
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alessandra Bearz | -- | 3136 | 2022-07-20 09:13:27 | | | |
| 2 | Vivi Li | -13 word(s) | 3123 | 2022-07-21 03:32:32 | | |
Video Upload Options
The scenario of neoadjuvant and adjuvant settings in non-small cell lung cancer (NSCLC) is rapidly evolving. As already happened for the advanced disease, also early stages have entered the era of precision medicine, with molecular analysis and Programmed death-ligand 1 (PD-L1) evaluation that by now can be considered a routine assessment. New treatment options have been approved, with osimertinib now part of clinical practice for Epidermal Growth Factor Receptor mutated (EGFRm) patients, and immune checkpoint inhibitors (ICIs) available after FDA approval both in the adjuvant (atezolizumab) and neoadjuvant (nivolumab) setting. Several clinical trials with specific-tyrosine kinase inhibitors (TKIs) and ICIs are ongoing, both with and without concomitant chemotherapy. As therapeutic strategies are rapidly expanding, quite a few questions remain unsettled, such as the optimal duration of adjuvant targeted therapy or the effective benefit of ICIs in early-stage EGFRm or ALK (Anaplastic Lymphoma Kinase) rearranged patients, or the possibility to individuate high-risk patients after surgical resection assessing minimal residual disease (MRD) by ctDNA evaluation.
1. Introduction
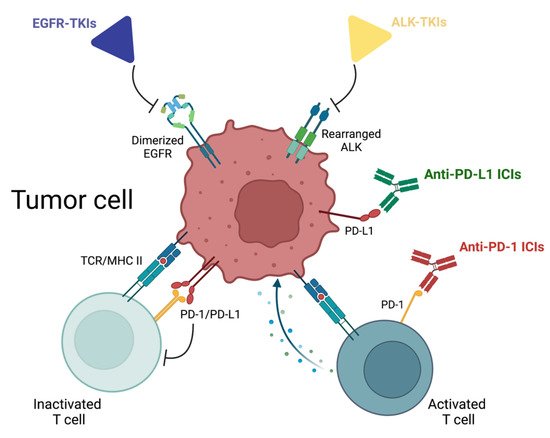
2. EGFR Tyrosine Kinase Inhibitors
2.1. Adjuvant Setting
| Clinical Trial | Phase | N° pts a | Years | Stage | Treatment Arms | DFS | OS |
|---|---|---|---|---|---|---|---|
| BR19 [8] (NCT00049543) | III | 503 (EGFRm- unselected) |
2002–2005 | IB-IIIA | Gefitinib × 2 y vs. placebo (after adj CT) (1:1) |
No difference (HR 1.22, 95% CI 0.93–1.61, p = 0.15) |
No difference (HR 1.24, 95% CI 0.94–1.64, p = 0.14) |
| ADJUVANT-CTONG1104 [9] (NCT01405079) | III | 222 | 2011–2014 | II-IIIA | Gefitinib × 2 y vs. adj CT (1:1) |
30.8 vs. 19.8 m (HR 0.56, 95% CI 0.40–0.79, p = 0.001) |
75.5 vs. 62.8 m (HR 0.92, 95% CI 0.62–1.36, p = 0.674) |
| IMPACT [10] (UMIN000006252) |
III | 234 | 2011–2015 | II-III | Gefitinib × 2 y vs. adj CT (1:1) |
35.9 vs. 25.1 m (HR 0.92, 95% CI 0.67–1.28, p = 0.63) |
No difference (HR 1.03, 95% CI 0.65–1.65, p = 0.89) |
| RADIANT [11] (NCT00373425) |
III | 973 (‘EGFR- positive’) |
2007–2010 | IB-IIIA | Erlotinib × 2 y vs. placebo (after adj CT) (2:1) |
50.5 vs. 48.2 m (HR 0.90, 95% CI 0.74–1.10, p = 0.324) |
Not reached (HR 1.13, 95% CI 0.88–1.45, p = 0.335) |
| SELECT [12] (NCT00567359) | II | 100 | 2008–2012 | IA-IIIA | Erlotinib × 2 y (after adj CT) |
Not reached (5-year DFS rate 56%) |
Not reached (5-year OS rate 86%) |
| EVAN [13][14] (NCT01683175) | II | 102 | 2012–2015 | IIIA | Erlotinib × 2 y vs. adj CT (1:1) |
42.4 vs. 21.0 m (HR 0.27, 95% CI 0.14–0.53, p < 0.0001) |
84.2 vs. 61.1 m (HR 0.32, 95% CI 0.15–0.67) |
| EVIDENCE [15] (NCT02448797) | III | 322 | 2015–2019 | II-IIIA | Icotinib × 2 y vs. adj CT (1:1) |
47.0 vs. 22.1 m (HR 0.36, 95% CI 0.24–0.55, p < 0.0001) |
Not reached (HR 0.91, 95% CI 0.42–1.94) |
| ADAURA [16][17] (NCT02511106) | III | 682 | 2015–2019 | IB-IIIA | Osimertinib × 3 y vs. placebo (after adj CT or not) (1:1) |
Not reached vs. 27.5 m (HR 0.20, 99% CI 0.14–0.30, p < 0.001) b |
Not reached (2-year OS rate 98% vs. 85%) b |
| Clinical Trial | Phase | N° pts | Estimated Primary Completion | Stage | Treatment Arms | Primary Endpoint |
|---|---|---|---|---|---|---|
| NCT02518802 | III | 220 | Jan 2018 | II-IIIA | Gefitinib × 2 y started during or after CT vs. adj CT |
DFS |
| NCT03381430 | II | 50 | Mar 2023 | IIIA N2 | Gefitinib × 2 y + adj RT | DFS |
| NCT02193282 | III | 450 a | Oct 2026 | IB-IIIA | Erlotinib × 2 y vs. placebo (after adj CT) |
OS |
| ICWIP [18] (NCT02125240) |
III | 124 | Dec 2018 | II-IIIA | Icotinib × 3 y vs. placebo | DFS |
| ICTAN (NCT01996098) |
III | 318 | Jan 2020 | II-IIIA | Icotinib × 6 m vs. icotinib × 12 m vs. observation (after adj CT) |
DFS |
| NCT03983811 | III | 174 | Oct 2021 | IIB-IIIA | Icotinib/placebo on days 8–15 during adj q21 CT cycles, then × 2 y |
DFS |
| CORIN (NCT02264210) |
II | 128 | Dec 2025 | IB | Icotinib × 12 m vs. observation | DFS |
| NCT01746251 | II | 92 | Nov 2020 | I-III | Afatinib × 3 m vs. afatinib × 2 y | RFS |
| ADAURA2 (NCT05120349) | III | 380 | Aug 2027 | IA2-IA3 | Osimertinib × 3 y vs. placebo | DFS |
| FORWARD (NCT04853342) | III | 318 | Dec 2023 | II-IIIA | Furmonertinib vs. placebo (after adj CT) |
DFS |
| ATHEM (NCT05165355) |
II | 90 | Nov 2024 | IB-IIA b | Furmonertinib × 3 y | DFS |
| NCT04687241 | III | 192 | Jan 2026 | II-IIIB N2 | Almonertinib vs. placebo (after adj CT) |
DFS |
| APEX (NCT04762459) |
III | 606 | May 2026 | II-IIIA | Almonertinib × 3 y vs. almonertinib + adj CT vs. adj CT (3:2:1) |
DFS |
2.2. Neoadjuvant Setting
| Clinical Trial | Phase | N° pts | Estimated Primary Completion | Stage | Treatment Arms | Primary Endpoint |
|---|---|---|---|---|---|---|
| NCT03656393 | III | 48 | Jul 2020 | II-IIIA | Gefitinib × 56 d vs. CT × 6 w (+ adj CT if not responding disease) |
2-year DFS rate |
| NCT03203590 | III | 590 | Jan 2026 | II-IIIA | Gefitinib × 8 w vs. CT × 2 cycles | 2-year DFS rate |
| NCT03749213 | II | 36 | Feb 2022 | IIIA N2 | Neoadj icotinib × 8 w, then × 2 y after surgery |
ORR |
| Neoafa (NCT04470076) |
II | 30 | Dec 2021 | II-IIIB | Neoadj CT + afatinib (48 h after and until 24 h before CT) × 3 cycles, then adj afatinib × 2 y after surgery |
MPR, ORR |
| NCT03433469 | II | 27 | Dec 2022 | I-IIIA | Neoadj osimertinib × 1–2 cycles | MPR |
| NeoADAURA [26] (NCT04351555) |
III | 328 | Mar 2024 | II-IIIB N2 | Neoadj osimertinib + CT × 3 cycles vs. placebo + CT vs. osimertinib alone (1:1:1) | MPR |
3. ALK Tyrosine Kinase Inhibitors
| Clinical Trial | Phase | N° pts | Estimated Primary Completion | Stage | Treatment Arms | Primary Endpoint |
|---|---|---|---|---|---|---|
| ALCHEMIST [32] (NCT02194738) | III | 8300 a | Sep 2026 | IB-IIIA | Crizotinib × 2 y vs. observation (after adj CT) |
OS |
| ALINA [33] (NCT03456076) |
III | 257 | Jun 2023 | IB-IIIA | Alectinib × 2 y vs. adj CT | DFS |
| NCT05341583 | III | 202 | Jun 2025 | II-IIIB | Ensartinib × 2 y vs. placebo | DFS |
| NCT05186506 | II | 152 | Dec 2025 | II-IIIA | Ensartinib × 2 y vs. adj CT | DFS |
| NCT05241028 | II | 80 | Feb 2027 | IB-IIIA | Ensartinib × 3 y (after adj CT) | 3-year DFS rate |
| ALNEO [34] (NCT05015010) | II | 33 | May 2023 | III | Neoadj alectinib × 8 w, then adj × 96 w after surgery | MPR |
| NAUTIKA1 (NCT04302025) | II | 80 a | Mar 2023 | IB-III | Neoadj alectinib × 8 w, then adj CT and alectinib × 2 y |
MPR |
References
- Pignon, J.-P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559.
- Burdett, S.; Pignon, J.P.; Tierney, J.; Tribodet, H.; Stewart, L.; Le Pechoux, C.; Aupérin, A.; Le Chevalier, T.; Stephens, R.J.; Arriagada, R.; et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst. Rev. 2015, 3, CD011430.
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51.
- Zhang, Y.-L.; Yuan, J.-Q.; Wang, K.-F.; Fu, X.-H.; Han, X.-R.; Threapleton, D.; Yang, Z.-Y.; Mao, C.; Tang, J.-L. The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 2016, 7, 78985–78993.
- Rodig, S.J.; Mino-Kenudson, M.; Dacic, S.; Yeap, B.Y.; Shaw, A.; Barletta, J.A.; Stubbs, H.; Law, K.; Lindeman, N.; Mark, E.; et al. Unique Clinicopathologic Features Characterize ALK-Rearranged Lung Adenocarcinoma in the Western Population. Clin. Cancer Res. 2009, 15, 5216–5223.
- Blackhall, F.H.; Peters, S.; Bubendorf, L.; Dafni, U.; Kerr, K.M.; Hager, H.; Soltermann, A.; O’Byrne, K.J.; Dooms, C.; Sejda, A.; et al. Prevalence and Clinical Outcomes for Patients With ALK-Positive Resected Stage I to III Adenocarcinoma: Results From the European Thoracic Oncology Platform Lungscape Project. J. Clin. Oncol. 2014, 32, 2780–2787.
- Takahashi, T.; Sakai, K.; Kenmotsu, H.; Yoh, K.; Daga, H.; Ohira, T.; Ueno, T.; Aoki, T.; Hayashi, H.; Yamazaki, K.; et al. Predictive value of EGFR mutation in non–small-cell lung cancer patients treated with platinum doublet postoperative chemotherapy. Cancer Sci. 2021, 113, 287–296.
- Goss, G.D.; O’Callaghan, C.; Lorimer, I.; Tsao, M.-S.; Masters, G.A.; Jett, J.; Edelman, M.J.; Lilenbaum, R.; Choy, H.; Khuri, F.; et al. Gefitinib Versus Placebo in Completely Resected Non–Small-Cell Lung Cancer: Results of the NCIC CTG BR19 Study. J. Clin. Oncol. 2013, 31, 3320–3326.
- Zhong, W.-Z.; Wang, Q.; Mao, W.-M.; Xu, S.-T.; Wu, L.; Wei, Y.-C.; Liu, Y.-Y.; Chen, C.; Cheng, Y.; Yin, R.; et al. Gefitinib Versus Vinorelbine Plus Cisplatin as Adjuvant Treatment for Stage II-IIIA (N1-N2) EGFR-Mutant NSCLC: Final Overall Survival Analysis of CTONG1104 Phase III Trial. J. Clin. Oncol. 2021, 39, 713–722.
- Tada, H.; Mitsudomi, T.; Misumi, T.; Sugio, K.; Tsuboi, M.; Okamoto, I.; Iwamoto, Y.; Sakakura, N.; Sugawara, S.; Atagi, S.; et al. Randomized Phase III Study of Gefitinib Versus Cisplatin Plus Vinorelbine for Patients with Resected Stage II-IIIA Non–Small-Cell Lung Cancer with EGFR Mutation (IMPACT). J. Clin. Oncol. 2022, 40, 231–241.
- Kelly, K.; Altorki, N.K.; Eberhardt, W.E.E.; O’Brien, M.E.R.; Spigel, D.R.; Crinò, L.; Tsai, C.-M.; Kim, J.-H.; Cho, E.K.; Hoffman, P.C.; et al. Adjuvant Erlotinib Versus Placebo in Patients with Stage IB-IIIA Non–Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2015, 33, 4007–4014.
- Pennell, N.A.; Neal, J.W.; Chaft, J.E.; Azzoli, C.G.; Jänne, P.A.; Govindan, R.; Evans, T.L.; Costa, D.B.; Wakelee, H.A.; Heist, R.S.; et al. SELECT: A Phase II Trial of Adjuvant Erlotinib in Patients with Resected Epidermal Growth Factor Receptor–Mutant Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2019, 37, 97–104.
- Yue, D.; Xu, S.; Wang, Q.; Li, X.; Shen, Y.; Zhao, H.; Chen, C.; Mao, W.; Liu, W.; Liu, J.; et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): A randomised, open-label, phase 2 trial. Lancet Respir. Med. 2018, 6, 863–873.
- Yue, D.; Xu, S.-D.; Wang, Q.; Li, X.; Shen, Y.; Zhao, H.; Chen, C.; Mao, W.; Liu, W.; Liu, J.; et al. Updated overall survival (OS) and exploratory analysis from the randomized, phase II EVAN study of erlotinib (E) versus vinorelbine plus cisplatin (NP) as adjuvant therapy in Chinese patients with stage IIIA EGFR+ NSCLC. J. Clin. Oncol. 2021, 39, 8520.
- He, J.; Su, C.; Liang, W.; Xu, S.; Wu, L.; Fu, X.; Zhang, X.; Ge, D.; Chen, Q.; Mao, W.; et al. Icotinib versus chemotherapy as adjuvant treatment for stage II–IIIA EGFR-mutant non-small-cell lung cancer (EVIDENCE): A randomised, open-label, phase 3 trial. Lancet Respir. Med. 2021, 9, 1021–1029.
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723.
- Wu, Y.-L.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Kim, S.-W.; Kato, T.; Laktionov, K.; Vu, H.V.; Wang, Z.; et al. Postoperative Chemotherapy Use and Outcomes From ADAURA: Osimertinib as Adjuvant Therapy for Resected EGFR-Mutated NSCLC. J. Thorac. Oncol. 2021, 17, 423–433.
- Liu, Y.-T.; Hao, X.-Z.; Liu, D.-R.; Cheng, G.; Zhang, S.-C.; Xiao, W.-H.; Hu, Y.; Liu, J.-F.; He, M.; Ding, C.-M.; et al. Icotinib as Adjuvant Treatment for Stage II-IIIA Lung Adenocarcinoma Patients with EGFR Mutation (ICWIP Study): Study Protocol for a Randomised Controlled Trial. Cancer Manag. Res. 2020, 12, 4633–4643.
- Yin, Q.; Xun, X.; Yang, G.; Cui, H.; Liu, H. Efficacy of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in the Adjuvant Setting for Patients with Resected Epidermal Growth Factor Receptor Mutant Non-Small Cell Lung Cancer: A Meta-Analysis with 11 Trials. Oncol. Res. Treat. 2021, 44, 344–353.
- Chen, R.-L.; Sun, L.-L.; Cao, Y.; Chen, H.-R.; Zhou, J.-X.; Gu, C.-Y.; Zhang, Y.; Wang, S.-Y.; Hou, W.; Lin, L.-Z. Adjuvant EGFR-TKIs for Patients with Resected EGFR-Mutant Non-Small Cell Lung Cancer: A Meta-Analysis of 1,283 Patients. Front. Oncol. 2021, 11, 629394.
- Colclough, N.; Chen, K.; Johnström, P.; Strittmatter, N.; Yan, Y.; Wrigley, G.L.; Schou, M.; Goodwin, R.; Varnäs, K.; Adua, S.J.; et al. “Preclinical Comparison of the Blood–brain barrier Permeability of Osimertinib with Other EGFR TKIs. Clin. Cancer Res. 2020, 27, 189–201.
- Lara-Guerra, H.; Waddell, T.K.; Salvarrey, M.A.; Joshua, A.M.; Chung, C.T.; Paul, N.; Boerner, S.; Sakurada, A.; Ludkovski, O.; Ma, C.; et al. Phase II Study of Preoperative Gefitinib in Clinical Stage I Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2009, 27, 6229–6236.
- Zhong, W.; Yang, X.; Yan, H.; Zhang, X.; Su, J.; Chen, Z.; Liao, R.; Nie, Q.; Dong, S.; Zhou, Q.; et al. Phase II study of biomarker-guided neoadjuvant treatment strategy for IIIA-N2 non-small cell lung cancer based on epidermal growth factor receptor mutation status. J. Hematol. Oncol. 2015, 8, 1–10.
- Xiong, L.; Li, R.; Sun, J.; Lou, Y.; Zhang, W.; Bai, H.; Wang, H.; Shen, J.; Jing, B.; Shi, C.; et al. Erlotinib as Neoadjuvant Therapy in Stage IIIA (N2) EGFR Mutation-Positive Non-Small Cell Lung Cancer: A Prospective, Single-Arm, Phase II Study. Oncologist 2018, 24, 157-e64.
- Wu, Y.-L.; Zhong, W.; Chen, K.-N.; Chen, C.; Yang, F.; Yang, X.-N.; Gu, C.; Mao, W.; Wang, Q.; Qiao, G.-B.; et al. CTONG1103: Final overall survival analysis of the randomized phase 2 trial of erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non–small cell lung cancer. J. Clin. Oncol. 2021, 39, 8502.
- Tsuboi, M.; Weder, W.; Escriu, C.; Blakely, C.; He, J.; Dacic, S.; Yatabe, Y.; Zeng, L.; Walding, A.; Chaft, J.E. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA. Futur. Oncol. 2021, 17, 4045–4055.
- Yang, P.; Kulig, K.; Boland, J.M.; Erickson-Johnson, M.R.; Oliveira, A.M.; Wampfler, J.; Jatoi, A.; Deschamps, C.; Marks, R.; Fortner, C.; et al. Worse Disease-Free Survival in Never-Smokers with ALK+ Lung Adenocarcinoma. J. Thorac. Oncol. 2012, 7, 90–97.
- Kim, M.H.; Shim, H.S.; Kang, D.R.; Jung, J.Y.; Lee, C.Y.; Kim, D.J.; Lee, J.G.; Bae, M.K.; Kim, H.R.; Lim, S.M.; et al. Clinical and prognostic implications of ALK and ROS1 rearrangements in never-smokers with surgically resected lung adenocarcinoma. Lung Cancer 2014, 83, 389–395.
- Seto, K.; Kuroda, H.; Yoshida, T.; Sakata, S.; Mizuno, T.; Sakakura, N.; Hida, T.; Yatabe, Y.; Sakao, Y. Higher frequency of occult lymph node metastasis in clinical N0 pulmonary adenocarcinoma with ALK rearrangement. Cancer Manag. Res. 2018, 10, 2117–2124.
- Paik, J.H.; Choi, C.-M.; Kim, H.; Jang, S.J.; Choe, G.; Kim, D.K.; Kim, H.J.; Yoon, H.; Lee, C.-T.; Jheon, S.; et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer. Lung Cancer 2011, 76, 403–409.
- Chaft, J.E.; Dagogo-Jack, I.; Santini, F.C.; Eng, J.; Yeap, B.Y.; Izar, B.; Chin, E.; Jones, D.R.; Kris, M.G.; Shaw, A.T.; et al. Clinical outcomes of patients with resected, early-stage ALK-positive lung cancer. Lung Cancer 2018, 122, 67–71.
- Sands, J.; Mandrekar, S.J.; Oxnard, G.R.; Kozono, D.E.; Hillman, S.L.; Dahlberg, S.E.; Sun, Z.; Chaft, J.E.; Govindan, R.; Gerber, D.E.; et al. ALCHEMIST: Adjuvant targeted therapy or immunotherapy for high-risk resected NSCLC. J. Clin. Oncol. 2020, 38, TPS9077.
- Solomon, B.J.; Ahn, J.S.; Barlesi, F.; Dziadziuszko, R.; Nishio, M.; Shaw, A.T.; Bordogna, W.; Meyenberg, C.; Wu, Y.-L. ALINA: A phase III study of alectinib versus chemotherapy as adjuvant therapy in patients with stage IB–IIIA anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2019, 37, TPS8569.
- Leonetti, A.; Minari, R.; Boni, L.; Gnetti, L.; Verzè, M.; Ventura, L.; Musini, L.; Tognetto, M.; Tiseo, M. Phase II, Open-label, Single-arm, Multicenter Study to Assess the Activity and Safety of Alectinib as Neoadjuvant Treatment in Surgically Resectable Stage III ALK-positive NSCLC: ALNEO Trial. Clin. Lung Cancer 2021, 22, 473–477.




