
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tricia Kuah | -- | 3423 | 2022-07-20 16:27:57 | | | |
| 2 | Camila Xu | -22 word(s) | 3401 | 2022-07-21 07:40:05 | | |
Video Upload Options
Metastatic Spinal Cord Compression (MSCC) is a feared complication in oncology patients due to its potential for severe pain, permanent neurological disability and mechanical instability of the spine. Radiotherapy constitutes one of the major treatment modalities for MSCC and includes conventional external beam radiotherapy (cEBRT) and stereotactic body radiotherapy (SBRT). SBRT is technically feasible in low-grade MSCC (up to Bilsky 1c) and is mainly indicated in the setting of oligometastatic disease, re-irradiation, and in the treatment of radioresistant primary tumors where durable local control and symptom relief are required. SBRT is a high-precision technique that delivers highly conformal ablative doses while minimizing radiation to the surrounding organs at risk.
1. Introduction
2. The Role of Imaging in Stereotactic Body Radiotherapy (SBRT)
-
Pre-treatment planning;
-
In-room imaging guidance;
-
Post-treatment follow-up.
2.1. Pre-Treatment Planning
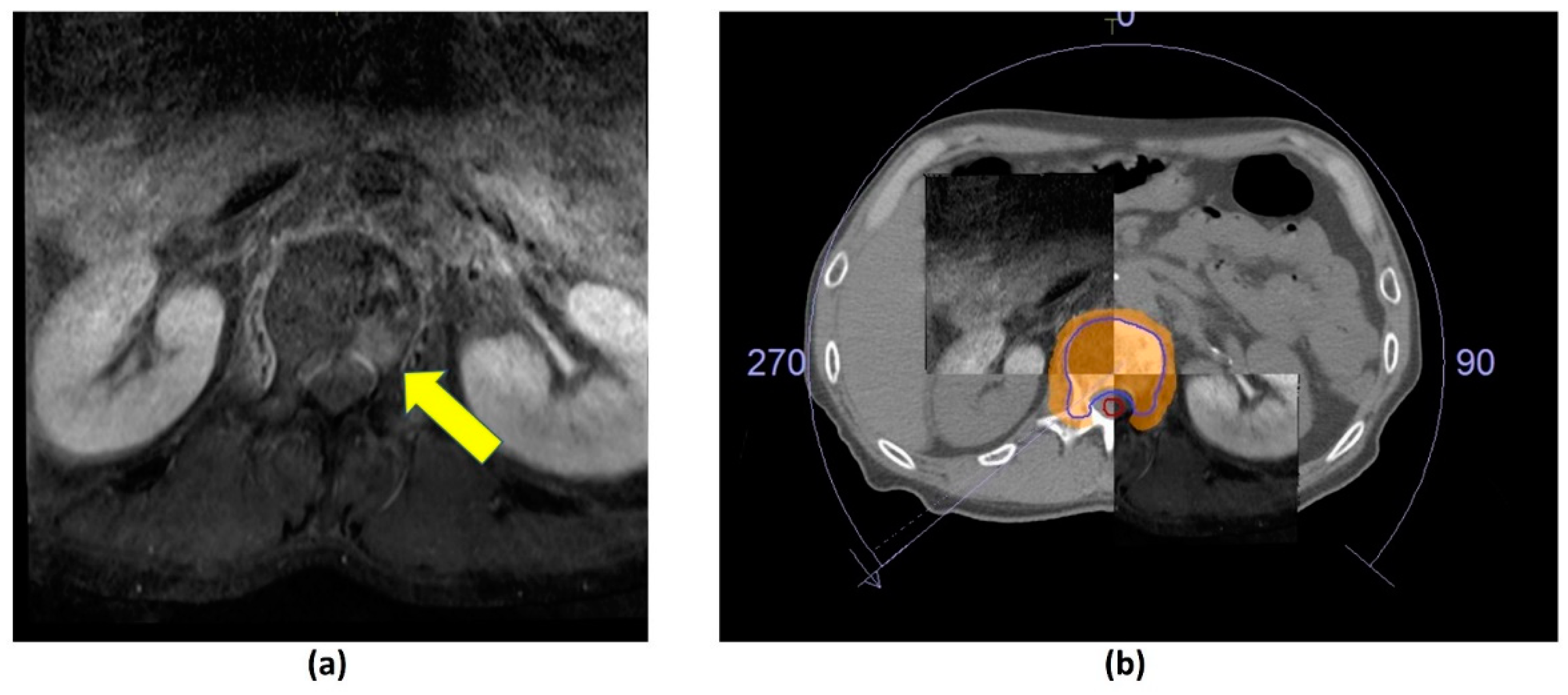 Figure 1. Axial post-contrast fat-suppressed T1-weighted MRI. (a) Metastatic lesion in the L1 vertebral body (yellow arrow). (b) CT and MR fusion for stereotactic body radiotherapy (SBRT) planning; 27 Gy over 3 fractions delivered using volumetric modulated arc therapy. Clinical target volume (CTV—blue outline), planning organ at risk volume (PRV, cord—red outline), 95% isodose (orange colour wash).
Figure 1. Axial post-contrast fat-suppressed T1-weighted MRI. (a) Metastatic lesion in the L1 vertebral body (yellow arrow). (b) CT and MR fusion for stereotactic body radiotherapy (SBRT) planning; 27 Gy over 3 fractions delivered using volumetric modulated arc therapy. Clinical target volume (CTV—blue outline), planning organ at risk volume (PRV, cord—red outline), 95% isodose (orange colour wash).2.2. Image-Guided Radiotherapy (IGRT)
2.3. Post-Treatment Follow-Up
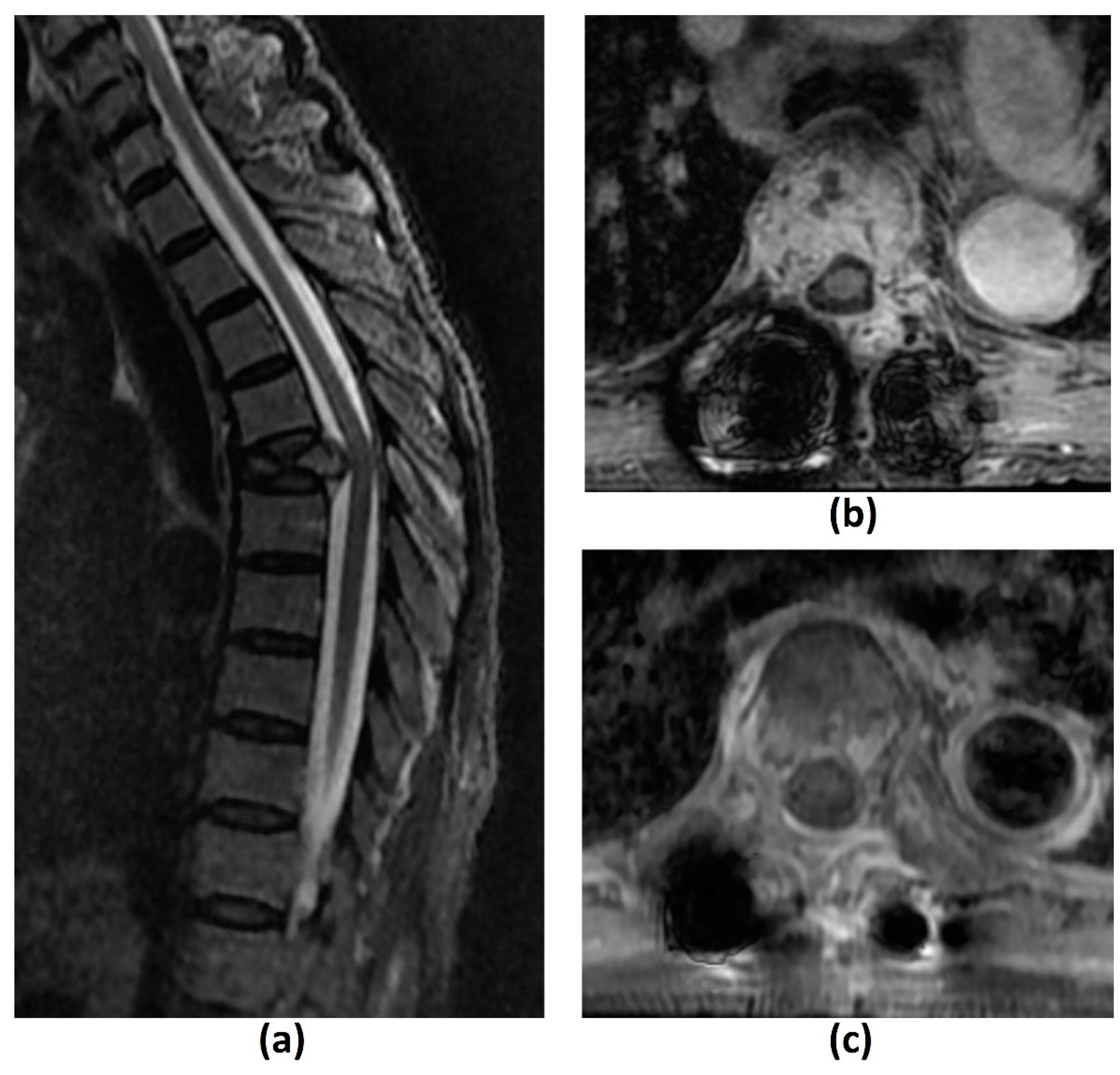 Figure 2. Sagittal T2-weighted image (a) of a 69-year-old female patient with metastatic rectal cancer to the spine shows a pathological T5 vertebral fracture. Axial post-contrast fat-suppressed T1-weighted images of the same patient, post separation surgery and planning for stereotactic body radiotherapy (SBRT) (b), and 6 months post-treatment (c). There is reduced tumour enhancement and bulk between (b) and (c) due to favourable post-SBRT response.
Figure 2. Sagittal T2-weighted image (a) of a 69-year-old female patient with metastatic rectal cancer to the spine shows a pathological T5 vertebral fracture. Axial post-contrast fat-suppressed T1-weighted images of the same patient, post separation surgery and planning for stereotactic body radiotherapy (SBRT) (b), and 6 months post-treatment (c). There is reduced tumour enhancement and bulk between (b) and (c) due to favourable post-SBRT response.3. Summary
MSCC is a debilitating complication in cancer patients with spinal metastasis, and its incidence is expected to rise due to improving cancer treatments and survival. Radiotherapy, including cEBRT and SBRT, constitutes one of the major treatment arms of MSCC. Imaging plays an important role in SBRT for pre-treatment planning, in-room image-guidance, and post-treatment follow-up. Recommendations from the SPINO group have been made on the definitions of local progression and local control post SBRT. However, there remain inherent difficulties in determining treatment response due to factors like pseudoprogression, and the uncertainty in signal changes indicating treatment response in bone metastasis. Functional MRI may help in the latter.
VCF is the most common complication post SBRT, and may be related to local tumour progression or the SBRT treatment itself. Current imaging modalities like MRI and CT have limited ability to distinguish between these two processes, and further research into the role of functional imaging in evaluating VCF post SBRT is warranted.
References
- National Institute for Health and Care Excellence. Metastatic Spinal Cord Compression: Diagnosis and Management of Adults at Risk of and with Metastatic Spinal Cord Compression NICE Guidelines (CG75); NICE: London, UK, 2008.
- Laufer, I.; Schiff, D.; Kelly, H.R.; Bilsky, M. Clinical Features and Diagnosis of Neoplastic Epidural spinal Cord Compression; Eichler, A.F., Ed.; UpToDate: Waltham, MA, USA.
- McLinton, A.; Hutchison, C. Malignant spinal cord compression: A retrospective audit of clinical practice at a UK regional cancer centre. Br. J. Cancer. 2006, 94, 486–491.
- Laur, O.; Nandu, H.; Titelbaum, D.S.; Nunez, D.B.; Khurana, B. Nontraumatic Spinal Cord Compression: MRI Primer for Emergency Department Radiologists. Radiographics 2019, 39, 1862–1880.
- Macdonald, A.G.; Lynch, D.; Garbett, I.; Nazeer, N. Malignant spinal cord compression. J. R Coll. Physicians Edinb. 2019, 49, 151–156.
- Barzilai, O.; Fisher, C.G.; Bilsky, M.H. State of the Art Treatment of Spinal Metastatic Disease. Neurosurgery 2018, 82, 757–769.
- Mak, K.S.; Lee, L.K.; Mak, R.H.; Wang, S.; Pile-Spellman, J.; Abrahm, J.L.; Prigerson, H.G.; Balboni, T.A. Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998–2006. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 824–831.
- Savage, P.; Sharkey, R.; Kua, T.; Schofield, L.; Richardson, D.; Panchmatia, N.; Papanastasopoulos, P.; Williams, M.; Falconer, A.; Power, D.; et al. Malignant spinal cord compression: NICE guidance, improvements and challenges. QJM 2014, 107, 277–282.
- Schiff, D.; O’Neill, B.P.; Suman, V.J. Spinal epidural metastasis as the initial manifestation of malignancy: Clinical features and diagnostic approach. Neurology 1997, 49, 452–456.
- Robson, P. Metastatic spinal cord compression: A rare but important complication of cancer. Clin. Med. 2014, 14, 542–545.
- Decroisette, C.; Monnet, I.; Berard, H.; Quere, G.; Le Caer, H.; Bota, S.; Audigier-Valette, C.; Geriniere, L.; Vernejoux, J.M.; Chouaid, C. Groupe Français de Pneumo-Cancérologie 0601 Team. Epidemiology and treatment costs of bone metastases from lung cancer: A French prospective, observational, multicenter study (GFPC 0601). J. Thorac. Oncol. 2011, 6, 576–582.
- Laufer, I.; Bilsky, M.; Schiff, D.; Brown, P. Treatment and Prognosis of Neoplastic Epidural Spinal Cord Compression; Eichler, A.F., Savarese, D.M.F., Eds.; UpToDate: Waltham, MA, USA.
- Laufer, I.; Zuckerman, S.L.; Bird, J.E.; Bilsky, M.H.; Lazáry, Á.; Quraishi, N.A.; Fehlings, M.G.; Sciubba, D.M.; Shin, J.H.; Mesfin, A.; et al. Predicting Neurologic Recovery after Surgery in Patients with Deficits Secondary to MESCC: Systematic Review. Spine 2016, 41 (Suppl. 20), S224–S230.
- Hussain, I.; Barzilai, O.; Reiner, A.S.; DiStefano, N.; McLaughlin, L.; Ogilvie, S.; Bilsky, M.; Laufer, I. Patient-reported outcomes after surgical stabilization of spinal tumors: Symptom-based validation of the Spinal Instability Neoplastic Score (SINS) and surgery. Spine J. 2018, 18, 261–267.
- Levack, P.; Graham, J.; Collie, D.; Grant, R.; Kidd, J.; Kunkler, I.; Gibson, A.; Hurman, D.; McMillan, N.; Rampling, R.; et al. A Prospective Audit of the Diagnosis, Management and Outcome of Malignant Spinal Cord Compression; Clinical Resource and Audit Group (CRAG) 97/08; CRAG: Edinburgh, Scotland, 2001.
- van Tol, F.R.; Versteeg, A.L.; Verkooijen, H.M.; Öner, F.C.; Verlaan, J.J. Time to Surgical Treatment for Metastatic Spinal Disease: Identification of Delay Intervals. Glob. Spine J. 2021, 2192568221994787.
- Laufer, I.; Rubin, D.G.; Lis, E.; Cox, B.W.; Stubblefield, M.D.; Yamada, Y.; Bilsky, M.H. The NOMS framework: Approach to the treatment of spinal metastatic tumors. Oncologist 2013, 18, 744–751.
- Tseng, C.L.; Eppinga, W.; Charest-Morin, R.; Soliman, H.; Myrehaug, S.; Maralani, P.J.; Campbell, M.; Lee, Y.K.; Fisher, C.; Fehlings, M.G.; et al. Spine Stereotactic Body Radiotherapy: Indications, Outcomes, and Points of Caution. Glob. Spine J. 2017, 7, 179–197.
- Ito, K.; Nakamura, N.; Shimizuguchi, T.; Ogawa, H.; Karasawa, K. Appropriate endpoints for stereotactic body radiotherapy for bone metastasis: Classification into five treatment groups. Rep. Pr. Oncol. Radiother. 2020, 25, 150–153.
- Gerszten, P.C.; Mendel, E.; Yamada, Y. Radiotherapy and radiosurgery for metastatic spine disease: What are the options, indications, and outcomes? Spine 2009, 34 (Suppl. 22), S78–S92.
- Ito, K.; Ogawa, H.; Shimizuguchi, T.; Nihei, K.; Furuya, T.; Tanaka, H.; Karasawa, K. Stereotactic Body Radiotherapy for Spinal Metastases: Clinical Experience in 134 Cases from a Single Japanese Institution. Technol. Cancer Res. Treat. 2018, 17, 1533033818806472.
- Dunne, E.M.; Fraser, I.M.; Liu, M. Stereotactic body radiation therapy for lung, spine and oligometastatic disease: Current evidence and future directions. Ann. Transl. Med. 2018, 6, 283.
- Vellayappan, B.A.; Kumar, N.; Chang, E.L.; Sahgal, A.; Sloan, A.E.; Lo, S.S. Novel multidisciplinary approaches in the management of metastatic epidural spinal cord compression. Future Oncol. 2018, 14, 1665–1668.
- Yamada, Y.; Katsoulakis, E.; Laufer, I.; Lovelock, M.; Barzilai, O.; McLaughlin, L.A.; Zhang, Z.; Schmitt, A.M.; Higginson, D.S.; Lis, E.; et al. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg. Focus. 2017, 42, E6.
- Yamada, Y.; Bilsky, M.H.; Lovelock, D.M.; Venkatraman, E.S.; Toner, S.; Johnson, J.; Zatcky, J.; Zelefsky, M.J.; Fuks, Z. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 484–490.
- Thibault, I.; Chang, E.L.; Sheehan, J.; Ahluwalia, M.S.; Guckenberger, M.; Sohn, M.J.; Ryu, S.; Foote, M.; Lo, S.S.; Muacevic, A.; et al. Response assessment after stereotactic body radiotherapy for spinal metastasis: A report from the SPIne response assessment in Neuro-Oncology (SPINO) group. Lancet Oncol. 2015, 16, e595–e603.
- Gerszten, P.C.; Burton, S.A.; Ozhasoglu, C.; Welch, W.C. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine 2007, 32, 193–199.
- Sahgal, A.; Larson, D.A.; Chang, E.L. Stereotactic body radiosurgery for spinal metastases: A critical review. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 652–665.
- Husain, Z.A.; Sahgal, A.; De Salles, A.; Funaro, M.; Glover, J.; Hayashi, M.; Hiraoka, M.; Levivier, M.; Ma, L.; Mar-tínez-Alvarez, R.; et al. Stereotactic body radiotherapy for de novo spinal metastases: Systematic review. J. Neurosurg. Spine 2017, 27, 295–302.
- Sahgal, A.; Myrehaug, S.D.; Siva, S.; Masucci, G.L.; Maralani, P.J.; Brundage, M.; Butler, J.; Chow, E.; Fehlings, M.G.; Foote, M.; et al. trial investigators. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: An open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2021, 22, 1023–1033.
- Mossa-Basha, M.; Gerszten, P.C.; Myrehaug, S.; Mayr, N.A.; Yuh, W.T.; Jabehdar Maralani, P.; Sahgal, A.; Lo, S.S. Spinal metastasis: Diagnosis, management and follow-up. Br. J. Radiol. 2019, 92, 20190211.
- Das, I.J.; McGee, K.P.; Tyagi, N.; Wang, H. Role and future of MRI in radiation oncology. Br. J. Radiol. 2019, 92, 20180505.
- Neumann, J.O.; Giese, H.; Biller, A.; Nagel, A.M.; Kiening, K. Spatial Distortion in MRI-Guided Stereotactic Procedures: Evaluation in 1.5-, 3- and 7-Tesla MRI Scanners. Stereotact. Funct. Neurosurg. 2015, 93, 380–386.
- Rogé, M.; Henni, A.H.; Neggaz, Y.A.; Mallet, R.; Hanzen, C.; Dubray, B.; Colard, E.; Gensanne, D.; Thureau, S. Evaluation of a Dedicated Software “Elements™ Spine SRS, Brainlab®” for Target Volume Definition in the Treatment of Spinal Bone Metastases with Stereotactic Body Radiotherapy. Front. Oncol. 2022, 12, 827195.
- Aselmaa, A.; van Herk, M.; Song, Y.; Goossens, R.H.M.; Laprie, A. The influence of automation on tumor contouring. Cogn. Technol. Work 2017, 19, 795–808.
- Sterzing, F.; Engenhart-Cabillic, R.; Flentje, M.; Debus, J. Image-guided radiotherapy: A new dimension in radiation oncology. Dtsch. Arztebl. Int. 2011, 108, 274–280.
- Cubillos Mesí as, M.; Boda-Heggemann, J.; Thoelking, J.; Lohr, F.; Wenz, F.; Wertz, H. Quantification and Assessment of Interfraction Setup Errors Based on Cone Beam CT and Determination of Safety Margins for Radiotherapy. PLoS ONE 2016, 11, e0150326.
- Tseng, C.L.; Sussman, M.S.; Atenafu, E.G.; Letourneau, D.; Ma, L.; Soliman, H.; Thibault, I.; Cho, B.C.; Simeonov, A.; Yu, E.; et al. Magnetic resonance imaging assessment of spinal cord and cauda equina motion in supine patients with spinal metastases planned for spine stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 995–1002.
- Hyde, D.; Lochray, F.; Korol, R.; Davidson, M.; Wong, C.S.; Ma, L.; Sahgal, A. Spine stereotactic body radiotherapy utilizing cone-beam CT image-guidance with a robotic couch: Intrafraction motion analysis accounting for all six degrees of freedom. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e555–e562.
- Oztek, M.A.; Mayr, N.A.; Mossa-Basha, M.; Nyflot, M.; Sponseller, P.A.; Wu, W.; Hofstetter, C.P.; Saigal, R.; Bowen, S.R.; Hippe, D.S.; et al. The Dancing Cord: Inherent Spinal Cord Motion and Its Effect on Cord Dose in Spine Stereotactic Body Radiation Therapy. Neurosurgery 2020, 87, 1157–1166.
- Boda-Heggemann, J.; Lohr, F.; Wenz, F.; Flentje, M.; Guckenberger, M. kV cone-beam CT-based IGRT: A clinical review. Strahlenther Onkol. 2011, 187, 284–291.
- Kumar, M.; Shanavas, M.; Sidappa, A.; Kiran, M. Cone beam computed tomography—Know its secrets. J. Int. Oral. Health 2015, 7, 64–68.
- Chan, M.F.; Lim, S.B.; Li, X.; Tang, X.; Zhang, P.; Shi, C. Commissioning and Evaluation of a Third-Party 6 Degrees-of-Freedom Couch Used in Radiotherapy. Technol. Cancer Res. Treat. 2019, 18, 1533033819870778.
- Li, G. Patient radiation dose and protection from cone-beam computed tomography. Imaging Sci. Dent. 2013, 43, 63–69.
- Lechuga, L.; Weidlich, G.A. Cone Beam CT vs. Fan Beam CT: A Comparison of Image Quality and Dose Delivered Between Two Differing CT Imaging Modalities. Cureus 2016, 8, e778.
- Sajja, S.; Lee, Y.; Eriksson, M.; Nordström, H.; Sahgal, A.; Hashemi, M.; Mainprize, J.G.; Ruschin, M. Technical Principles of Dual-Energy Cone Beam Computed Tomography and Clinical Applications for Radiation Therapy. Adv. Radiat. Oncol. 2019, 5, 1–16.
- Nagarajappa, A.K.; Dwivedi, N.; Tiwari, R. Artifacts: The downturn of CBCT image. J. Int. Soc. Prev. Community Dent. 2015, 5, 440–445.
- Schulze, R.; Heil, U.; Gross, D.; Bruellmann, D.D.; Dranischnikow, E.; Schwanecke, U.; Schoemer, E. Artefacts in CBCT: A review. Dentomaxillofac. Radiol. 2011, 40, 265–273.
- Llorente, R.; Spieler, B.O.; Victoria, J.; Takita, C.; Yechieli, R.; Ford, J.C.; Brown, K.; Samuels, M.A.; Mellon, E.A. MRI-guided stereotactic ablative radiation therapy of spinal bone metastases: A preliminary experience. Br. J. Radiol. 2020, 93, 20190655.
- Choi, C.H.; Kim, J.H.; Kim, J.I.; Park, J.M. Comparison of treatment plan quality among MRI-based IMRT with a linac, MRI-based IMRT with tri-Co-60 sources, and VMAT for spine SABR. PLoS ONE 2019, 14, e0220039.
- Pollard, J.M.; Wen, Z.; Sadagopan, R.; Wang, J.; Ibbott, G.S. The future of image-guided radiotherapy will be MR guided. Br. J. Radiol. 2017, 90, 20160667.
- Grégoire, V.; Guckenberger, M.; Haustermans, K.; Lagendijk, J.J.W.; Ménard, C.; Pötter, R.; Slotman, B.J.; Tanderup, K.; Thorwarth, D.; van Herk, M.; et al. Image guidance in radiation therapy for better cure of cancer. Mol. Oncol. 2020, 14, 1470–1491.
- Schmidt, M.A.; Payne, G.S. Radiotherapy planning using MRI. Phys. Med. Biol. 2015, 60, R323–R361.
- Ranger, M.R.; Irwin, G.J.; Bunbury, K.M.; Peutrell, J.M. Changing body position alters the location of the spinal cord within the vertebral canal: A magnetic resonance imaging study. Br. J. Anaesth. 2008, 101, 804–809.
- Koo, J.; Nardella, L.; Degnan, M.; Andreozzi, J.; Yu, H.M.; Penagaricano, J.; Johnstone, P.A.S.; Oliver, D.; Ahmed, K.; Rosenberg, S.A.; et al. Triggered kV Imaging During Spine SBRT for Intrafraction Motion Management. Technol. Cancer Res. Treat. 2021, 20, 15330338211063033.
- Xiao, Y.; Kry, S.F.; Popple, R.; Yorke, E.; Papanikolaou, N.; Stathakis, S.; Xia, P.; Huq, S.; Bayouth, J.; Galvin, J.; et al. Flattening filter-free accelerators: A report from the AAPM Therapy Emerging Technology Assessment Work Group. J. Appl. Clin. Med. Phys. 2015, 16, 5219.
- Hwang, Y.J.; Sohn, M.J.; Lee, B.H.; Kim, S.Y.; Seo, J.W.; Han, Y.H.; Lee, J.Y.; Cha, S.J.; Kim, Y.H. Radiosurgery for metastatic spinal tumors: Follow-up MR findings. AJNR Am. J. Neuroradiol. 2012, 33, 382–387.
- Zhou, J.; Jawad, M.S.; Harb, J.G.; Yee, S.; Yan, D.; Grills, I.S. Quantifying Follow-up T2-weighted MR Image in Local Failure Spinal Tumors after Stereotactic Body Radiation Therapy (SBRT). Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, s6.
- Kumar, K.A.; Peck, K.K.; Karimi, S.; Lis, E.; Holodny, A.I.; Bilsky, M.H.; Yamada, Y. A Pilot Study Evaluating the Use of Dynamic Contrast-Enhanced Perfusion MRI to Predict Local Recurrence After Radiosurgery on Spinal Metastases. Technol. Cancer Res. Treat. 2017, 16, 857–865.
- Chu, S.; Karimi, S.; Peck, K.K.; Yamada, Y.; Lis, E.; Lyo, J.; Bilsky, M.; Holodny, A.I. Measurement of blood perfusion in spinal metastases with dynamic contrast-enhanced magnetic resonance imaging: Evaluation of tumor response to radiation therapy. Spine 2013, 38, E1418–E1424.
- Lee, J.H.; Yoo, G.S.; Yoon, Y.C.; Park, H.C.; Kim, H.S. Diffusion-weighted and dynamic contrast-enhanced magnetic resonance imaging after radiation therapy for bone metastases in patients with hepatocellular carcinoma. Sci. Rep. 2021, 11, 10459.
- Byun, W.M.; Shin, S.O.; Chang, Y.; Lee, S.J.; Finsterbusch, J.; Frahm, J. Diffusion-weighted MR imaging of metastatic disease of the spine: Assessment of response to therapy. AJNR Am. J. Neuroradiol. 2002, 23, 906–912.
- Gwak, H.S.; Youn, S.M.; Chang, U.; Lee, D.H.; Cheon, G.J.; Rhee, C.H.; Kim, K.; Kim, H.J. Usefulness of (18)F-fluorodeoxyglucose PET for radiosurgery planning and response monitoring in patients with recurrent spinal metastasis. Min-Minim. Invasive Neurosurg. 2006, 49, 127–134.
- Choi, J.; Kim, J.W.; Jeon, T.J.; Lee, I.J. The 18F-FDG PET/CT response to radiotherapy for patients with spinal metastasis correlated with the clinical outcomes. PLoS ONE 2018, 13, e0204918.
- O’Sullivan, S.; McDermott, R.; Keys, M.; O’Sullivan, M.; Armstrong, J.; Faul, C. Imaging response assessment following stereotactic body radiotherapy for solid tumour metastases of the spine: Current challenges and future directions. J. Med. Imaging Radiat. Oncol. 2020, 64, 385–397.
- Bilsky, M.H.; Laufer, I.; Fourney, D.R.; Groff, M.; Schmidt, M.H.; Varga, P.P.; Vrionis, F.D.; Yamada, Y.; Gerszten, P.C.; Kuklo, T.R. Reliability analysis of the epidural spinal cord compression scale. J. Neurosurg. Spine 2010, 13, 324–328.
- Correia, D.; Moullet, B.; Cullmann, J.; Heiss, R.; Ermiş, E.; Aebersold, D.M.; Hemmatazad, H. Response assessment after stereotactic body radiation therapy for spine and non-spine bone metastases: Results from a single institutional study. Radiat. Oncol. 2022, 17, 37.
- Balagamwala, E.H.; Naik, M.; Reddy, C.A.; Angelov, L.; Suh, J.H.; Djemil, T.; Magnelli, A.; Chao, S.T. Pain flare after stereotactic radiosurgery for spine metastases. J. Radiosurg. SBRT 2018, 5, 99–105.
- McDonald, R.; Chow, E.; Rowbottom, L.; DeAngelis, C.; Soliman, H. Incidence of pain flare in radiation treatment of bone metastases: A literature review. J. Bone Oncol. 2014, 3, 84–89.
- Amini, B.; Beaman, C.B.; Madewell, J.E.; Allen, P.K.; Rhines, L.D.; Tatsui, C.E.; Tannir, N.M.; Li, J.; Brown, P.D.; Ghia, A.J. Osseous Pseudoprogression in Vertebral Bodies Treated with Stereotactic Radiosurgery: A Secondary Analysis of Prospective Phase I/II Clinical Trials. AJNR Am. J. Neuroradiol. 2016, 37, 387–392.
- Bahig, H.; Simard, D.; Létourneau, L.; Wong, P.; Roberge, D.; Filion, E.; Donath, D.; Sahgal, A.; Masucci, L. A Study of Pseudoprogression After Spine Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 848–856.
- Stutz, E.; Wartenberg, M.; Hemmatazad, H. Epidural tumor pseudoprogression after spine SBRT: A case report and a mini review of the literature. RAS Oncol. Ther. 2021, 2.
- Jabehdar Maralani, P.; Winger, K.; Symons, S.; Machnowska, M.; Heyn, C.; Helmi, A.; Chan, A.; Tseng, C.L.; Sahgal, A. Incidence and Time of Onset of Osseous Pseudoprogression in Patients with Metastatic Spine Disease from Renal Cell or Prostate Carcinoma After Treatment with Stereotactic Body Radiation Therapy. Neurosurgery 2019, 84, 647–654.
- Faruqi, S.; Tseng, C.L.; Whyne, C.; Alghamdi, M.; Wilson, J.; Myrehaug, S.; Soliman, H.; Lee, Y.; Maralani, P.; Yang, V.; et al. Vertebral Compression Fracture After Spine Stereotactic Body Radiation Therapy: A Review of the Pathophysiology and Risk Factors. Neurosurgery 2018, 83, 314–322.
- Cunha, M.V.; Al-Omair, A.; Atenafu, E.G.; Masucci, G.L.; Letourneau, D.; Korol, R.; Yu, E.; Howard, P.; Lochray, F.; da Costa, L.B.; et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): Analysis of predictive factors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e343–e349.
- Rose, P.S.; Laufer, I.; Boland, P.J.; Hanover, A.; Bilsky, M.H.; Yamada, J.; Lis, E. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J. Clin. Oncol. 2009, 27, 5075–5079.
- Al-Omair, A.; Smith, R.; Kiehl, T.R.; Lao, L.; Yu, E.; Massicotte, E.M.; Keith, J.; Fehlings, M.G.; Sahgal, A. Radiation-induced vertebral compression fracture following spine stereotactic radiosurgery: Clinicopathological correlation. J. Neurosurg. Spine 2013, 18, 430–435.
- Ozdemir, Y.; Torun, N.; Guler, O.C.; Yildirim, B.A.; Besen, A.A.; Yetisken, A.G.; Onal, H.C.; Topkan, E. Local control and vertebral compression fractures following stereotactic body radiotherapy for spine metastases. J. Bone Oncol. 2019, 15, 100218.
- Chen, W.T.; Shih, T.T.; Chen, R.C.; Lo, H.Y.; Chou, C.T.; Lee, J.M.; Tu, H.Y. Blood perfusion of vertebral lesions evaluated with gadolinium-enhanced dynamic MRI: In comparison with compression fracture and metastasis. J. Magn. Reson. Imaging 2002, 15, 308–314.
- Gui, C.; Chen, X.; Sheikh, K.; Mathews, L.; Lo, S.L.; Lee, J.; Khan, M.A.; Sciubba, D.M.; Redmond, K.J. Radiomic modeling to predict risk of vertebral compression fracture after stereotactic body radiation therapy for spinal metastases. J. Neurosurg. Spine 2021, 1–9.




