Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiaoyan Liu | -- | 987 | 2022-07-21 21:36:20 | | | |
| 2 | Conner Chen | + 1 word(s) | 988 | 2022-07-25 05:09:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, X.; Claesson, P.M. Bio-Lubricants. Encyclopedia. Available online: https://encyclopedia.pub/entry/25415 (accessed on 07 February 2026).
Liu X, Claesson PM. Bio-Lubricants. Encyclopedia. Available at: https://encyclopedia.pub/entry/25415. Accessed February 07, 2026.
Liu, Xiaoyan, Per M. Claesson. "Bio-Lubricants" Encyclopedia, https://encyclopedia.pub/entry/25415 (accessed February 07, 2026).
Liu, X., & Claesson, P.M. (2022, July 21). Bio-Lubricants. In Encyclopedia. https://encyclopedia.pub/entry/25415
Liu, Xiaoyan and Per M. Claesson. "Bio-Lubricants." Encyclopedia. Web. 21 July, 2022.
Copy Citation
An extremely efficient lubrication system is achieved in synovial joints by means of bio-lubricants and sophisticated nanostructured surfaces that work together.
aqueous boundary lubrication
friction
wear resistance
1. Introduction
The origin of friction is the energy dissipation processes that occur as two surfaces slide against each other [1]. Friction forces between two surfaces are often characterized by the effective friction coefficient, μeff, that is, calculated by taking the ratio of friction force (FFriction) and applied load (FLoad). The friction coefficient in synovial joints in mammals was found to be as low as 0.001 as measured by hip function simulator machines, even though values reported in different studies vary significantly [2]. It can be safely concluded that the friction coefficient is well below 0.01. For example, Gale et al. have reported values in the range of 0.002~0.006 [3]. This extremely efficient aqueous lubrication ability of the synovial joint is achieved by an association of lubricin, hyaluronan, phospholipids, and aggrecan [4][5][6]. Lubricin and aggrecan, bottlebrush-like biomacromolecules that have densely grafted pendant chains are abundant in synovial joints [7][8]. It is believed that these bottlebrush structured biomacromolecules play key roles for a number of critical biological functions, for example, hydration, aqueous boundary lubrication, wear resistance as well as mediating the rheological and mechanical properties under pressure [8][9].
2. Bio-Lubricants
Bio-lubricants including lubricin, hyaluronan, and phospholipids as well as aggrecan, which is important for the mechanical response of cartilage, play important roles in reducing friction forces and providing shock absorbing mechanical responses to articular cartilage surfaces [8]. These molecular structures are very different, but they share a common feature that they contain highly hydrophilic groups that bind water and allow for the hydration lubrication mechanism [10] to be operative. Lubricin is a glycoprotein with a bottlebrush structure. It is composed of the N-terminal by 2-somatomedin B (SMB)-like domains, the C-terminal with a hemopexin (PEX)-like domain, chondroitin sulfate (CS) side chain, and a densely glycosylated and mucin-like domain in the middle (Figure 1) [11][12]. It has been suggested that lubricin is able to counteract damages of the superficial zone of cartilage, and contributes to the preservation of chondrocytes in the joints [13]. The bottlebrush structure in lubricin and mucins also reduces the interpenetration zone, that is, the region where polymer chains on two opposing surfaces carrying such molecules overlap. This suggests that the bottlebrush structured polymers are promising candidates for reducing friction forces as the energy dissipation related to dragging polymer chains through the interpenetration zone is minimized. The interfacial lubrication studies of various types of mucins have shown that friction forces between surfaces can be reduced by mucins, which can be attributed to their extensive hydration, which arises from the large amount of oligomeric carbohydrate side chains [14][15][16][17][18][19]. Aggrecan also has a bottlebrush structure domain in the middle (Figure 1) [20][21]. The friction forces between surfaces with covalently attached cartilage aggrecan was found to be rather low (μeff = 0.03 ± 0.01~0.11 ± 0.01, depending on the ionic strength of the solution) [22], even though this molecule’s most important function in the synovial joint area is found within the cartilage where it is associated with hyaluronan, and together with collagen, builds the intricate nanostructure of cartilage. For instance, the compressive resistance of articular cartilage has important contributions from hierarchical brush-on-brush structures formed by one hyaluronan associated with as many as 100 aggrecan molecules [23].
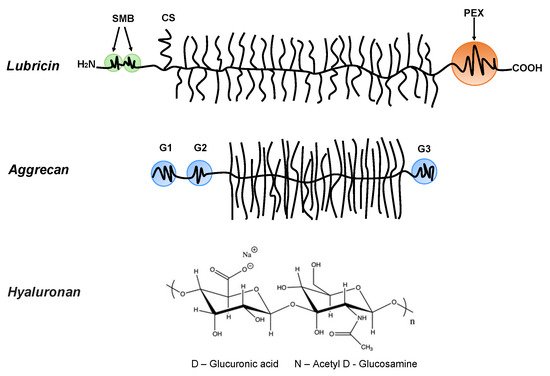
Figure 1. Lubricin: containing the N-terminal 2-somatomedin B (SMB)-like domains and the C-terminal hemopexin (PEX)-like domain. Lubricin also contains a chondroitin sulfate (CS) side chain, and in the middle region, it has a densely glycosylated and mucin-like domain. Aggrecan: containing three globular domains (G1, G2, and G3), and in the middle, it has a large extended domain heavily modified with glycosaminoglycans. Hyaluronan (HA): the anionic disaccharide building unit of hyaluronan.
Hyaluronan and phospholipids also play important roles to achieve efficient aqueous lubrication systems [5][24][25][26][27][28][29][30]. Hyaluronan is a linear anionic polysaccharide (Figure 1). In fact, hyaluronan alone is not enough to reduce friction forces between surfaces [31][32]. However, hyaluronan is responsible for the viscous and elastic properties of the synovial fluid, which is very important for reducing the friction forces between articular cartilage [33][34]. It has also been reported that hyaluronan/aggrecan aggregates can achieve better lubrication than hyaluronan alone, which is due to the highly charged glycosaminoglycan segments on the aggrecan core protein [34]. In addition, the friction forces between damaged human cartilage can be reduced by self-assembled structures formed by hyaluronan and phospholipids [35]. The favorable interfacial lubrication properties of hyaluronan associated with phospholipids have also been observed in model systems [36][37][38]. The results of the friction force measurements between model silica surfaces coated with supported DPPC bilayers in the presence of hyaluronan have clearly indicated that the aggregates of hyaluronan/phospholipids are able to achieve low friction up to the pressure of 56 MPa, [36] which is higher than the pressure (25 ± 5.2 MPa) [39] that leads to the damage of the hyaline cartilage. Recently, multilayers have been prepared by the co-adsorption of HA and DPPC vesicles by Raj et al. [37], and the investigation showed that the friction coefficient between the layers was below 0.01 up to the pressure of 20 MPa. Over the last decade, Dedinaite and co-workers have investigated the intermolecular synergistic mechanism of the bio-lubricants in synovial fluid such as synergy pairs of hyaluronan and phospholipids [32][36][37][38][39][40][41][42]. Their studies indicate that synergistic lubrication can be achieved by the bio-lubricants working together. Some of the observed synergistic effects of bio-lubricants regarding their interfacial lubricating properties are summarized in Table 1.
Table 1. The lubrication performance of the bio-lubricant aggregates. The table provides data for the effective friction coefficient, µeff, and the highest pressure, P, investigated.
| Compositions | Substrate | Interfacial Lubricating Properties | Ref. |
|---|---|---|---|
| HA + DPPC liposomes | Damaged human cartilage | The reduction in friction was 69.5%, P = 1.3 MPa |
[34] |
| HA + Aggrecan | Mica | μeff = 0.01, P = 1.6 MPa | [33] |
| HA + DPPC vesicles | Macroscopic glass surfaces | μeff = 0.1, P = 210 MPa | [32] |
| HA + DPPC bilayer | Silica | μeff = 0.03, P = 56 MPa | [35] |
| HA + DPPC vesicles | Silica | μeff < 0.01, P = 23 MPa | [36] |
| COMP + lubricin | PMMA | μeff = 0.06, P = 7 MPa | [40] |
| cross-linked HA + DOPC | Mica | μeff > 0.5, P = 2 MPa | [43] |
| HA + Lubricin | Mica | μeff = 0.09–0.4, P = 4 MPa | [44] |
| HA + Lubricin + Type II collagen |
Gold versus SiO2 | μeff = 0.01, P = 0.013 MPa | [45] |
References
- Singer, I.L.; Pollock, H. Fundamentals of Friction: Macroscopic and Microscopic Processes; Springer: Berlin/Heidelberg, Germany, 2012.
- Forster, H.; Fisher, J. The Influence of Loading Time and Lubricant on the Friction of Articular Cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1996, 210, 109–119.
- Gale, L.R.; Coller, R.; Hargreaves, D.; Hills, B.A.; Crawford, R. The role of SAPL as a boundary lubricant in prosthetic joints. Tribol. Int. 2007, 40, 601–606.
- Wright, V.; Dowson, D. Lubrication and cartilage. J. Anat. 1976, 121, 107–118.
- Hills, B.A. Surface-active phospholipid: A Pandora’s box of clinical applications. Part II. Barrier and lubricating properties. Intern. Med. J. 2002, 32, 242–251.
- Klein, J. Molecular mechanisms of synovial joint lubrication. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2006, 220, 691–710.
- Levangie, P.K.; Norkin, C.C. Joint Structure and Function: A Comprehensive Analysis, 5th ed.; F. A. Davis Company: Philadelphia, PA, USA, 2011.
- Dėdinaitė, A. Biomimetic lubrication. Soft Matter 2012, 8, 273–284.
- Abbasi, M.; Faust, L.; Wilhelm, M. Comb and Bottlebrush Polymers with Superior Rheological and Mechanical Properties. Adv. Mater. 2019, 31, 1806484.
- Ma, L.; Gaisinskaya-Kipnis, A.; Kampf, N.; Klein, J. Origins of hydration lubrication. Nat. Commun. 2015, 6, 6060.
- Estrella, R.P.; Whitelock, J.M.; Packer, N.H.; Karlsson, N.G. The glycosylation of human synovial lubricin: Implications for its role in inflammation. Biochem. J. 2010, 429, 359–367.
- Swann, D.A.; Sotman, S.; Dixon, M.; Brooks, C. The isolation and partial characterization of the major glycoprotein (LGP-I) from the articular lubricating fraction from bovine synovial fluid. Biochem. J. 1977, 161, 473–485.
- Jay, G.D.; Waller, K.A. The biology of Lubricin: Near frictionless joint motion. Matrix Biol. 2014, 39, 17–24.
- Pettersson, T.; Dėdinaitė, A. Normal and friction forces between mucin and mucin–chitosan layers in absence and presence of SDS. J. Colloid Interface Sci. 2008, 324, 246–256.
- Efremova, N.V.; Huang, Y.; Peppas, N.A.; Leckband, D.E. Direct Measurement of Interactions between Tethered Poly(ethylene glycol) Chains and Adsorbed Mucin Layers. Langmuir 2002, 18, 836–845.
- Harvey, N.M.; Yakubov, G.E.; Stokes, J.R.; Klein, J. Normal and Shear Forces between Surfaces Bearing Porcine Gastric Mucin, a High-Molecular-Weight Glycoprotein. Biomacromolecules 2011, 12, 1041–1050.
- Zappone, B.; Patil, N.J.; Madsen, J.B.; Pakkanen, K.I.; Lee, S. Molecular Structure and Equilibrium Forces of Bovine Submaxillary Mucin Adsorbed at a Solid–Liquid Interface. Langmuir 2015, 31, 4524–4533.
- An, J.; Dėdinaitė, A.; Nilsson, A.; Holgersson, J.; Claesson, P.M. Comparison of a Brush-with-Anchor and a Train-of-Brushes Mucin on Poly(methyl methacrylate) Surfaces: Adsorption, Surface Forces, and Friction. Biomacromolecules 2014, 15, 1515–1525.
- An, J.; Jin, C.; Dėdinaitė, A.; Holgersson, J.; Karlsson, N.G.; Claesson, P.M. Influence of Glycosylation on Interfacial Properties of Recombinant Mucins: Adsorption, Surface Forces, and Friction. Langmuir 2017, 33, 4386–4395.
- Seog, J.; Dean, D.; Plaas, A.H.K.; Wong-Palms, S.; Grodzinsky, A.J.; Ortiz, C. Direct Measurement of Glycosaminoglycan Intermolecular Interactions via High-Resolution Force Spectroscopy. Macromolecules 2002, 35, 5601–5615.
- Hardingham, T.E.; Fosang, A.J. Proteoglycans: Many forms and many functions. FASEB J. 1992, 6, 861–870.
- Han, L.; Dean, D.; Ortiz, C.; Grodzinsky, A.J. Lateral Nanomechanics of Cartilage Aggrecan Macromolecules. Biophys. J. 2007, 92, 1384–1398.
- Horkay, F.; Basser, P.J.; Hecht, A.-M.; Geissler, E. Gel-like behavior in aggrecan assemblies. J. Chem. Phys. 2008, 128, 135103.
- Goldberg, R.; Schroeder, A.; Silbert, G.; Turjeman, K.; Barenholz, Y.; Klein, J. Boundary Lubricants with Exceptionally Low Friction Coefficients Based on 2D Close-Packed Phosphatidylcholine Liposomes. Adv. Mater. 2011, 23, 3517–3521.
- Goldberg, R.; Schroeder, A.; Barenholz, Y.; Klein, J. Interactions between Adsorbed Hydrogenated Soy Phosphatidylcholine (HSPC) Vesicles at Physiologically High Pressures and Salt Concentrations. Biophys. J. 2011, 100, 2403–2411.
- Goldberg, R.; Klein, J. Liposomes as lubricants: Beyond drug delivery. Chem. Phys. Lipids 2012, 165, 374–381.
- Sivan, S.; Schroeder, A.; Verberne, G.; Merkher, Y.; Diminsky, D.; Priev, A.; Maroudas, A.; Halperin, G.; Nitzan, D.; Etsion, I.; et al. Liposomes Act as Effective Biolubricants for Friction Reduction in Human Synovial Joints. Langmuir 2009, 26, 1107–1116.
- Dėdinaitė, A.; Wieland, D.C.F.; Bełdowski, P.; Claesson, P.M. Biolubrication synergy: Hyaluronan–Phospholipid interactions at interfaces. Adv. Colloid Interface Sci. 2019, 274, 102050.
- Cao, Y.; Kampf, N.; Klein, J. Boundary Lubrication, Hemifusion, and Self-Healing of Binary Saturated and Monounsaturated Phosphatidylcholine Mixtures. Langmuir 2019, 35, 15459–15468.
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338.
- Tadmor, R.; Chen, N.; Israelachvili, J. Normal and Shear Forces between Mica and Model Membrane Surfaces with Adsorbed Hyaluronan. Macromolecules 2003, 36, 9519–9526.
- Li, S.; Macakova, L.; Bełdowski, P.; Claesson, P.M.; Dėdinaitė, A. Phospholipids and Hyaluronan: From Molecular Interactions to Nano- and Macroscale Friction. Colloids Interfaces 2022, 6, 38.
- Lapcik, L., Jr.; Lapcik, L.; De Smedt, S.; Demeester, J.; Chabreček, P. Hyaluronan: Preparation, Structure, Properties, and Applications. Chem. Rev. 1998, 98, 2663–2684.
- Seror, J.; Merkher, Y.; Kampf, N.; Collinson, L.; Day, A.J.; Maroudas, A.; Klein, J. Articular Cartilage Proteoglycans As Boundary Lubricants: Structure and Frictional Interaction of Surface-Attached Hyaluronan and Hyaluronan–Aggrecan Complexes. Biomacromolecules 2011, 12, 3432–3443.
- Forsey, R.W.; Fisher, J.; Thompson, J.; Stone, M.H.; Bell, C.; Ingham, E. The effect of hyaluronic acid and phospholipid based lubricants on friction within a human cartilage damage model. Biomaterials 2006, 27, 4581–4590.
- Wang, M.; Liu, C.; Thormann, E.; Dėdinaitė, A. Hyaluronan and Phospholipid Association in Biolubrication. Biomacromolecules 2013, 14, 4198–4206.
- Raj, A.; Wang, M.; Zander, T.; Wieland, D.F.; Liu, X.; An, J.; Garamus, V.M.; Willumeit-Römer, R.; Fielden, M.; Claesson, P.M.; et al. Lubrication synergy: Mixture of hyaluronan and dipalmitoylphosphatidylcholine (DPPC) vesicles. J. Colloid Interface Sci. 2017, 488, 225–233.
- Liu, C.; Wang, M.; An, J.; Thormann, E.; Dėdinaitė, A. Hyaluronan and phospholipids in boundary lubrication. Soft Matter 2012, 8, 10241–10244.
- Spahn, G.; Wittig, R. Spannungs-und Bruchverhalten des gesunden Gelenkknorpels unter axialer Belastung. Eine biomechanische Untersuchung. Zent. Für Chir. 2003, 128, 78–82.
- Bełdowski, P.; Weber, P.; Dėdinaitė, A.; Claesson, P.M.; Gadomski, A. Physical crosslinking of hyaluronic acid in the presence of phospholipids in an aqueous nano-environment. Soft Matter 2018, 14, 8997–9004.
- Raj, A.; Wang, M.; Liu, C.; Ali, L.; Karlsson, N.G.; Claesson, P.M.; Dėdinaitė, A. Molecular synergy in biolubrication: The role of cartilage oligomeric matrix protein (COMP) in surface-structuring of lubricin. J. Colloid Interface Sci. 2017, 495, 200–206.
- Dėdinaitė, A.; Claesson, P.M. Synergies in lubrication. Phys. Chem. Chem. Phys. 2017, 19, 23677–23689.
- Yu, J.; Banquy, X.; Greene, G.; Lowrey, D.D.; Israelachvili, J.N. The Boundary Lubrication of Chemically Grafted and Cross-Linked Hyaluronic Acid in Phosphate Buffered Saline and Lipid Solutions Measured by the Surface Forces Apparatus. Langmuir 2011, 28, 2244–2250.
- Das, S.; Banquy, X.; Zappone, B.; Greene, G.W.; Jay, G.; Israelachvili, J.N. Synergistic Interactions between Grafted Hyaluronic Acid and Lubricin Provide Enhanced Wear Protection and Lubrication. Biomacromolecules 2013, 14, 1669–1677.
- Majd, S.E.; Kuijer, R.; Köwitsch, A.; Groth, T.; Schmidt, T.A.; Sharma, P.K. Both Hyaluronan and Collagen Type II Keep Proteoglycan 4 (Lubricin) at the Cartilage Surface in a Condition That Provides Low Friction during Boundary Lubrication. Langmuir 2014, 30, 14566–14572.
More
Information
Subjects:
Chemistry, Physical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
25 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




