The rise of multidrug-resistant (MDR) pathogens has become a global health threat and an economic burden in providing adequate and effective treatment for many infections. This large-scale concern has emerged mainly due to mishandling of antibiotics (ABs) and has resulted in the rapid expansion of antimicrobial resistance (AMR). Nowadays, there is an urgent need for more potent, non-toxic and effective antimicrobial agents against MDR strains. In this regard, clinicians, pharmacists, microbiologists and the entire scientific community are encouraged to find alternative solutions in treating infectious diseases cause by these strains. In its “10 global issues to track in 2021”, the World Health Organization (WHO) has made fighting drug resistance a priority.
1. Introduction
Antimicrobial resistance (AMR) is a process that occurs naturally and has been known for more than 50 years, when
Staphylococcus aureus began to develop penicillin resistance
[1]. Nowadays, AMR has become a public health emergency, mainly due to inappropriate use of antibiotics (ABs)
[2]. In addition, socioeconomic aspects such as: poor community hygiene, poor control of hospital infections, use of antibiotics in the animal and food industry are key determinants for the development of AMR
[3][4].
In 2017, the World Health Organization (WHO) published a list of bacteria that are in urgent need for new ABs. The following bacteria were classified as having critical priority:
Acinetobacter baumannii,
Pseudomonas aeruginosa and
Enterobacteriaceae (Enterobacterales).
Enterobacterales include:
Klebsiella pneumoniae,
Escherichia coli,
Enterobacter spp.,
Serratia spp.,
Proteus spp.,
Providencia spp. and
Morganella spp. Next on the list are the priority ones:
Enterococcus faecium,
Staphylococcus aureus,
Helicobacter pylori,
Campylobacter spp.,
Salmonellae and
Neisseria gonorrhoeae [5].
The reality of AMR in Europe is highlighted by the “Surveillance Atlas of Infectious Diseases”—an ample database conceived by European Centre for Disease Prevention and Control. Situation varies broadly from country to country, but south-eastern region seems to be the most affected
[4][6]. For instance, in 2020, Bulgaria was the most affected European country by
K. pneumoniae resistance to 3rd-generation cephalosporins (
Table 1). Even worse,
Acinetobacter spp. were resistant to carbapenems in most European countries, with Croatia ranking first (
Table 2)
[6]. These facts are by no means recent. According to previous preliminary findings of a European study, southern and eastern Europe have greater rates of AMR than northern Europe, since these regions consume more ABs per person
[7]. Additionally, people in several southern European nations are more fearful of getting sick than those in northern Europe. Other influences are variations in prescription reimbursement programs, the accessibility of over-the-counter ABs, and drug company marketing
[8].
Table 1. European prevalence of critical priority bacteria that acquired 3rd generation cephalosporin-resistance
[6].
| Bacteria |
% of Bacteria Resistant to
3rd Generation Cephalosporins |
Country |
| Klebsiella pneumoniae |
79.1 |
Bulgaria |
| 74.5 |
Greece |
| 67.9 |
Romania |
| 63.0 |
Poland |
| 54.7 |
Cyprus |
| 54.4 |
Slovakia |
| Escherichia coli |
41.4 |
Bulgaria |
| 29.8 |
Cyprus |
| 27.1 |
Slovakia |
| 26.4 |
Italy |
| 24.1 |
Latvia |
| 21.9 |
Greece |
Table 2. European prevalence of critical priority bacteria that acquired carbapenem-resistance
[6].
| Bacteria |
% of Bacteria Resistant to Carbapenems |
Country |
| Acinetobacter spp. |
96.4 |
Croatia |
| 94.6 |
Greece |
| 93.3 |
Romania |
| 91.1 |
Lithuania |
| 82.9 |
Bulgaria |
| 82.7 |
Latvia |
| Pseudomonas aeruginosa |
48.9 |
Slovakia |
| 43.9 |
Romania |
| 42.9 |
Bulgaria |
| 35.7 |
Greece |
| 33.8 |
Hungary |
| 30.3 |
Croatia |
| Klebsiella pneumoniae |
66.3 |
Greece |
| 48.3 |
Romania |
| 29.5 |
Italy |
| 28.1 |
Bulgaria |
| 19.8 |
Cyprus |
| 19.1 |
Croatia |
| Escherichia coli |
0.8 |
Bulgaria |
| 0.7 |
Romania |
| 0.5 |
Greece |
| 0.5 |
Italy |
| 0.4 |
Spain |
| 0.2 |
Portugal |
A very important factor in increasing global AMR is strongly connected to disruptions in the “one health” principles
[9]. The massive and inadequate use of ABs in humans, animals or agriculture has led to the growth in microorganisms resistant to the drugs available today
[4][9]. Antibiotic-resistant microorganisms can be transmitted in various ways, such as via water, food or waste
[10].
The animal industry represents a key element in the development of AMR. In developed countries, 60–80% of all ABs are purchased for animals, especially poultry, pigs and cattle
[11][12]. Moreover, the highest rate of AMR targets the most commonly used ABs in the animal industry: penicillin, tetracyclines and sulfonamides
[11]. In addition to ABs, the use of metal ions contributes to the occurrence of AMR. For example, cattle are fed zinc and copper to promote growth and productivity. This elevated AMR of fecal bacteria, since copper-resistant bacteria have shown resistance to ABs such as ampicillin and sulfanilamide
[12][13]. Moreover, smaller farms seem to be better protected from antibiotic-resistant microorganisms than larger ones through better management
[12][13]. This is due to the superior stringency in cleaning animals or stables, and the effective separation of healthy animals from sick ones
[13].
One other important factor involved in AMR is related to the way water is processed
[14][15]. A fraction of ABs ingested by humans or animals are subsequently excreted and reach the sewer
[14][15]. From there, some ABs pass into natural water sources used in agriculture or for animals. In Italy, a study revealed that 273
E. coli strains isolated from water used in agriculture were resistant to ampicillin, tetracycline, sulfamethoxazole and streptomycin
[14].
In 2015, the WHO has developed an action plan to combat AMR. Among its objectives, hygiene and sanitation measures were mentioned, as well as reducing the use of antibiotics in human and animal health and increasing investment in new drugs and vaccines, as part of the main strategies to combat the spread of resistant restrains
[16].
Since 2017, nine new antibacterial active ingredients were approved by the Food and Drug Administration (delafloxacin, eravacycline, meropenem—vaborbactam, plazomicin, omadacycline, cefiderocol, pretomanid, relebactam—imipenem/cilastatin and lefamulin) and one vaccine for tuberculosis
[17]. Nearly half of the recently approved ABs act against carbapenem-resistant
Enterobacterales, Oxacillinase-48-producing
Enterobacterales and β-lactamase-producing
Enterobacterales [17]. A total of 32 antibiotics act on the pathogens mentioned in the WHO’s priority pathogens list
[17]. However, new treatment options for carbapenem-resistant
A. baumannii and carbapenem-resistant
P. aeruginosa are still lacking
[18]. It is worth mentioning that carbapenems are considered as a last line of drugs for the treatment of severe infections. Thus, the increasing frequency of Gram-negative bacteria producing extended spectrum enzymes able to inactivate carbapenems is a major public health concern
[19].
Essential oils (EOs) are lipophilic, volatile compounds extracted from various parts of the plants (such as: leaves, roots, stem, flowers, buds, fruits, seeds, and woods), known for their antipathogen properties (such as: antibacterial, antifungal, antiviral, and insecticidal properties)
[20]. They are secondary metabolites produced by the plants in order to counter fight the aggression of pests and predators, enhance seed dispersal or to attract the pollinators
[20]. They represent a source of robust antimicrobial agent or reversal substances of drug-resistant strains against aggressive microbes
[21]. Their wide spectrum of applications encompasses cosmetics (including perfumery and soap or even insect repellents), the beverage and food industry (such as flavoring agents, preservation additives, and disinfecting agents), agriculture (such as pesticides, fungicides or insecticides), as well as the drug industry (potent antimicrobial agents used in controlling healthcare related infections or as an alternative to medico-therapeutic techniques)
[22].
Several studies reported EOs which possess direct antibacterial properties, as well as sensitizing or re-sensitizing actions on drug-resistant strains against pathogenic bacteria
[23]. EOs are seen as agents with a great antimicrobial potential based on their natural origin. The high antibacterial effect of EOs was observed especially in members of
Lamiaceae family and was attributed to the high content of thymol and carvacrol (monoterpenoid phenols, derivates of cymene)
[24], even if, in some genera, the composition of essential oils can change, even considerably, depending on the different environmental conditions
[25][26]. Data regarding the bioavailability of EOs in humans are extremely limited, with oral, pulmonary and dermal absorption being considered effective
[20]. Due to their lipophilic structure, EOs can easily cross cell membranes, including the blood–brain barrier (with psychological effects in several central nervous system (CNS) diseases such as: anxiety, depression, insomnia, etc.)
[27]. A dose-dependent selectivity of EOs was noticed, as well as a non-toxic effect against normal human cells has been demonstrated, although for the moment, limited data are available regarding they safety
[20][28]. Among the commonly reported adverse effects of EOs are sensitization, irritation, photosensitization, dermatitis, neurotoxicity, organ toxicity, as well as endocrine imbalances
[29]. Due to their low molecular weight, lipophilicity and protein binding ability, they can easily cross also the placenta, arriving to the fetal circulation and causing fetotoxicity
[30][31][32]. Therefore, their safety profile in humans should be closely monitored and more intensively investigated, as what is currently known about the adverse effects of EOs is derived from non-systematic investigation and absence of spontaneous reporting systems, contrary to authorized medicinal products
[29].
2. WHO Critical Priority Pathogens
2.1. Carbapenem-Resistant Acinetobacter baumannii
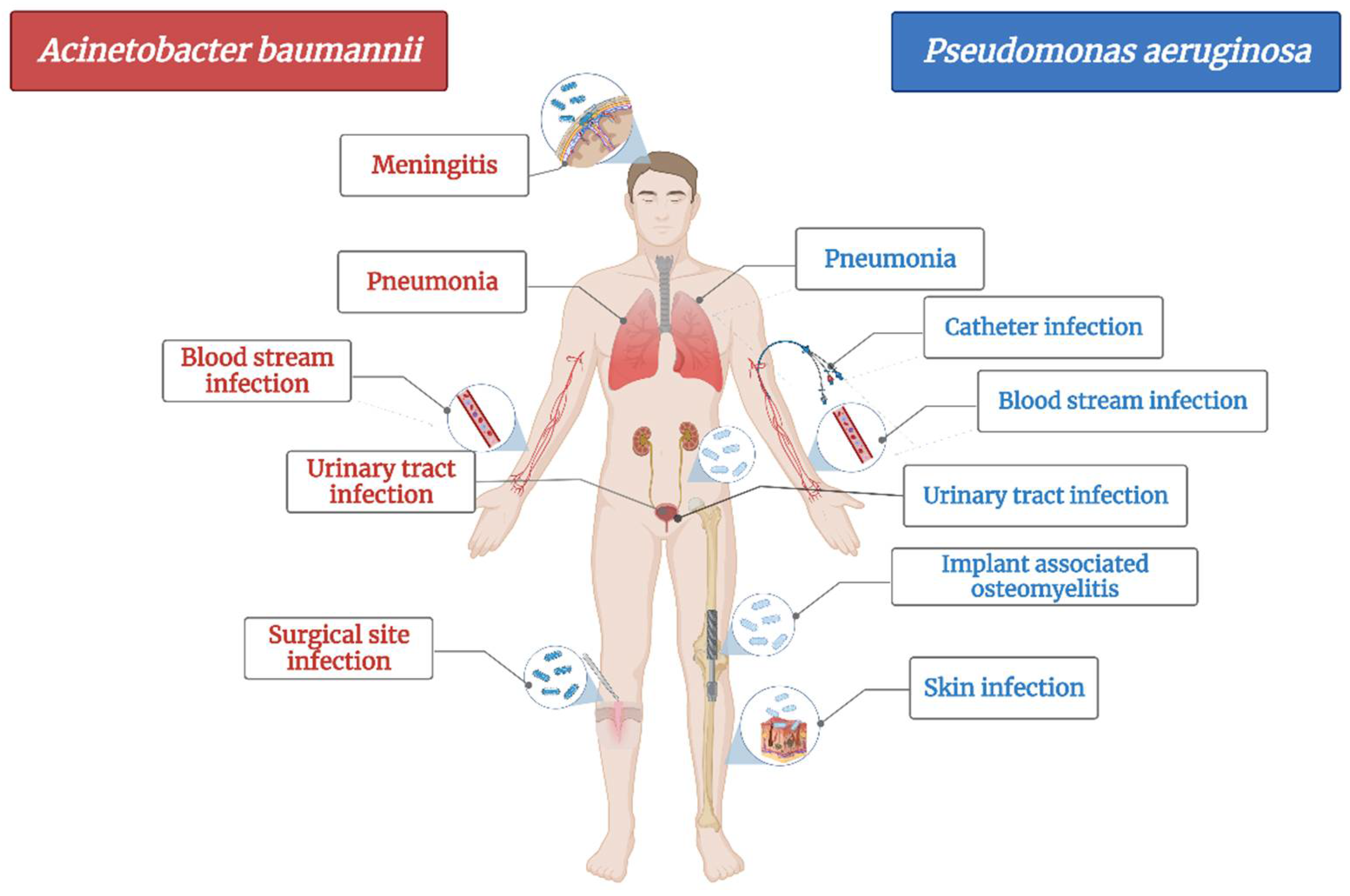
A. baumannii is a cause of serious healthcare associated infections. Some clinical mani-festations are pneumonia, bloodstream infections, infections of lower respiratory tract, urinary tract and wounds, skin infections, meningitis, osteomyelitis and endocarditis (
Figure 1)
[33]. It has the ability to survive on abiotic surfaces and against disinfectant, which turns it into a “successful” nosocomial pathogen
[34]. Plasticity of
Acinetobacter spp. led to its rapid evolution in terms of developing resistance and turned it into a serious threat to hospitalized patients as treatment options are shrinking
[35].
Figure 1. The main diseases induced by Acinetobacter baumannii and Pseudomonas aeruginosa.
Among “ESKAPE” pathogens (
E. faecium,
S. aureus,
K. pneumoniae,
A. baumanni,
P. aeruginosa and
E. cloacae),
A. baumannii has been classified as being one of the most severe bacteria in terms of antimicrobial resistance (considered nowadays to be multidrug-resistant (MDR), extensively drug-resistant (XDR), and even pandrug-resistant strain (PDR)) as it shows resistance for almost all first-line antibiotics used for healthcare associated infections (problem that has emerged since the late ’70s)
[33][36].
Hospitalized patients have a higher risk of infection with
A. baumanii due to its ability to penetrate through skin and respiratory devices
[33]. The most common healthcare associated infection induced by
A. baumannii is pneumonia, encountered mainly in patients in the intensive care unit as well as in immunocompromised ones
[33][34][36].
A. baumannii strains can develop multiple mechanisms of antibiotic resistance. Currently, these strains are resistant to the broad-spectrum β-lactam antibiotics, carboxypenicillins, third generation of cephalosporins, and most recently to carbapenems
[34]. Colistin and tigecycline have been reported to still be effective on MDR strains, alone or in combination, with some exceptions
[34][37].
An important virulence factor in
A. baumannii is the outer membrane protein (OmpA), which increases cell death by targeting mitochondria and the nucleus
[33][36]. In addition, pure OMP38 induces apoptosis of human monocytes and epithelial cells
[33][36]. Apoptosis of epithelial cells can reduce the surface of the mucosa and provide a way for bacteria to penetrate deeper into the tissues. Biofilms form on surfaces, and their matrix is composed of carbohydrates, nucleic acids, proteins, and other macromolecules
[38]. The biofilm protects bacteria from environmental damage by host responses, antibiotics, detergents and disinfectants. Therefore, biofilms contribute to prolonged and more severe bacterial effects
[38]. Biofilm-producing
A. baumanii strains have a higher survival rate than those without biofilm. These biofilm-producing strains showed a 10–13-day survival rate on dry, untouched surfaces in intensive care units compared to other Gram-negative bacteria. In addition, these strains are able to survive on hospital bed rails and in humid environments
[38].
In a study conducted in 2019, the microscopic analysis of biofilm showed that the inhibition of biofilm formation is correlated with the duration of treatment and the dose of antibiotic administered. The inhibition was much more visible at longer administration and at a higher concentration
[39].
Numerous EOs have been shown to elicit antimicrobial activity and potency on
A. baumanii strains and could be therefore seen as antibiotic alternatives and adjuvants in treating these infections
[25][26][27][28][29][30].
Anchana SR et al., in 2021, evaluated the in vitro antibiofilm effect of
Ocimum sanctum L. EO on 73 strains of carbapenem-resistant
A. baumannii, by targeting csgA gene. The biofilm assay results highlighted a 58.9% high-grade, 31.5% low-grade and the rest (9.58%) non-biofilms formers. The minimum biofilm inhibitory concentration (MBEC50%) was 25 mcL and MBEC90% = 50 mcL, making
O. sanctum L. EO (through its main active ingredient: benzofuran) effective in targeting this gene in carbapenem-resistant
A. baumannii strains
[40].
Two articles were found regarding the effect of
Origanum vulgare L. EO on some of WHO List of critical pathogens
[41].
Origanum vulgare L. EO was found to induce destabilization and rupture of bacterial cell membrane, and thus, apoptosis, in
A. baumannii—metallo-β-lactamase and carbapenemase producer strains (clinical isolates) by the study performed by Amaral SC et al., in 2020. Moreover, associated with polymyxin B, it induced a synergic activity (FICI: 0.18–0.37; checkerboard assay) with a 16 fold reduction in polymyxin’s minimum inhibitory concentration (MIC)
[41]. The Fractional Inhibitory Concentration Index (FICI) is a mathematical expression, used for describing the effect of antimicrobial agent combinations (e.g., additive, synergistic, antagonistic effects)
[42]. It is defined as the MIC of drug A, in the presence of drug B, divided by the MIC of drug A alone (and vice versa). This concept gives an indication of the degree of drug interaction (e.g., synergy: FICI ≤ 0.5; no interaction: FICI > 0.5–4; antagonism: FICI > 4)
[42].
Three studies were found regarding
Mentha spp.’s antibacterial effect on carbapenem-resistant
A. baumannii strains
[43][44][45]. In the article published by Rinaldi F and colleagues in 2020, the effect of two EOs (
Thymus vulgaris L. and
Syzygium aromaticum L.) chitosan coated nanoformulations was analyzed
[46]. Both intranasal formulations manifested excellent antibacterial properties against carbapenem-resistant—
A. baumannii and
K. pneumoniae strains, with a MIC/MBC: 0.03%
v/
v. A remarkable antibacterial, concentration-dependent effect was observed in
T. vulgaris L. against both types of bacteria. When the two main phytocompounds of the EOs, thymol and eugenol, were analyzed separately, they showed an unpredicted performance, suggesting the fact that the potent antimicrobial activity of
T. vulgaris L. is attributed to the synergic actions of all active constituents of an EO, and not just one.
S. aromaticum L. chitosan coated nanoformulations induced a bactericidal effect against
A. baumannii-carbapenem-resistant strains after 2 h at a conc. of 0.125%
v/
v and after 6 h, at a conc. of 0.06%
v/
v, with absence of bacterial growth. In contrast,
T. vulgaris L. nanoformulations induced a more rapid bactericidal effect, after 2 h incubation at a conc. of 0.06%
v/
v. Therefore, both EOs chitosan nanoformulations make them promising solutions against MDR bacterial strains of clinical concern, as for most of the available drugs, blood–brain barrier is the major limiting factor for drugs to reach subarachnoid space
[46].
2.2. Carbapenem-Resistant Pseudomonas aeruginosa
Likewise,
Pseudomonas aeruginosa is also an opportunistic pathogen, the most common bacteria responsible for healthcare associated infections (
Figure 1) and ventilator-associated pneumonia. It mainly affects cystic fibrosis patients and immunocompromised people and less often, healthy individuals
[47]. Based on the fact that its genome is relatively large compared with other MDR strains, it has an enhanced capacity of coding regu-latory enzymes involved in the metabolism, transportation and efflux of organic substances, making it a bacterium with high adaptability and versatility to environmental changes
[48]. Therefore, just as
A. baumannii, it has developed the ability to resist most of the available antibiotics
[49]. It is currently resistant to β-lactams, aminoglycosides and quinolones
[47].
P. aeruginosa resistance to classical antibiotics has been classified and divided in three main types: intrinsic (by decreasing the outer membrane permeability, or over-expression of efflux pumps—which expel the antibiotic out of the cell or production of enzymes that inactivate the antibiotic), acquired (horizontal transfer of resistance genes = plasmids carrying genetic materials or mutations) and adaptive (formation of biofilm in the patient’s lungs that prevents antibiotic penetration and which induce prolonged and recurrent infections)
[47][50]. Moreover, the outer membrane of Gram-negative bacteria is another limiting factor for antibiotic penetration, as it acts as a selective barrier, planted with porins
[47][49]. More specifically, the outer membrane of
P. aeruginosa has a 12–100× decreased permeability compared with that of
E. coli [51].
P. aeruginosa’s resistance to broad-spectrum drugs such as carbapenems and cephalosporins is due to its ability to adapt by reducing the number of nonspecific porin proteins and replacing them with specific channels with low permeability to toxic chemicals
[48]. Many carbapenem-resistant
P. aeruginosa strains have been shown to be deficient in OprD porin (which is involved in antibiotic uptake and which contains the biding sites for carbapenems)
[48].
Multidrug efflux pumps contribute also to its antibiotic resistance: they expel toxic and antimicrobial materials out of the bacterial cell. The four well known active multidrug efflux pumps of
P. aeruginosa are MexAB-OprM, MexXY/OprM(OprA), MexCD-OprJ, and MexEF-OprN
[47][50][52].
The long presence of
P. aeruginosa in clinical settings is attributed to the formation of biofilms on lung epithelial cells surfaces, through the production of DNA, proteins and exopolysaccharides
[47][49]. These biofilms are characterized by effective cell-to-cell communication methods, known as quorum sensing. Three main quorum sensing systems (LasR, RhlI-RhlR, PQS-MvfR) are known contributors to the formation of mature and differentiated types of
P. aeruginosa biofilms
[47][49].
Modifications in the lipopolysaccharide (the central component of the Gram-negative bacterial membrane), production of bacterial enzymes (β-lactamases, metallo-β-lactamases, aminoglycoside modifying enzymes) as well as mutations are other mechanisms by which this pathogen can acquire resistance to classical antibiotics
[53].
Various EOs are effective against carbapenem-resistant
P. aeruginosa. Oliva A et al., investigated, in the study published in 2018, the antibacterial effect of
Melaleuca alternifolia L., tea tree oil (TTO) (used alone or in combination with classical antibiotics) against several MDR or PDR microorganisms (including carbapenem-resistant
P. aeruginosa, A. baumannii, K. pneumoniae, as well as methicillin-resistant
S. aureus), in both liquid and vapor phases. TTO expressed potent antibacterial activity, with MIC/MBCs of 0.25%/0.25%
v/
v for carbapenem-resistant
A. baumannii, K. pneumoniae and of 1%/1%
v/
v for carbapenem-resistant
P. aeruginosa. The MIC/MBC of TTO for methicillin-resistant
Staphylococcus aureus (MRSA) was 0.5%/2%
v/
v. For all tested strains, an absence of bacterial growth was observed after 24 h incubation-period. TTO expressed a potent synergistic activity at sub-inhibitory concentrations with cefazolin (lowering the MIC from 32 to 1 mcg/mL), oxacillin (from 64 to 2 mcg/mL) and with amikacin against MRSA. Regarding the synergistic activity expressed with the tested Gram-negative strains, it showed a good bactericidal action with meropenem, amikacin and colistin. Therefore, the authors postulated that TTO could be taken into account as a possible non-conventional inhalation therapy for lung infections (e.g., caused by carbapenem-resistant
A. baumannii) or other treatment regimens (used alone or in combination with classical antibiotics) against MDR and PDR strains
[54].