Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mary Virginia Orna | -- | 3221 | 2022-07-20 08:41:40 | | | |
| 2 | Conner Chen | -46 word(s) | 3175 | 2022-07-20 11:08:48 | | | | |
| 3 | Conner Chen | + 2 word(s) | 3177 | 2022-07-21 04:14:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Orna, M.V.; Fontani, M. The Modernity of Ancient Pigments. Encyclopedia. Available online: https://encyclopedia.pub/entry/25310 (accessed on 07 February 2026).
Orna MV, Fontani M. The Modernity of Ancient Pigments. Encyclopedia. Available at: https://encyclopedia.pub/entry/25310. Accessed February 07, 2026.
Orna, Mary Virginia, Marco Fontani. "The Modernity of Ancient Pigments" Encyclopedia, https://encyclopedia.pub/entry/25310 (accessed February 07, 2026).
Orna, M.V., & Fontani, M. (2022, July 20). The Modernity of Ancient Pigments. In Encyclopedia. https://encyclopedia.pub/entry/25310
Orna, Mary Virginia and Marco Fontani. "The Modernity of Ancient Pigments." Encyclopedia. Web. 20 July, 2022.
Copy Citation
Naturally occurring and synthetic ancient pigments have a history of use spanning thousands of years. Curiously, some of their newly discovered properties make them excellent candidates for semiconductors, anticounterfeiting agents and so much more.
daguerreotyping
Egyptian blue
genetic engineering
indigo
Maya blue
1. Indigo: From a Coveted Blue Dye to Genetic Engineering
Indigo is arguably one of the most ancient coloring materials known to humanity. It is also one of the few naturally occurring colorants extensively used to this day. Blue jeans alone soak up most of the 70,000 metric tons produced each year. Because the people who lived along the Indus River in India were once thought to be among the first to dye with it, they got the naming rights, but perhaps unjustly. Its use has been definitively identified on textile samples from Huaca Prieta, Peru, that date to 6000 BP [1]. Its use on a robe in ancient Thebes has been dated to about 5000 before present (BP) [2], and on a mummy wrapping, dated to approximately 3580 BP [3]. It was described by Marco Polo (1254–1324), who encountered indigo on his travels, but its large-scale importation into Europe had to wait until the 16th century.
“Indigo” is actually the term for the mix of colorants extracted from about three dozen plant species that produce it. The major historically cultivated types were Indigofera tinctoria in India, Isatis tinctoria in Europe and Indigofera suffruticosa in the Americas. The chief component is indigotin (C16H10N2O2), the formal chemical name of which is 3H-indol-3-one, 2-(1,3-dihydro-3-oxo-2H-indol-2-ylidene)-1,2-dihydro-, although the terms indigo and indigotin are synonymous in common parlance, denoting both the impure natural material and the purified chemical species.
Extracting the blue dye from the plant is both labor-intensive and difficult due to its rather complicated chemistry. It exists in the plant in the form of a precursor, indoxylic acid, a colorless glucoside. When the cellular structure of the plant is crushed in any way, such as by a feeding insect or a dye master, the plant’s chemical defenses come into play to form presumably unappetizing indigo. Chemically, indoxylic acid’s glucoside bond is ruptured enzymatically by ß-glucosidases also present in the indigo plant. These are only brought into contact with the indoxyl when the plant cells are broken open. Mashing and fermentation of the raw material in an alkaline solution (usually urine) for a few days was the ancient way to start the chain of chemical reactions; regular maceration (typically with bare feet) helped the process along [4][5]. Figure 1 shows indigo formation by (a) crushing the leaves and (b) the chemical sequence of events. Eventually, the desired deep blue indigo, insoluble in water, precipitated out and, when dried, could be cut into cakes for shipment or storage. Figure 1 is an archival photograph of workers in India cutting prepared indigo into cakes.
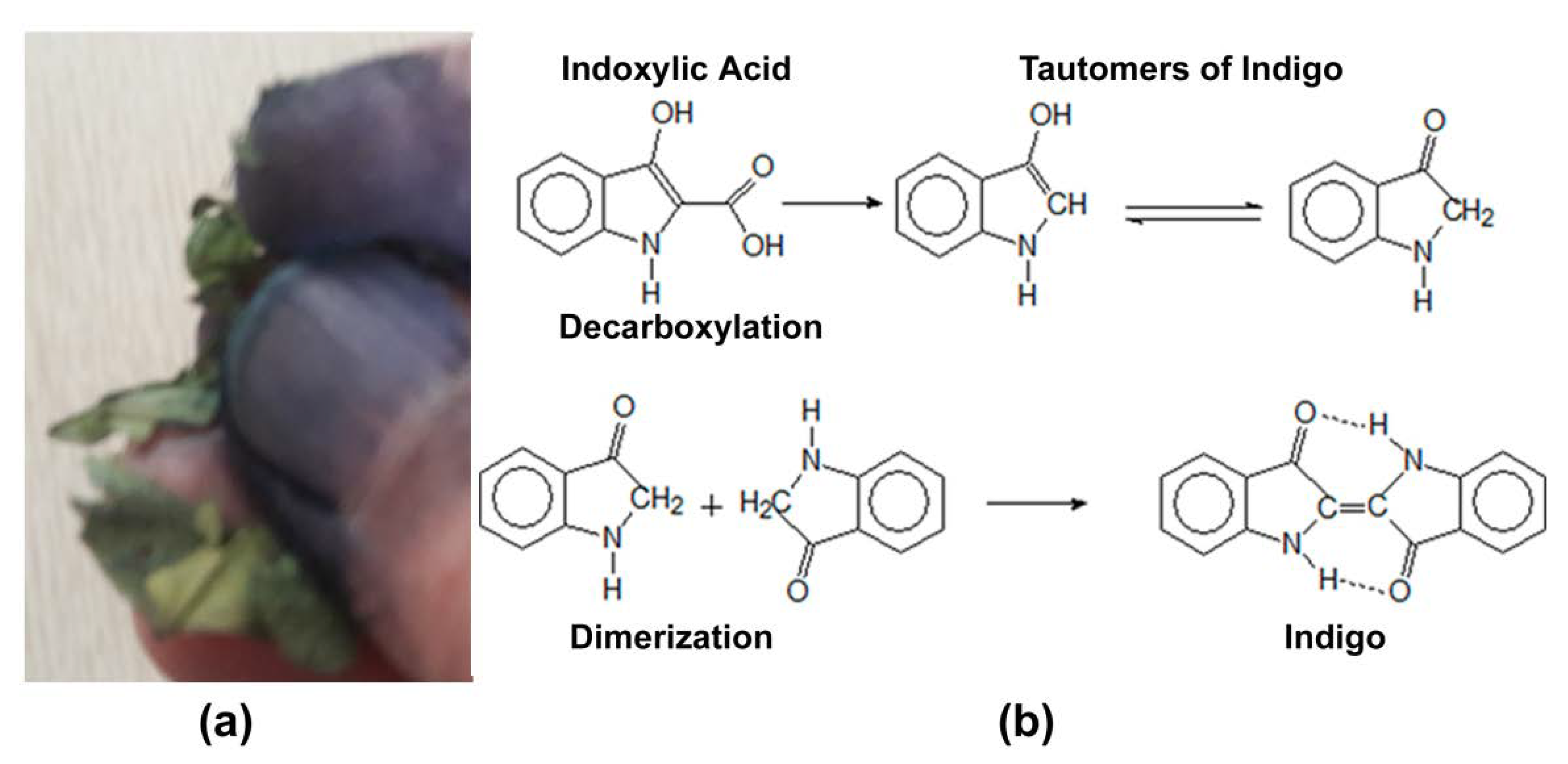
Figure 1. (a) Blue indigo formation from crushed Indigofera leaves; (b) formation of insoluble blue indigo from its indoxylic acid precursor.

Figure 2. Indigo workers in Allahabad, India, cutting the product into cakes (1877). Photograph: Oscar Mallitte. Public domain.
If, instead of a solid product, it is desired to directly dye textiles with indigo, then the fermentation bath should be maintained as an alkaline solution. When properly prepared, the broth should be near-colorless, indicating the presence of the soluble leuco form of indigo. After decanting off the plant solids, the materials to be dyed are soaked in the bath for a time and then hung out to air dry. The leuco form oxidizes spontaneously to insoluble blue indigo, which adheres to the textile fibers as tiny particles [6][7]. This process is completely reversible, the soluble, colorless leuco form being restored in the presence of a reducing agent, as shown in Figure 3.
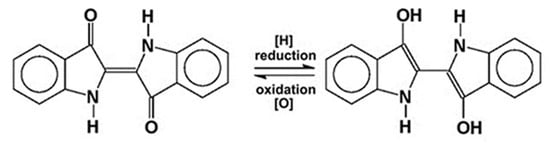
Figure 3. Reversible redox reaction of blue indigo (left) and its leuco form (right).
Because indigo was the most important industrial colorant throughout the 19th century, the great German chemist, Adolf von Baeyer (1835–1917), spent a great deal of time studying it. His aim was to determine the structure of the indigo molecule so as to develop a synthetic pathway that would do away with the wasteful and laborious processing of the natural product. Through step-by-step analysis of indigo’s degradation products, he deduced that its fundamental building block was indole, C8H7N. However, it took him another 15 years to deduce the correct structural formula in 1883 [8]. Only after laboring for another seven years was von Baeyer able to develop a commercially viable synthesis protocol, mainly because the starting materials were so expensive. The breakthrough came in 1890 when another chemist, Karl Heumann (1851–1894), at the Swiss Federal Institute accidentally discovered how to produce a feasible starting material, anthranilic acid, from cheap and plentiful naphthalene. A broken thermometer spilled mercury into the reaction vessel, and the mercury reacted with the sulfuric acid therein to form mercury sulfate, which catalyzed the reaction. Figure 4 shows the reaction sequence of the first commercially feasible process based on anthranilic acid, which it took BASF (Badische Anilinen und Soda Fabrik, a German multinational chemical company) seven years to develop. Indigo went into production using this method in 1897.
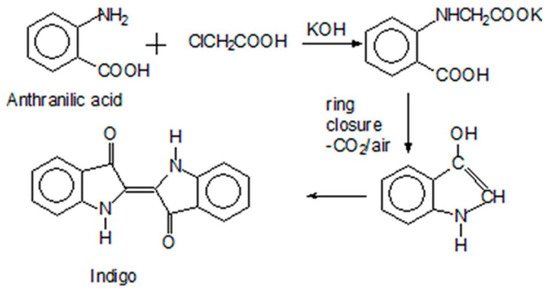
Figure 4. The 1897 synthetic pathway from anthranilic acid to indigo.
This single chemical reaction changed the world. It tolled the funeral knell of the natural products industry worldwide. The shockwave was first felt by the indigo plantations, especially those in India, where indigo was a single crop over a large part of the country. Within 17 years, from 1897 until 1914, the number of hectares of indigo planted in India diminished by an astonishing 83% [9]; worse yet, the drop in income was a crippling 93% [10].
World War I put an end to regular shipments of indigo. This situation forced industrially advanced countries, such as the United States, Britain and Switzerland, to develop their own synthetic indigo industries. By the end of the war, natural indigo had been relegated to a specialty material of interest only to diehard artists and historians. Furthermore, with today’s regulatory requirements for sustainable synthesis methods, those that require large amounts of petroleum-based feedstock, such as aniline, cyanide, formaldehyde and corrosive redox reagents, are clearly untenable.
Two historical asides can speak to indigo’s ease of synthesis and stability under stress. The first instance occurred in 1812, when Sir Henry Halford (1766–1844), physician extraordinaire to His Majesty King George III (1738–1820), reported the presence of a substance in the king’s urine that formed an insoluble blue ring around the container near the surface. Almost 200 years later, 21st century chemistry Professor Wilfred Arnold investigated. The fact that the blue substance formed near the surface meant that oxygen was involved, he reasoned, and indole chemistry was clearly involved. Arnold’s working hypothesis was as follows: enzymatic action on dietary tryptophan in the gut, abetted by putrefaction brought on by constipation, would lead to indole formation. If some of the indole was absorbed into the blood stream, it could be converted in the liver to indoxyl and then to the sulfate ester, i.e., indican. If the indican encountered a sulfatase, possibly from bacteria due to a urinary tract infection or contamination of the chamber pot, the indican would slowly hydrolyze to indoxyl, which, in the presence of air, would dimerize to insoluble blue indigo. Using this model, Arnold performed an experiment and found that the blue pigment did indeed develop after an incubation period of about two days and that the color was more intense if the pH was adjusted upward, which corresponds to the need for an alkaline dyebath [11]. Therefore, King George’s body, under optimum circumstances, sponataneously synthesized indigo. Too bad that Adolf von Baeyer had to work 17 long years to accomplish the same thing.
The second story speaks to indigo’s durability under certain stressful circumstances that also have to do with the digestive tract. Archaeological chemist Marvin Rowe was asked to date a Coptic textile that was contaminated by a mass of debris that turned out to be insect coprolites. Having no other food to munch on but the textile, the inevitable egestion of the waste material became embedded in the artifact. Rowe and his colleagues saw an opportunity to glean chronological data by analyzing the insect excrement, which was dark blue. They hand-picked about 3 mg of the fecal pellets and subjected them to plasma extraction preparatory to radiocarbon dating. The results revealed that the dark blue pellets were 1360 ± 70 years old. Although the moths were modern, the age of the carbon retained in the blue dye, more than likely indigo, remained unchanged during digestion. Durability indeed [12]!
Manufacturers of pigments and dyes, in an attempt to be more responsive to the growing concern over the tons of harmful pollutants entering the atmosphere and the world’s waterways, are seeking to change direction and try natural, rather than petroleum-based, substitutes. Although for centuries, we have been exploiting mushrooms and lichens in textile dyeing, the verdict seems to have come down on microorganisms as the potential answer to the problem. A pioneer in this area is Natsai Chieza, who is using bacteria to dye fabric instead [13]. Genetically engineered bacteria programmed to produce a given colorant in a fermentation tank can be a game changer in the industry. A startup called Tinctorium has received a great deal of attention lately. Its cofounder and chief scientific officer, Tammy Hsu, is intent on producing greener blue jeans with genetically modified Escherichia coli. Her approach is to trick the bacterium into synthesizing indigo’s water-soluble precursor directly in the dye bath. The eureka moment is when the indican is hydrolyzed right on the fabric to blue, insoluble indigo, thus avoiding the pollution engendered by conventional methods [14]. Talented biochemists can also utilize new gene-splicing techniques, such as CRISPR (clustered regularly interspaced short palindromic repeats), to program bacteria to produce any desired dye with the snip of a genetic scissor. A Korean team succeeded in synthesizing blue-pigmented actinorhodin from Streptomyces coelicolor with this method [15].
Once upon a time, the transistor radio was the hottest thing on the teenage circuit. Semiconductor technology allowed bulky vacuum tubes to be replaced with tiny silicon- or germanium-based chips, leading to easily portable electronic devices. However, no one ever dreamed that transistor circuitry might, in turn, give way to simple dyes and pigments that have been used for a very different purpose from ancient times.
The revolution began quietly enough in 1977, when the team of Hideki Shirakawa (b. 1936), Alan MacDiarmid (1927–2007) and Alan Heeger (b. 1936), working at the University of Pennsylvania, discovered that certain organic polymers could conduct electricity [16]. When their groundbreaking publication garnered the 2000 Nobel Prize in Chemistry, it spawned a tsunami-like wave of electronic innovations, such as organic light-emitting diodes (OLEDs), which are essential for smartphones and high-definition television.
However, it took at least another decade before the semiconducting properties of pigments received much attention. Because many of them are inexpensive, sustainable, biodegradable and biocompatible, they are ideal substitutes for traditional semiconductors. In particular, indigo, dibromoindigo (Tyrian purple) [17] and isoindigo (a geometric isomer of indigo) [18] derivatives have shown potential for applications in regenerative medicine, in the biomedical field as resorbable implants and for in vivo medical imaging. Additional applications inspired by natural indigo are in the areas of organic lasers, transistors, photovoltaics, photodetectors and photoacoustic applications [19]—truly an ancient colorant with a very modern twist!
2. Ultramarine: From Priceless Gemstone to Workhorse Blue
The land that lies beyond (ultra) the sea (marine) bestows its location on the name of its once most precious export, ultramarine blue, or lapis lazuli. From beyond the Caspian Sea, Afghans have wrested this shimmering gemstone from the intransigent bedrock of the Sar-e-Sang Mine for the past 7000 years. For millions of years before that, Mother Nature was slowly depositing this treasure in one of the most inaccessible corners of the earth, the High Pamirs of Afghanistan’s Hindu Kush. The renowned international journalist Victoria Finlay put it this way: “After all, part of the mystery of lapis was that although for millennia it had travelled to Europe and Egypt it was always known to come from a mythical land so far away that…[e]ven Alexander had not managed to cast his greatly acquisitive eyes on the mines when he conquered the area 2300 years ago, and Marco Polo in 1271 had only nodded in its direction from another mountain range to the north [20].” Even Finlay had to try twice before she eventually reached the mine in 2001, the first woman in many a month, as Afghan women were forbidden to dwell there [20] (p. 307). Upon arrival, she ticked off the milestones of the whole of art history, from Egyptian tombs to Poussin’s paintings, by analogizing the first 1000 feet of the mine as a timeline.
The raw mineral lazurite is the principal component of the rock, lapis lazuli, in combination with two other minerals, white calcite (CaCO3) and white-gold pyrite (FeS2). High-quality lapis lazuli contains smaller amounts of calcite and pyrite. The latter occurs as tiny, scattered grains and is responsible for the glitter in the gemstone. Because the blue material is intimately mixed with a high proportion of colorless silicates and aluminates, its extraction from these materials is a very laborious and time-consuming process [21].
Couple this processing with numerous price markups by middlemen, to say nothing of the transport costs from Afghanistan; all of these factors make ultramarine the most expensive colorant ever known, worth more than pure gold. Figure 5a shows an ingot of lazurite; the inset (b) shows the ground material to form a low-quality impure pigment.
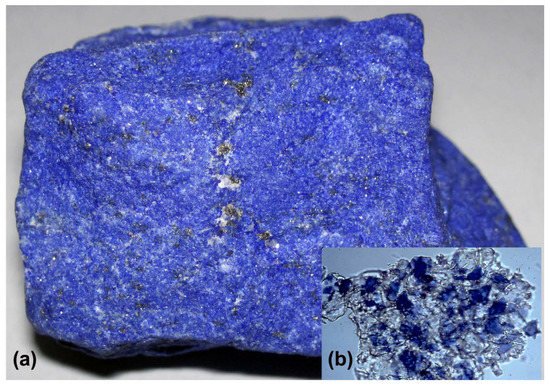
Figure 5. Ultramarine. (a) A fine example of lapis lazuli (lazuritic metamorphite) from the Sar-e-Sang deposit, Precambrian Sakhi Formation, 4.5 cm width; photo: James St. John. (b) Inset: natural ultramarine (photographed at 50× magnification). Note the large proportion of colorless material surrounding the blue portions; this indicates that the pigment was simply crushed lapis lazuli and that no attempt had been made to purify it further. Photo: Mary Virginia Orna.
Chemically, the pigment is an alumino-silicate clathrate, with the formula ((Na,Ca)8(AlSiO4)6(S,Cl,SO4,OH)2), which encloses in isolated cages the unique chromophore responsible for the deep blue color, S3−, the trisulfide radical anion (Figure 6). The nature of this unit was the subject of speculation for centuries until F. Albert Cotton and colleagues finally solved the puzzle in 1976 [22]. They showed that a strong absorption band centered at around 625 nm was responsible for the blue color and that increasing the sulfur content appreciably deepened the blue. Because lapis lazuli rock was formed by contact metamorphism, the presence of sulfur in the pyrite may have contributed to the presence of sulfur in the clathrate. However, this arrangement is one of host crystal–guest ion, and replacing the guest ion with, for example, selenium, turns the pigment to a blood-red color. Furthermore, it is the trisulfide/disulfide radical anion, S3−/S2−, ratio that is key to the color, yielding blues to blue greens to greens to yellow greens to yellow as the disulfide concentration increases [23].
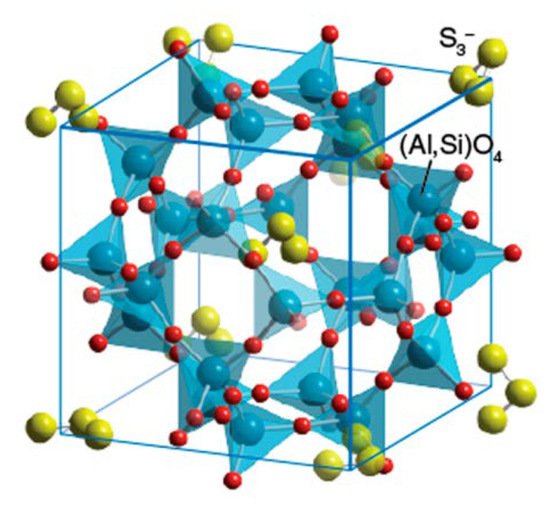
Figure 6. Ultramarine structure: trisulfide radical anions (shown as three yellow balls) in a host cage-like structure [24]. Color key: yellow–sulfur; red–oxygen; blue–silicon or aluminum.
Thanks to the superb analytical work of the great French chemist Nicolas Vauquelin (1763–1829), in 1814, the chemical composition of ultramarine and of a bright blue compound found in lime kilns was shown to be identical, although the series of analyses and attempted syntheses was far more complicated, stretching over decades from about the late 1780s to 1850. The biggest sticking point was the assignment of the chromophore, which almost everyone assumed had to be metallic. All the analysts who puzzled over the problem realized that the presence of sulfur was intimately involved; however, it was too much of a step to postulate that sulfur, in a unique configuration, just might be the chromophore [25]. Meanwhile, the French Société d’Encouragement pour l’Industrie Nationale decided to offer prize of FF (pre-1960 French francs) 6000 to the individual who devised a cost-effective route to the production of artificial ultramarine. A Frenchman, Jean-Baptiste Guimet (1795–1871), managed to produce and market an acceptable substitute for the real thing at a cost of about FF 850 per kilogram, which won him the prize, despite the fact that his price did not match the original requirement of FF 300 per kilogram. This is a far cry from 1541, when the price of natural ultramarine hit an all-time high, which amounted to about 55 times the daily wage paid to a skilled worker at the time [26]. Considering the price of the natural material, at FF 11,000/kg in Guimet’s time, or USD 105.00/g at today’s prices, Guimet’s product was a bargain at USD 8.00/g, a price differential of 13:1 [27]. However, a German chemistry professor, Christian Gottlieb Gmelin (1792–1860), also claimed the prize for his independently and near-simultaneously developed method. As they were pursuing their priority dispute, other manufacturers got into the act, making every effort to keep their processes secret. By the end of the 19th century, almost three dozen firms were in the business, and the price differential between the natural and artificial product widened to eighty to one, quickly transforming a luxury blue into a mainstay commonplace colorant. Today, the synthetic product can be produced for between USD 0.08 and 0.17 per gram [28].
Price was not the only factor that propelled ultramarine’s metamorphosis from luxury item to workhorse pigment. It is one of the few brilliant pigments that does not contain a transition metal as the chromophore—a real ecological plus. In fact, it is so safe that it can be used with impunity in food containers, cosmetics, children’s toys and any other applications in which the substance comes into contact with the skin or the mouth. It is also a superb pigment that can be used in virtually any kind of paint medium—oil, water, tempera, acrylic, alkyd, polyurethane and water-based emulsion coatings, with a single caveat: it decolorizes on contact with acid. It also finds many uses in inks, coatings, plastics and fibers [29]. Pete Cole, president of the paint manufacturer Gamblin Artists Colors in Portland, OR put it this way: “If you’re trying to paint the colors of the natural world, there are colors you struggle with and there are colors that practically do it for you—ultramarine blue is one of those colors…It does its job so incredibly well [30]”.
Aside from all these pedestrian applications, ultramarine ultimately rocks! Due to the prominence afforded by the high visual impact and global publicity of Yves Klein’s (1928–1962) IKB (International Klein Blue), synthetic ultramarine has mounted the throne and displaced the iconic natural pigment with its own reality. (See Figure 7).
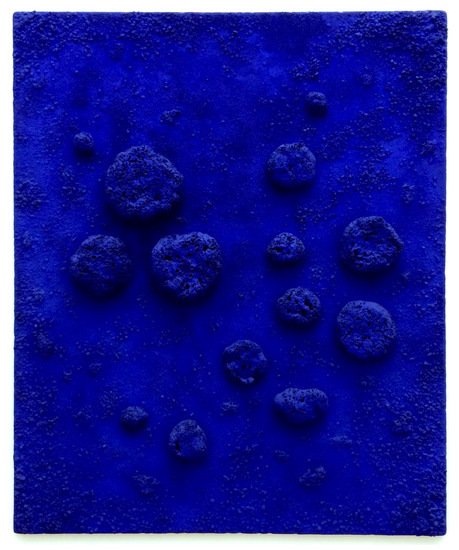
Figure 7. L’Accord Bleu. Mixed media painting, 1960. Yves Klein. Foundation Stedelijk Museum Amsterdam Collection [31].
The deep, luscious blue color has shaken off the captivity of form forever. Now immaterial, it has pervaded music, film, literature, academia, television and any other medium you might care to name to become the first postmodern pigment [32].
Unfortunately, synthetic ultramarine is also pervading the environment, as noxious air pollutants, such as SO2 and H2S, are needed for its formation. A Polish team has described a new approach whereby sulfur radical anions are encapsulated into zeolites, forming ultramarine-like pigments with a range of colors [33]. The jury is still out regarding the acceptability of these products into the ultramarine family.
References
- Splitstoser, J.C.; Dillehay, T.D.; Wouters, J.; Claro, A. Early pre-Hispanic use of indigo blue in Peru. Sci. Adv. 2016, 2, e1501623.
- Glen, C. The dyes of the Ancients. J. Soc. Dyers Colour. 1911, 27, 293–296.
- Ruggli, P. Die Geschichte der Färberei. In Die Geschichte der Textil-Industrie; Johannsen, E.H.O., Fahrbach, R., Eds.; Süd-Verlag o.J.: Stuttgart, Germany; Zürich, Switzerland, 1932; p. 345.
- Struckmaier, S. Naturfarbstoffe: Farben mit Geschichte. Chem. Unserer Zeit 2003, 37, 402–409.
- Maier, W.; Schumann, B.; Gröger, D. Biosynthesis of indoxyl derivatives in Isatis tinctoria and Polygonum tinctorium. Phytochemistry 1990, 29, 817–819.
- Perkin, A.G. XCIII. Indoxylic acid. J. Chem. Soc. Trans. 1909, 95 Pt 2, 847–853.
- Schweppe, H. Indigo and woad. In Artists’ Pigments: A Handbook of Their History and Characteristics; West FitzHugh, E., Ed.; National Gallery of Art: Washington, DC, USA, 1997; Volume 3, pp. 80–107.
- Travis, A.S. The Rainbow Makers: The Origins of the Synthetic Dyestuff Industry in Western Europe; Lehigh University Press: Bethlehem, PA, USA, 1993; p. 223.
- Schaefer, B. Natural Products in the Chemical Industry; Springer: New York, NY, USA; Heidelberg, Germany, 2014; pp. 23–28.
- Royal Economic Society. The rise and fall of the indigo industry in India. Asiat. Econ. J. 1912, 22, 237–247.
- Arnold, W.N. George III’s urine and indigo blue. Lancet 1996, 347, 1811–1813.
- Rowe, M.; Blinman, E.; Jones, S. Everybody poops, even insects. Insect waste used to date ancient textile. New Mexico Archaeology, 4–5 November 2021.
- Peters, A. These Gorgeous Colors Come from Dye Made by Bacteria, not Chemicals. Available online: https://www.fastcompany.com/90257662/these-gorgeous-colors-come-from-dye-made-by-bacteria-not-chemicals (accessed on 8 January 2022).
- Hsu, T.M.; Welner, D.M.; Russ, Z.M.; Cervantes, B.; Prathuri, R.L.; Adams, P.D.; Dueber, J.E. Employing a biochemical protecting group for a sustainable indigo dyeing strategy. Nat. Chem. Biol. 2018, 14, 256–261.
- Cho, S.; Shin, J.; Cho, B.-K. Applications of CRISPR/Cas system to bacterial metabolic engineering. Int. J. Mol. Sci. 2018, 19, 1089.
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580.
- Glowacki, E.D.; Voss, G.; Leonat, L.; Irimia-Vladu, M.; Bauer, S.; Sariciftci, N.S. Indigo and Tyrian purple—From ancient natural dyes to modern organic semiconductors. Isr. J. Chem. 2012, 52, 540–551.
- Wang, Y.; Yu, Y.; Liao, H.; Zhou, Y.; McCulloch, I.; Yue, W. The chemistry and applications of heteroisoindigo units as enabling links for semiconducting materials. Acc. Chem. Res. 2020, 53, 2855–2868.
- Fallon, K.J.; Bronstein, H. Indolonaphthyridine: A versatile chromophore for organic electronics inspired by natural indigo dye. Acc. Chem. Res. 2021, 54, 182–193.
- Finlay, V. Color: A Natural History of the Palette; Random House: New York, NY, USA, 2004; p. 282.
- Plesters, J. Ultramarine blue, natural and artificial. In Artists’ Pigments: A Handbook of Their History and Characteristics; Roy, A., Ed.; National Gallery of Art: Washington, DC, USA, 1993; Volume 2, pp. 37–54.
- Cotton, F.A.; Harmon, J.B.; Hedges, R.M. Calculation of the ground state electronic structures and electronic spectra of di- and trisulfide radical anions by the scattered wave-SCF-X.alpha. method. J. Am. Chem. Soc. 1976, 98, 1417–1424.
- Hamerton, I.; Tedaldi, L.; Eastaugh, N. A systematic examination of colour development in synthetic ultramarine according to historical methods. PLoS ONE 2013, 8, e50364. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0050364 (accessed on 3 June 2022).
- Greeves, N.; University of Liverpool. Ultramarine—Na86.(S3)2. Available online: https://www.chemtube3d.com/ss-ultramarine/ (accessed on 13 December 2021).
- Mertens, J. The history of artificial ultramarine (1787–1844): Science, industry and secrecy. Ambix 2004, 51, 219–244.
- Kubersky-Piredda, S. The market for painters’ materials in Renaissance Florence. In Trade in Artists’ Materials: Markets and Commerce in Europe to 1700; Cannon, J., Kirby, J., Nash, S., Eds.; Archetype Publications: London, UK, 2010; pp. 223–243.
- UCoin.net Coin Catalog. Available online: https://en.ucoin.net/coin/france-1-franc-1825-1830/?tid=73762 (accessed on 18 December 2021).
- Delamare, F. Blue Pigments. 5000 Years of Art and Industry; Archetype Publications: London, UK, 2013; p. 250.
- Virtual Visit: Ultramarine Manufacture at Holliday Pigments, Hull. Available online: https://www.york.ac.uk/org/seg/salters/ChemistryArchive/V_VisitHP/applications.html. (accessed on 18 December 2021).
- Ables, K. Now Supply-Chain Woes Have Come to the Color Blue. Washington Post. 23 December 2021. Available online: https://www.washingtonpost.com/entertainment/museums/blue-paint-shortage-art/2021/12/21/486c667c-5df9-11ec-ae5b-5002292337c7_story.html (accessed on 27 December 2021).
- The File is Licensed under the Creative Commons Attribution-Share Alike 3.0 Unported (https://creativecommons.org/licenses/by-sa/3.0/deed.en) license. User: Jaredzimmerman. Available online: https://commons.wikimedia.org/w/index.php?title=File:L%27accord_bleu_(RE_10),_1960.jpg&oldid=493966780 (accessed on 18 December 2021).
- International Klein Blue. Available online: https://en.wikipedia.org/wiki/International_Klein_Blue (accessed on 18 December 2021).
- Jankowska, A.; Kowalak, S. New methods of ultramarine pigments synthesis from zeolites. Wiadomości Chemisczne 2008, 62, 659–682. (In Polish)
More
Information
Subjects:
Art
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
3 times
(View History)
Update Date:
21 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




