uptake of glucose to produce ATP, which can alleviate the ATP deficiency caused by oxidative phosphorylation coupling in the mitochondria [21]. It has been shown that cold stimulation can promote browning of white adipose to counteract the disruption of glucolipid metabolism in obese mice [48]. In addition, the transplantation of brown fat plays an active role in combating obesity and improving insulin sensitivity in mice [49]. In addition, transplantation of beige fat from humans into mice also improved energy metabolism in mice [50]. Interestingly, a recent study has shown that local heat therapy also activates the thermogenesis of beige adipose tissue to improve the body’s energy metabolism and combat obesity [51]. Recent studies have demonstrated that the effect of beige adipose tissue on energy metabolism in mice is less than the contribution of brown fat [52]. Therefore, a better understanding of the activators of brown fat as well as the browning agents of white adipose tissue is important to improve the disorders of glucolipid metabolism in obese patients.
References
- Cui, J.X.; Zeng, Q.F.; Chen, W.; Zhang, H.; Zeng, Y.Q. Analysis and preliminary validation of the molecular mechanism of fat deposition in fatty and lean pigs by high-throughput sequencing. Mamm. Genome 2019, 30, 71–80.
- Unger, R.H.; Clark, G.O.; Scherer, P.E.; Orci, L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. BBA—Mol. Cell Biol. Lipids 2010, 1801, 209–214.
- Feng, D.; Yao, H.; Xiaowei, H.E.; Yaxuan, D.U.; Zuo, X. Metabolic Regulation of Adipose Tissue on Reproduction. Feed Rev. 2012, 9, 13–16.
- Mazzone, T. Adipose Tissue and the Vessel Wall. Curr. Drug Targets 2007, 8, 1190–1195.
- Kita, S.; Maeda, N.; Shimomura, I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J. Clin. Investig. 2019, 129, 4041–4049.
- Zhao, Y.; Zhao, M.F.; Jiang, S.; Wu, J.; Liu, J.; Yuan, X.W.; Shen, D.; Zhang, J.Z.; Zhou, N.; He, J.; et al. Liver governs adipose remodelling via extracellular vesicles in response to lipid overload. Nat. Commun. 2020, 11, 719.
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10.
- Chau, Y.Y.; Bandiera, R.; Serrels, A.; Martínez-Estrada, O.M.; Qing, W.; Lee, M.; Slight, J.; Thornburn, A.; Berry, R.; McHaffie, S.; et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 2014, 16, 367–375.
- Saely, C.H.; Geiger, K.; Drexel, H. Brown versus white adipose tissue: A mini-review. Gerontology 2012, 58, 15–23.
- Westcott, G.P.; Emont, M.P.; Li, J.; Jacobs, C.; Tsai, L.; Rosen, E.D. Mesothelial cells are not a source of adipocytes in mice. Cell Rep. 2021, 36, 109388.
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental origin of fat: Tracking obesity to its source. Cell 2007, 131, 242–256.
- Rockstroh, D.; Landgraf, K.; Wagner, I.V.; Gesing, J.; Tauscher, R.; Lakowa, N.; Kiess, W.; Bühligen, U.; Wojan, M.; Till, H.; et al. Direct evidence of brown adipocytes in different fat depots in children. PLoS ONE 2015, 10, e0117841.
- Saito, S.; Saito, C.T.; Shingai, R. Adaptive evolution of the uncoupling protein 1 gene contributed to the acquisition of novel nonshivering thermogenesis in ancestral eutherian mammals. Gene 2008, 408, 37–44.
- Lidell, M.E. Brown Adipose Tissue in Human Infants. Handb. Exp. Pharmacol. 2019, 251, 107–123.
- Alkhawaldeh, K.; Alavi, A. Quantitative assessment of FDG uptake in brown fat using standardized uptake value and dual-time-point scanning. Clin. Nucl. Med. 2008, 33, 663–667.
- Martinez-Lopez, N.; Athonvarangkul, D.; Sahu, S.; Coletto, L.; Zong, H.; Bastie, C.C.; Pessin, J.E.; Schwartz, G.J.; Singh, R. Autophagy in Myf5+ progenitors regulates energy and glucose homeostasis through control of brown fat and skeletal muscle development. EMBO Rep. 2013, 14, 795–803.
- Shan, T.; Liang, X.; Bi, P.; Zhang, P.; Liu, W.; Kuang, S. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J. Lipid Res. 2013, 54, 2214–2224.
- Loncar, D. Development of thermogenic adipose tissue. Int. J. Dev. Biol. 1991, 35, 321–333.
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468.
- Matesanz, N.; Bernardo, E.; Acín-Pérez, R.; Manieri, E.; Pérez-Sieira, S.; Hernández-Cosido, L.; Montalvo-Romeral, V.; Mora, A.; Rodríguez, E.; Leiva-Vega, L.; et al. MKK6 controls T3-mediated browning of white adipose tissue. Nat. Commun. 2017, 8, 856.
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702.
- Gulyaeva, O.; Dempersmier, J.; Sul, H.S. Genetic and epigenetic control of adipose development. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 3–12.
- Oguri, Y.; Shinoda, K.; Kim, H.; Alba, D.L.; Bolus, W.R.; Wang, Q.; Brown, Z.; Pradhan, R.N.; Tajima, K.; Yoneshiro, T.; et al. CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling. Cell 2020, 182, 563–577.e520.
- Li, H.; Zhang, Y.; Wang, F.; Donelan, W.; Zona, M.C.; Li, S.; Reeves, W.; Ding, Y.; Tang, D.; Yang, L. Effects of irisin on the differentiation and browning of human visceral white adipocytes. Am. J. Transl. Res. 2019, 11, 7410–7421.
- Wu, J.; Bostrm, P.; Sparks, L.; Li, Y.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.; Nuutila, P.; Schaart, G. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376.
- Shabalina, I.G.; Petrovic, N.; de Jong, J.M.; Kalinovich, A.V.; Cannon, B.; Nedergaard, J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013, 5, 1196–1203.
- Rui, L. Brown and Beige Adipose Tissues in Health and Disease. Compr. Physiol. 2017, 7, 1281–1306.
- Betz, M.J.; Enerbäck, S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat. Rev. Endocrinol. 2018, 14, 77–87.
- Lo, K.A.; Sun, L. Turning WAT into BAT: A review on regulators controlling the browning of white adipocytes. Biosci. Rep. 2013, 33, 711–719.
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809.
- Gregoire, F.M. Adipocyte differentiation: From fibroblast to endocrine cell. Exp. Biol. Med. 2001, 226, 997–1002.
- Wagstaff, A.J.; Goa, K.L. Rosiglitazone: A review of its use in the management of type 2 diabetes mellitus. Drugs 2002, 62, 1805–1837.
- Morley, L.C.; Tang, T.; Yasmin, E.; Norman, R.J.; Balen, A.H. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst. Rev. 2017, 11, Cd003053.
- Arya, S.; Hansen, K.R.; Wild, R.A. Metformin, rosiglitazone, or both for obese women with polycystic ovary syndrome? Fertil. Steril. 2020, 113, 87–88.
- Haemmerle, G.; Moustafa, T.; Woelkart, G.; Büttner, S.; Schmidt, A.; van de Weijer, T.; Hesselink, M.; Jaeger, D.; Kienesberger, P.C.; Zierler, K.; et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 2011, 17, 1076–1085.
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967.
- Kajimura, S.; Seale, P.; Kubota, K.; Lunsford, E.; Frangioni, J.V.; Gygi, S.P.; Spiegelman, B.M. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 2009, 460, 1154–1158.
- Becerril, S.; Gómez-Ambrosi, J.; Martín, M.; Moncada, R.; Sesma, P.; Burrell, M.A.; Frühbeck, G. Role of PRDM16 in the activation of brown fat programming. Relevance to the development of obesity. Histol. Histopathol. 2013, 28, 1411–1425.
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54.
- Dixen, K.; Basse, A.L.; Murholm, M.; Isidor, M.S.; Hansen, L.H.; Petersen, M.C.; Madsen, L.; Petrovic, N.; Nedergaard, J.; Quistorff, B.; et al. ERRγ enhances UCP1 expression and fatty acid oxidation in brown adipocytes. Obesity 2013, 21, 516–524.
- Desjardins, E.M.; Steinberg, G.R. Emerging Role of AMPK in Brown and Beige Adipose Tissue (BAT): Implications for Obesity, Insulin Resistance, and Type 2 Diabetes. Curr. Diabetes Rep. 2018, 18, 80.
- Bombak, A. Obesity, health at every size, and public health policy. Am. J. Public Health 2014, 104, e60–e67.
- Puhl, R.M.; Heuer, C.A. Obesity stigma: Important considerations for public health. Am. J. Public Health 2010, 100, 1019–1028.
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts 2017, 10, 207–215.
- Johnstone, A. Fasting for weight loss: An effective strategy or latest dieting trend? Int. J. Obes. 2015, 39, 727–733.
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956.
- Marlatt, K.L.; Ravussin, E. Brown Adipose Tissue: An Update on Recent Findings. Curr. Obes. Rep. 2017, 6, 389–396.
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat. Protoc. 2012, 7, 606–615.
- Villarroya, F.; Giralt, M. The Beneficial Effects of Brown Fat Transplantation: Further Evidence of an Endocrine Role of Brown Adipose Tissue. Endocrinology 2015, 156, 2368–2370.
- Min, S.Y.; Kady, J.; Nam, M.; Rojas-Rodriguez, R.; Berkenwald, A.; Kim, J.H.; Noh, H.L.; Kim, J.K.; Cooper, M.P.; Fitzgibbons, T.; et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat. Med. 2016, 22, 312–318.
- Li, Y.; Wang, D.; Ping, X.; Zhang, Y.; Zhang, T.; Wang, L.; Jin, L.; Zhao, W.; Guo, M.; Shen, F.; et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell 2022, 185, 949–966.e919.
- Challa, T.D.; Dapito, D.H.; Kulenkampff, E.; Kiehlmann, E.; Moser, C.; Straub, L.; Sun, W.; Wolfrum, C. A Genetic Model to Study the Contribution of Brown and Brite Adipocytes to Metabolism. Cell Rep. 2020, 30, 3424–3433.e3424.
More
Information
Subjects:
Endocrinology & Metabolism
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
3 times
(View History)
Update Date:
26 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




