
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lory S. Rochín Hernández | -- | 1953 | 2022-07-19 20:11:22 | | | |
| 2 | Amina Yu | + 5 word(s) | 1958 | 2022-07-22 04:56:12 | | |
Video Upload Options
Endophytes, microorganisms that live in the internal tissues and organs of plants, are known to produce numerous bioactive compounds, including, at times, some phytochemicals of their host plant. So, endophytes have been quoted as a potential source for discovering bioactive compounds, particularly, of medical interest, including compounds that inhibit the formation or prevent an excessive accumulation of Advanced glycation end products (AGEs). The high levels of AGEs in body tissues are linked with the pathogenesis and development of some non-communicable diseases (NCDs) that are threatening global human health, noticeably: diabetes, neurodegenerative diseases, cancer, and other ailments linked to chronic inflammation and ageing. For that reason, endophytes as a source of compounds able to reduce AGEs could represent a possible treatment alternative for some NCDs.
1. Endophytes, an Exceptional Source of Bioactive Compounds
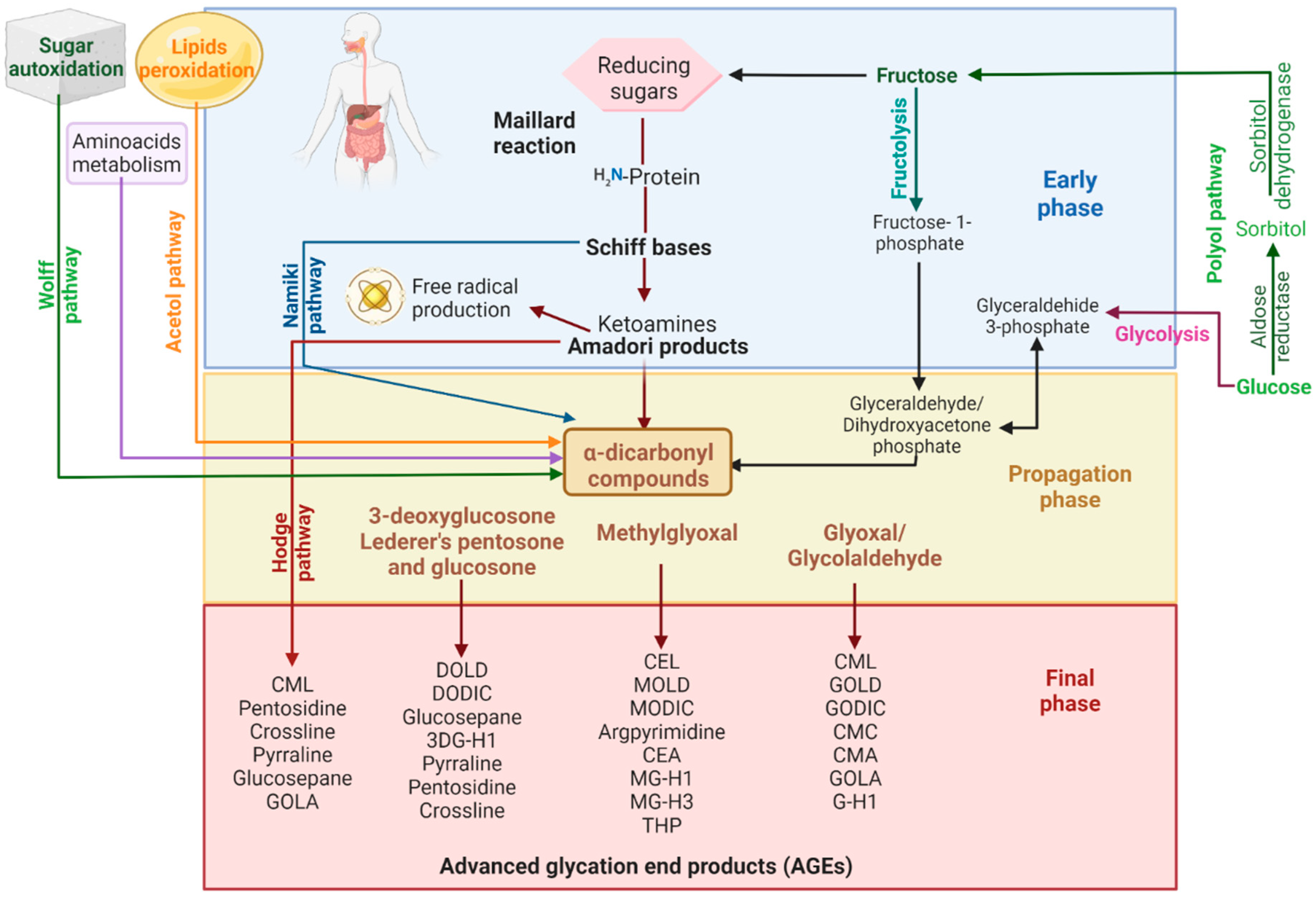
2. Advanced Glycation End Products
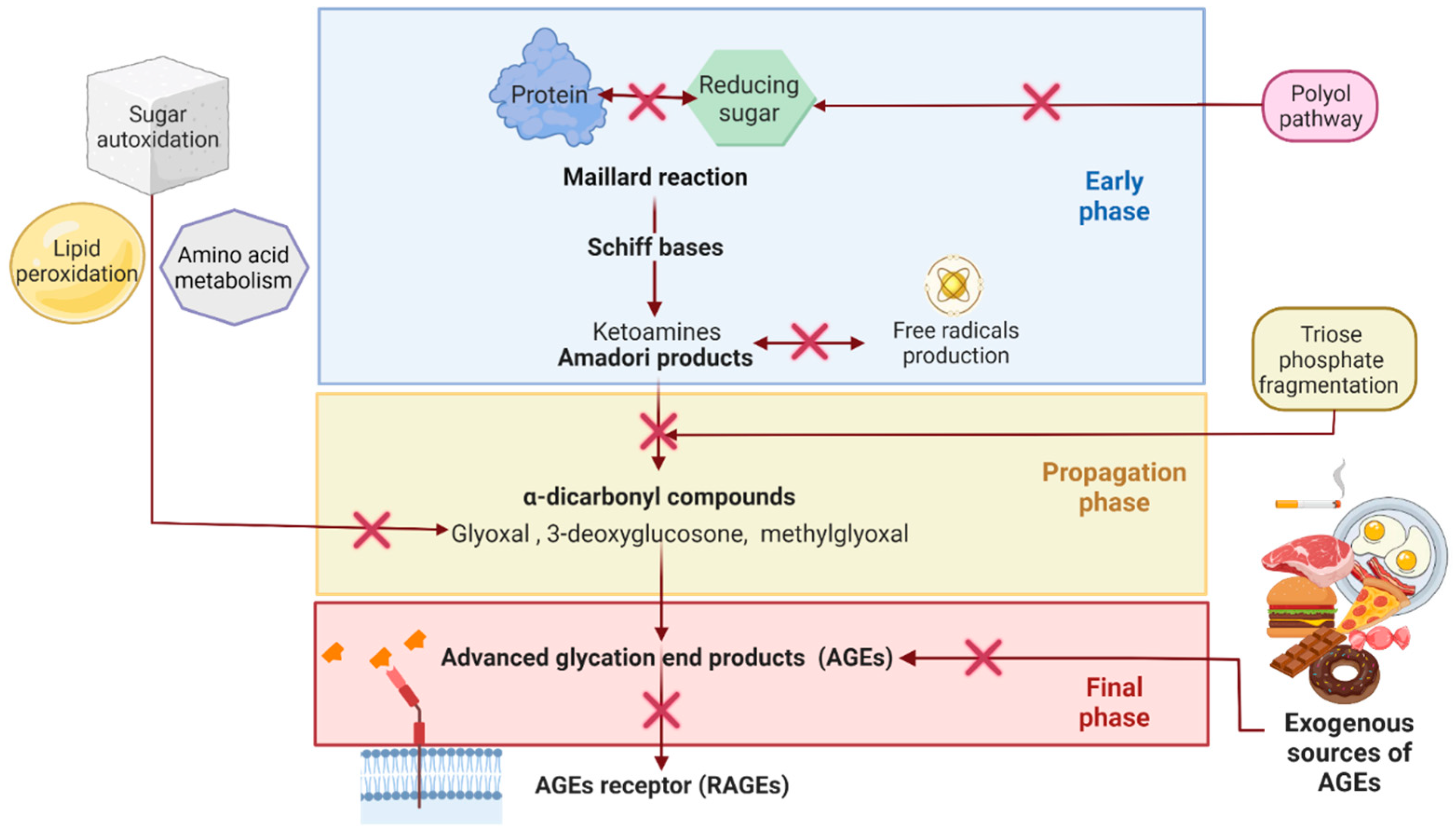
2.2. Reducing AGEs Accumulation as a Potential Treatment Strategy for Some NCDs
-
Blocking the carbonyl groups of reducing sugars or stabilizing the protein structure to inhibit the Maillard reaction or the formation of Schiff bases and Amadori products;
-
Scavenging of free radicals and chelating metal ions. Consequently, fewer reactive carbonyl groups and fewer radical-based reactions occur;
-
Blocking or breaking the AGEs cross-links to lessen protein aggregation;
-
Disrupting the AGEs–RAGE interaction, thus preventing inflammatory process and oxidative stress;
-
Some indirect mechanisms may be stimulating the glyoxalase system and other dicarbonyl detoxification systems to reduce the available AGEs precursors. Inhibition of polyol pathway enzymes (aldose reductase and sorbitol dehydrogenase) to reduce fructose intake and hypoglycemic activity to reduce sugar availability and so on. [37][39].
2.3. Synthetic AntiAGEs Compounds
3. Plant antiAGEs Compounds Have Also Been Reported in Endophytes
Endophytes are a rich source of a wide variety of chemical compounds such as alkaloids, phenols, tannins, amino acids, carbohydrates, saponins, terpenes, flavonoids, and sterols [49]. Various metabolites and crude extracts of endophytes have shown antioxidant activity, which is known to inhibit the formation of AGEs [50][51]. Gutiérrez-García et al. [52] explored the antiAGEs compounds produced by endophytes from Piper auritum. They found that 2,4-diacetylphloroglucinol (DAPG) and congeners such as 5-hydroxyferulic acid synthesized by endophytic Pseudomonas strains inhibit, in vitro, the formation of Amadori products and fluorescent-AGEs.
Natural antiAGEs compounds have been studied and found primarily in plants. However, some of these plant-derived antiAGEs compounds have also been reported as metabolites synthesized by endophytes [53][54][44][55]. Some of this compounds are protocatechuic acid [56][57], gallic acid [58][59][60][61], coumaric acid [60][61], caffeic acid [58][60][61][62][63][64][65], ferulic acid [56][58][61][62], rosmarinic acid [58][61][66], and chlorogenic acid [60][61][63], apigenin and derivatives such as vitexin and isovitexin [58][61][64][67][68][69], kaempferol and derivatives [65][70][71], luteolin [58][61][72], quercetin and derivatives [61][62][64][65][72], catechin [58], daidzein [73], genistein [64][73], icariin [58], rutin and derivatives [58][61][65], resveratrol, [74][75][76][77][78], tyrosol [79][80][81][82], ellagic acid [60][65], and 2,4-diacetyl-phloroglucinol [52], ginsenosides (Rb, Rd, Rg) [83][84][85], tanshinones [86], and stigmasterol [87], emodin [88][89][90], umbelliferone [60], hypericin [90] and matrine [91].References
- Stone, J.K.; Bacon, C.W.; White, J.F. An overview of endophytic microbes: Endophytism defined. In Microbial endophytes; Marcel Dekker: New York, NY, USA, 2000; pp. 29–33.
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end products: A review. Diabetologia 2001, 44, 129–146. https://doi.org/10.1007/s001250051591.
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. https://doi.org/10.1128/MMBR.00050-14.
- Kloepper, J.W.; McInroy, J.A.; Liu, K.; Hu, C.-H. Symptoms of fern distortion syndrome resulting from inoculation with opportunistic endophytic fluorescent Pseudomonas spp. PLoS ONE 2013, 8, e58531. https://doi.org/10.1371/journal.pone.0058531.
- Hsieh, C.-W.; Chuang, Y.-Y.; Lee, M.-Z.; Kirschner, R. First inventory of fungi in symptomless and symptomatic Chinese mesona indicates phytopathological threat. Plant Dis. 2020, 104, 2391–2397. https://doi.org/10.1094/PDIS-03-20-0475-RE.
- Frank, A.; Saldierna Guzmán, J.; Shay, J. Transmission of bacterial endophytes. Microorganisms 2017, 5, 70. https://doi.org/10.3390/microorganisms5040070.
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. https://doi.org/10.1016/j.soilbio.2009.11.024.
- Papik, J.; Folkmanova, M.; Polivkova-Majorova, M.; Suman, J.; Uhlik, O. The invisible life inside plants: Deciphering the riddles of endophytic bacterial diversity. Biotechnol. Adv. 2020, 44, 107614. https://doi.org/10.1016/j.biotechadv.2020.107614.
- Verma, S.K.; Sahu, P.K.; Kumar, K.; Pal, G.; Gond, S.K.; Kharwar, R.N.; White, J.F. Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J. Appl. Microbiol. 2021, 131, jam.15111. https://doi.org/10.1111/jam.15111.
- Aly, A.H.; Debbab, A.; Kjer, J.; Proksch, P. Fungal endophytes from higher plants: A prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 2010, 41, 1–16. https://doi.org/10.1007/s13225-010-0034-4.
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. https://doi.org/10.3389/fmicb.2018.02732.
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. https://doi.org/10.1128/MMBR.67.4.491-502.2003.
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites (1987 to 2000). Nat. Prod. Rep. 2001, 18, 448–459. https://doi.org/10.1039/b100918o.
- Singh, A.; Singh, D.K.; Kharwar, R.N.; White, J.F.; Gond, S.K. Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: Insights, avenues, and challenges. Microorganisms 2021, 9, 197. https://doi.org/10.3390/microorganisms9010197.
- Mishra, S.; Sahu, P.K.; Agarwal, V.; Singh, N. Exploiting endophytic microbes as micro-factories for plant secondary metabolite production. Appl. Microbiol. Biotechnol. 2021, 105, 6579–6596. https://doi.org/10.1007/s00253-021-11527-0.
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116. https://doi.org/10.1042/bj3440109.
- Ahmed, N. Advanced glycation end products—role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. https://doi.org/10.1016/j.diabres.2004.09.004.
- Kalapos, M.P. Methylglyoxal and glucose metabolism: A historical perspective and future avenues for research. Drug Metabol. Drug Interact. 2008, 23, 69–92. https://doi.org/10.1515/DMDI.2008.23.1-2.69.
- Henning, C.; Glomb, M.A. Pathways of the Maillard reaction under physiological conditions. Glycoconj. J. 2016, 33, 499–512. https://doi.org/10.1007/s10719-016-9694-y.
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461. https://doi.org/10.1152/physrev.00001.2019.
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced glycation end products (AGEs): Biochemistry, signaling, analytical methods, and epigenetic effects. Oxid. Med. Cell. Longev. 2020, 1-18. https://doi.org/10.1155/2020/3818196.
- Sourris, K.C.; Watson, A.; Jandeleit-Dahm, K. Inhibitors of advanced glycation end product (AGE) formation and accumulation. In Reactive Oxygen Species; Schmidt, H.H.H.W., Ghezzi, P., Cuadrado, A., Eds.; Handbook of experimental pharmacology; Springer International Publishing: Cham, Switzerland, 2020; Volume 264, pp 395–423. https://doi.org/10.1007/164_2020_391.
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. https://doi.org/10.1016/j.jada.2010.03.018.
- Cerami, C.; Founds, H.; Nicholl, I.; Mitsuhashi, T.; Giordano, D.; Vanpatten, S.; Lee, A.; Al-Abed, Y.; Vlassara, H.; Bucala, R.; et al. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA 1997, 94, 13915–13920. https://doi.org/10.1073/pnas.94.25.13915.
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. https://doi.org/10.1016/j.biopha.2021.111750.
- Brownlee, M. Negative consequences of glycation. Metabolism 2000, 49, 9–13.
- Shen, C.-Y.; Lu, C.-H.; Wu, C.-H.; Li, K.-J.; Kuo, Y.-M.; Hsieh, S.-C.; Yu, C.-L. The development of Maillard reaction, and advanced glycation end product (AGE)-Receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related diseases. Molecules 2020, 25, 5591. https://doi.org/10.3390/molecules25235591.
- Goh, S.-Y.; Cooper, M.E. The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. https://doi.org/10.1210/jc.2007-1817.
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1. https://doi.org/10.4196/kjpp.2014.18.1.1.
- Groener, J.B.; Oikonomou, D.; Cheko, R.; Kender, Z.; Zemva, J.; Kihm, L.; Muckenthaler, M.; Peters, V.; Fleming, T.; Kopf, S.; et al. Methylglyoxal and advanced glycation end products in patients with diabetes—What we know so far and the missing links. Exp. Clin. Endocrinol. Diabetes 2019, 127, 497–504. https://doi.org/10.1055/s-0043-106443.
- Li, J.; Liu, D.; Sun, L.; Lu, Y.; Zhang, Z. Advanced glycation end products and neurodegenerative diseases: Mechanisms and perspective. J. Neurol. Sci. 2012, 317, 1–5. https://doi.org/10.1016/j.jns.2012.02.018.
- Tarannum, Akhlas; Arif, Zarina; Alam, Khursheed; Ahmad, Shafeeque; Uddin, Moin. Nitroxidized-albumin advanced glycation end product and rheumatoid arthritis. Arch. Rheumatol. 2019, 34, 461–475. https://doi.org/10.5606/ArchRheumatol.2019.7285.
- de Leeuw, K.; Graaff, R.; de Vries, R.; Dullaart, R.P.; Smit, A.J.; Kallenberg, C.G.; Bijl, M. Accumulation of advanced glycation endproducts in patients with systemic lupus erythematosus. Rheumatology 2007, 46, 1551–1556. https://doi.org/10.1093/rheumatology/kem215.
- Papagrigoraki, A.; Maurelli, M.; del Giglio, M.; Gisondi, P.; Girolomoni, G. Advanced glycation end products in the pathogenesis of psoriasis. Int. J. Mol. Sci. 2017, 18, 2471. https://doi.org/10.3390/ijms18112471.
- Prasad, K.; Bhanumathy, K.K. AGE-RAGE axis in the pathophysiology of chronic lower limb ischemia and a novel strategy for its treatment. Int. J. Angiol. 2020, 29, 156–167. https://doi.org/10.1055/s-0040-1710045.
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2019, 148, 54–63. https://doi.org/10.1016/j.diabres.2018.11.016.
- Jahan, H.; Choudhary, I. Glycation, carbonyl stress and AGEs inhibitors: A patent review. Expert Opin. Ther. Pat. 2015, pp 1267–1284.
- Palimeri, S.; Diamanti-Kandarakis, E.; Palioura, E. Current perspectives on the health risks associated with the consumption of advanced glycation end products: Recommendations for dietary management. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 415. https://doi.org/10.2147/DMSO.S63089.
- Aldini, G.; Vistoli, G.; Stefek, M.; Chondrogianni, N.; Grune, T.; Sereikaite, J.; Sadowska-Bartosz, I.; Bartosz, G. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radic. Res. 2013, 47 (suppl. 1), 93–137. https://doi.org/10.3109/10715762.2013.792926.
- FDA. FDA Approves Oral Form for the Treatment of Adults with Amyotrophic Lateral Sclerosis (ALS). FDA 2022. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-oral-form-treatment-adults-amyotrophic-lateral-sclerosis-als (accessed on 29 May 2022).
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. https://doi.org/10.1021/acs.jnatprod.9b01285.
- Chinchansure, A.A.; Korwar, A.M.; Kulkarni, M.J.; Joshi, S.P. Recent Development of plant products with anti-glycation activity: A review. RSC Adv. 2015, 5, 31113–31138. https://doi.org/10.1039/C4RA14211J.
- Sadowska-Bartosz, I.; Bartosz, G. Prevention of Protein glycation by natural compounds. Molecules 2015, 20, 3309–3334. https://doi.org/10.3390/molecules20023309.
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: A comprehensive review. Food Res. Int. 2020, 130, 108933. https://doi.org/10.1016/j.foodres.2019.108933.
- Parveen, A.; Sultana, R.; Lee, S.M.; Kim, T.H.; Kim, S.Y. Phytochemicals against antidiabetic complications: Targeting the advanced glycation end product signaling pathway. Arch. Pharm. Res. 2021, 44, 378–401. https://doi.org/10.1007/s12272-021-01323-9.
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally occurring inhibitors against the formation of advanced glycation end products. Food Funct. 2011, 2, 289. https://doi.org/10.1039/c1fo10034c.
- Velichkova, S.; Foubert, K.; Pieters, L. Natural products as a source of inspiration for novel inhibitors of advanced glycation end products (AGEs) formation. Planta Med. 2021, 87, 780–801. https://doi.org/10.1055/a-1527-7611.
- Zhao, J.; Shan, T.; Mou, Y.; Zhou, L. Plant-derived bioactive compounds produced by endophytic fungi. Mini-Rev. Med. Chem. 2011, 11, 159–168. https://doi.org/10.2174/138955711794519492.
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. https://doi.org/10.3389/fmicb.2016.01538.
- Toghueo, R.M.; Boyom, F.F. Endophytes from ethno-pharmacological plants: Sources of novel antioxidants- a systematic review. Biocatal. Agric. Biotechnol. 2019, 22, 101430. https://doi.org/10.1016/j.bcab.2019.101430.
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A. Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs. metalloproteinases inhibition. Crit. Rev. Food Sci. Nutr. 2018, 58, 893–904. https://doi.org/10.1080/10408398.2016.1229657.
- Gutiérrez-García, K.; Neira-González, A.; Pérez-Gutiérrez, R.M.; Granados-Ramírez, G.; Zarraga, R.; Wrobel, K.; Barona-Gómez, F.; Flores-Cotera, L.B. Phylogenomics of 2,4-diacetylphloroglucinol-producing Pseudomonas and novel antiglycation endophytes from Piper auritum. J. Nat. Prod. 2017, 80, 1955–1963. https://doi.org/10.1021/acs.jnatprod.6b00823.
- West, B.J.; Deng, S.; Uwaya, A.; Isami, F.; Abe, Y.; Yamagishi, S.; Jensen, C.J. Iridoids are natural glycation inhibitors. Glycoconj. J. 2016, 33, 671–681. https://doi.org/10.1007/s10719-016-9695-x.
- Vijaykrishnaraj, M.; Wang, K. Dietary natural products as a potential inhibitor towards advanced glycation end products and hyperglycemic complications: A phytotherapy approaches. Biomed. Pharmacother. 2021, 144, 112336. https://doi.org/10.1016/j.biopha.2021.112336.
- Wan-Ju Yeh; Shih-Min Hsia; Wei-Hwa Lee; Chi-Hao Wu; Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. Journal of Food and Drug Analysis 2016, 25, 84-92, 10.1016/j.jfda.2016.10.017.
- Shaaban, M.; Abdel-Razek, A.S.; Previtali, V.; Clausen, M.H.; Gotfredsen, C.H.; Laatsch, H.; Ding, L. Sulochrins and alkaloids from a fennel endophyte Aspergillus sp. FVL2. Nat. Prod. Res. 2021, 2021, 1–11. https://doi.org/10.1080/14786419.2021.2005054.
- Pan, F.; Su, T.-J.; Cai, S.-M.; Wu, W. Fungal endophyte-derived Fritillaria unibracteata var. Wabuensis: Diversity, antioxidant capacities in vitro and relations to phenolic, flavonoid or saponin compounds. Sci. Rep. 2017, 7, 42008. https://doi.org/10.1038/srep42008.
- Qi, F.-H.; Jing, T.-Z.; Wang, Z.-X.; Zhan, Y.-G. Fungal endophytes from Acer ginnala Maxim: Isolation, identification and their yield of gallic acid. Lett. Appl. Microbiol. 2009, 49, 98–104. https://doi.org/10.1111/j.1472-765X.2009.02626.x.
- Singh, B.; Sharma, P.; Kumar, A.; Chadha, P.; Kaur, R.; Kaur, A. Antioxidant and in vivo genoprotective effects of phenolic compounds identified from an endophytic Cladosporium velox and their relationship with its host plant Tinospora Cordifolia. J. Ethnopharmacol. 2016, 194, 450–456. https://doi.org/10.1016/j.jep.2016.10.018.
- Parvandi, M.; Rezadoost, H.; Farzaneh, M. Introducing Alternaria tenuissima SBUp1, as an endophytic fungus of Ferula assa-foetida from Iran, which is a rich source of rosmarinic acid. Lett. Appl. Microbiol. 2021, 73, 569–578. https://doi.org/10.1111/lam.13542.
- Das, M.; Prakash, H.S.; Nalini, M.S. Bioactive sesquiterpene, plasticizer, and phenols from the fungal endophytes of Polygonum chinense L. Ann. Microbiol. 2018, 68, 595–609. https://doi.org/10.1007/s13213-018-1367-6.
- Wang, X.; Qin, L.; Zhou, J.; Li, Y.; Fan, X. A novel design to screen chlorogenic acid-producing microbial strains from the environment. Sci. Rep. 2018, 8, 14756. https://doi.org/10.1038/s41598-018-32968-0.
- Gagana, S.L.; Kumaraswamy, B.E.; Shivanna, M.B. Diversity, antibacterial and antioxidant activities of the fungal endophytes associated with Schleichera oleosa (Lour.) Merr. South Afr. J. Bot. 2020, 134, 369–381. https://doi.org/10.1016/j.sajb.2020.06.012.
- Kaur, N.; Arora, D.S.; Kalia, N.; Kaur, M. Antibiofilm, antiproliferative, antioxidant and antimutagenic activities of an endophytic fungus Aspergillus fumigatus from Moringa oleifera. Mol. Biol. Rep. 2020, 47, 2901–2911. https://doi.org/10.1007/s11033-020-05394-7.
- Chen, X.; Sang, X.; Li, S.; Zhang, S.; Bai, L. Studies on a chlorogenic acid-producing endophytic fungi isolated from Eucommia ulmoides Oliver. J. Ind. Microbiol. Biotechnol. 2010, 37, 447–454. https://doi.org/10.1007/s10295-010-0690-0.
- Gu, C.B.; Ma, H.; Ning, W.J.; Niu, L.L.; Han, H.Y.; Yuan, X.H.; Fu, Y.J. Characterization, culture medium optimization and antioxidant activity of an endophytic vitexin-producing fungus Dichotomopilus funicola Y3 from pigeon pea [Cajanus cajan (L.) Millsp.]. J. Appl. Microbiol. 2018, 125, 1054–1065. https://doi.org/10.1111/jam.13928.
- Tang, P.; Zhang, Z.; Niu, L.; Gu, C.; Zheng, W.; Cui, H.; Yuan, X. Fusarium solani G6, a novel vitexin-producing endophytic fungus: Characterization, yield improvement and osteoblastic proliferation activity. Biotechnol. Lett. 2021, 43, 1371–1383. https://doi.org/10.1007/s10529-021-03118-w.
- Gao, Y.; Zhao, J.; Zu, Y.; Fu, Y.; Liang, L.; Luo, M.; Wang, W.; Efferth, T. Antioxidant properties, superoxide dismutase and glutathione reductase activities in HepG2 cells with a fungal endophyte producing apigenin from pigeon pea [Cajanus cajan (L.) Millsp.]. Food Res. Int. 2012, 49, 147–152. https://doi.org/10.1016/j.foodres.2012.08.001.
- Huang, J.-X.; Zhang, J.; Zhang, X.-R.; Zhang, K.; Zhang, X.; He, X.-R. Mucor fragilis as a novel source of the key pharmaceutical agents podophyllotoxin and kaempferol. Pharm. Biol. 2014, 52, 1237–1243. https://doi.org/10.3109/13880209.2014.885061.
- Tijith, K.G.; Joy, A.; Divya, K.; Jisha, M.S. In vitro and in silico docking studies of antibacterial compounds derived from endophytic Penicillium setosum. Microb. Pathog. 2019, 131, 87–97. https://doi.org/10.1016/j.micpath.2019.03.033.
- Ebada, S.S.; Eze, P.; Okoye, F.B.C.; Esimone, C.O.; Proksch, P. The fungal endophyte Nigrospora oryzae produces quercetin monoglycosides previously known only from plants. ChemistrySelect 2016, 1, 2767–2771. https://doi.org/10.1002/slct.201600478.
- Hsieh, P.-W.; Hsu, L.-C.; Lai, C.-H.; Wu, C.-C.; Hwang, T.-L.; Lin, Y.-K.; Wu, Y.-C. Evaluation of the bioactivities of extracts of endophytes isolated from Taiwanese herbal plants. World J. Microbiol. Biotechnol. 2009, 25, 1461–1469. https://doi.org/10.1007/s11274-009-0036-0.
- Shi, J.; Zeng, Q.; Liu, Y.; Pan, Z. Alternaria sp. MG1, a resveratrol-producing fungus: Isolation, identification, and optimal cultivation conditions for resveratrol production. Appl. Microbiol. Biotechnol. 2012, 95, 369–379. https://doi.org/10.1007/s00253-012-4045-9.
- Dwibedi, V.; Saxena, S. Arcopilus aureus, a resveratrol-producing endophyte from Vitis vinifera. Appl. Biochem. Biotechnol. 2018, 186, 476–495. https://doi.org/10.1007/s12010-018-2755-x.
- Dwibedi, V.; Saxena, S. Diversity and phylogeny of resveratrol-producing culturable endophytic fungi from Vitis species in India. 3 Biotech 2019, 9, 182. https://doi.org/10.1007/s13205-019-1712-x.
- Dwibedi, V.; Saxena, S. Effect of precursor feeding, dietary supplementation, chemical elicitors and co-culturing on resveratrol production by Arcopilus aureus. Prep. Biochem. Biotechnol. 2022, 52, 404–412. https://doi.org/10.1080/10826068.2021.1955709.
- Liu, Y.; Nan, L.; Liu, J.; Yan, H.; Zhang, D.; Han, X. Isolation and identification of resveratrol-producing endophytes from wine grape Cabernet Sauvignon. SpringerPlus 2016, 5, 1029. https://doi.org/10.1186/s40064-016-2571-0.
- McMullin, D.R.; Green, B.D.; Prince, N.C.; Tanney, J.B.; Miller, J.D. Natural products of Picea endophytes from the Acadian Forest. J. Nat. Prod. 2017, 80, 1475–1483. https://doi.org/10.1021/acs.jnatprod.6b01157.
- Borges Coutinho Gallo, M.; Coêlho Cavalcanti, B.; Washington Araújo Barros, F.; Odorico de Moraes, M.; Veras Costa-Lotufo, L.; Pessoa, C.; Kenupp Bastos, J.; Tallarico Pupo, M. Chemical constituents of Papulaspora immersa, an endophyte from Smallanthus sonchifolius (Asteraceae), and their cytotoxic activity. Chem. Biodivers. 2010, 7, 2941–2950. https://doi.org/10.1002/cbdv.201000011.
- Cui, J.; Guo, T.; Chao, J.; Wang, M.; Wang, J. Potential of the endophytic fungus Phialocephala fortinii Rac56 found in Rhodiola plants to produce salidroside and p-tyrosol. Molecules 2016, 21, 502. https://doi.org/10.3390/molecules21040502.
- Rathnayake, G.R.N.; Savitri Kumar, N.; Jayasinghe, L.; Araya, H.; Fujimoto, Y. Secondary metabolites produced by an endophytic fungus Pestalotiopsis microspora. Nat. Prod. Bioprospect. 2019, 9, 411–417. https://doi.org/10.1007/s13659-019-00225-0.
- Jin, Z.; Gao, L.; Zhang, L.; Liu, T.; Yu, F.; Zhang, Z.; Guo, Q.; Wang, B. Antimicrobial activity of saponins produced by two novel endophytic fungi from Panax Notoginseng. Nat. Prod. Res. 2017, 31, 2700–2703. https://doi.org/10.1080/14786419.2017.1292265.
- Wu, H.; Yang, H.-Y.; You, X.-L.; Li, Y.-H. Diversity of endophytic fungi from roots of Panax ginseng and their saponin yield capacities. SpringerPlus 2013, 2, 107. https://doi.org/10.1186/2193-1801-2-107.
- Wu, H.; Yang, H.; You, X.; Li, Y. Isolation and characterization of saponin-producing fungal endophytes from Aralia elata in northeast China. Int. J. Mol. Sci. 2012, 13, 16255–16266. https://doi.org/10.3390/ijms131216255.
- Ming, Q.; Han, T.; Li, W.; Zhang, Q.; Zhang, H.; Zheng, C.; Huang, F.; Rahman, K.; Qin, L. Tanshinone IIA and tanshinone I production by Trichoderma atroviride D16, an endophytic fungus in Salvia miltiorrhiza. Phytomed. 2012, 19, 330–333. https://doi.org/10.1016/j.phymed.2011.09.076.
- Zhao, Y.; Wang, H.; Liu, T.; Xin, Z. The individual lipid compositions produced by Cunninghamella sp. salicorn 5, an endophytic oleaginous fungus from Salicornia bigelovii Torr. Eur. Food Res. Technol. 2014, 238, 621–633. https://doi.org/10.1007/s00217-013-2141-4.
- Alhadrami, H.A.; Sayed, A.M.; El-Gendy, A.O.; Shamikh, Y.I.; Gaber, Y.; Bakeer, W.; Sheirf, N.H.; Attia, E.Z.; Shaban, G.M.; Khalifa, B.A.; et al. A metabolomic approach to target antimalarial metabolites in the Artemisia annua fungal endophytes. Sci. Rep. 2021, 11, 2770. https://doi.org/10.1038/s41598-021-82201-8.
- Xie, J.; Strobel, G.A.; Feng, T.; Ren, H.; Mends, M.T.; Zhou, Z.; Geary, B. An endophytic Coniochaeta velutina producing broad spectrum antimycotics. J. Microbiol. 2015, 53, 390–397. https://doi.org/10.1007/s12275-015-5105-5.
- Kusari, S.; Zühlke, S.; Košuth, J.; Čellárová, E.; Spiteller, M. Light-independent metabolomics of endophytic Thielavia subthermophila provides insight into microbial hypericin biosynthesis. J. Nat. Prod. 2009, 72, 1825–1835. https://doi.org/10.1021/np9002977.
- Zhang, Q.; Li, Y.; Xu, F.; Zheng, M.; Xi, X.; Zhang, X.; Han, C. Optimization of submerged fermentation medium for matrine production by Aspergillus terreus, an endophytic fungus harboring seeds of Sophora flavescens, using response surface methodology. Mycobiology 2017, 45, 90–96. https://doi.org/10.5941/MYCO.2017.45.2.90.
- Qiang Zhang; Yujuan Li; Fangxue Xu; Mengmeng Zheng; Xiaozhi Xi; Xuelan Zhang; Chunchao Han; Optimization of Submerged Fermentation Medium for Matrine Production by Aspergillus terreus, an Endophytic Fungus Harboring Seeds of Sophora flavescens, Using Response Surface Methodology. Mycobiology 2017, 45, 90-96, 10.5941/myco.2017.45.2.90.




