Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mihayl Varbanov | -- | 3106 | 2022-07-19 17:12:13 | | | |
| 2 | Camila Xu | + 4 word(s) | 3110 | 2022-07-20 03:57:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mammari, N.; Hamblin, M.R.; Rauger, P.; Boyer, L.; Varbanov, M. Phototherapy-Based Treatment for Sexually Transmitted Infections. Encyclopedia. Available online: https://encyclopedia.pub/entry/25288 (accessed on 08 February 2026).
Mammari N, Hamblin MR, Rauger P, Boyer L, Varbanov M. Phototherapy-Based Treatment for Sexually Transmitted Infections. Encyclopedia. Available at: https://encyclopedia.pub/entry/25288. Accessed February 08, 2026.
Mammari, Nour, Michael R. Hamblin, Pauline Rauger, Laurence Boyer, Mihayl Varbanov. "Phototherapy-Based Treatment for Sexually Transmitted Infections" Encyclopedia, https://encyclopedia.pub/entry/25288 (accessed February 08, 2026).
Mammari, N., Hamblin, M.R., Rauger, P., Boyer, L., & Varbanov, M. (2022, July 19). Phototherapy-Based Treatment for Sexually Transmitted Infections. In Encyclopedia. https://encyclopedia.pub/entry/25288
Mammari, Nour, et al. "Phototherapy-Based Treatment for Sexually Transmitted Infections." Encyclopedia. Web. 19 July, 2022.
Copy Citation
Phototherapy (PT) is a major therapeutic approach based on the controlled administration of light in the visible, near infrared, or UV spectrum, with or without the application of an external photosensitizer. New therapeutic strategies are urgently needed to overcome drawbacks in the treatment of some infections, particularly sexually transmitted infections (STI). STIs are easily spread by the transmission of various bacteria, viruses, and parasites with some of the infections being incurable or even lethal, leading to a serious impact on reproductive health worldwide.
sexually transmitted infections
phototherapy
photodynamic therapy
1. Introduction
Sexually transmitted infections (STIs) are a major public health problem throughout the world [1]. They can be either ancient or emerging infections, with an increasing likelihood of being resistant to treatment [2]. Three types of viruses are often responsible for STIs [3]. First and foremost are human papillomavirus (HPV) infections, which have recently been brought under control by the development of highly effective vaccines [4]. Herpes virus infections (HSV) are characterized by high prevalence and morbidity [5]. No effective vaccine against viruses of the herpes group is currently available. This is also the case with HIV (human immunodeficiency virus) infections [6]. STIs can also be caused by non-viral pathogens such as bacteria, parasites, or fungi. Examples of these are Chlamydia trachomatis, Neisseria gonorrhoeae, Treponema pallidum, Mycoplasma genitalium and Trichomonas vaginalis [7]. While the site of infection is mainly urogenital, it can also be pharyngeal and/or anorectal [2]. Although pharyngeal infections with C. trachomatis and N. gonorrhoeae often resolve spontaneously, they can be transmitted by oral sex, suggesting a large oral reservoir of germs as a source of contamination [8][9]. Genital and extra-genital co-infection is far from rare and could explain the occurrence of re-infection and the sustainability of an epidemic [8]. The clinical presentation could be an erosive or ulcerative anorectal or genital lesion, more or less painful or raised [10]. The context is important to confirm the diagnosis, and possible co-infections should be systematically sought out because they are frequent, and this is essential in HIV-positive patients [11].
Moreover, the emergence of antimicrobial resistance in sexually acquired infection pathogens is an important global public health threat. There is an urgent need for novel STI treatment and prevention strategies to tackle the rising incidence of STIs in high-income settings, and the static incidence in low- and middle-income settings over the past decade [12]. Both the treatment and the prevention of these infections are often complex. Recent clinical studies have made it possible to use new light-based therapeutic approaches to treat skin lesions caused by sexually transmitted viral infections [13].
The primary care therapeutic approaches to treat benign skin lesions associated with HPV infection are quite diverse, such as topical therapy with agents such as podophyllin, trichloroacetic acid, salicylic acid, or 5-fluorouracil. However, 5-fluorouracil has the disadvantage of inducing a strong local inflammatory response, which makes its use on mucous membranes difficult [14]. Similar treatments have been used in cases of vaginal intraepithelial neoplasia with high-risk HPV infection, and other sites of this infection, including excision by surgery and intravaginal radiotherapy or chemotherapy [15]. Some physical methods constitute an alternative approach employed in current practice [16][17]. Several different approaches could be considered: cryotherapy [18]; photodynamic therapy (PDT) [19]; thermocoagulation [20]; CO2 laser ablation [21]. However, chemotherapy has certain disadvantages and side effects which can vary according to the individual patient, the drugs used, the doses, and the combination treatments. In some cases chemotherapy is performed during concomitant radiochemotherapy [22]. This therapeutic approach does not have the same side effects as chemotherapy alone, and it may be more effective than chemotherapy alone, because it targets the tissue with malignant cells in a spatially confined manner [23][24]. The undesirable effects often include digestive disorders, a drop in immune cells, red blood cells, and platelets, oral mucositis, and the appearance of skin disorders [25][26]. Locally applied laser and radiation therapies could improve the cure rate while avoiding the damage caused by surgery [27], but they could also cause damage to the vaginal mucosa resulting in scarring or vaginal stenosis, and other adverse effects such as burning sensations, pain, and dyspareunia [28][29]. Moreover, the development of therapeutic HPV vaccines could be used for the treatment of persistent infections, and to prevent the progression of HPV-associated cancers [6]. There are three vaccines called Cervarix (GlaxoSmithKline, London, United Kingdom), Gardasil and Gardasil9 (Merck Sharp & Dohme Corp, Whitehouse Station, Township of Readington, New Jersey, United States). Cervarix can protect against oncogenic HPV types HPV16 and HPV18. Gardasil provides protection against oncogenic types HPV16 and 18, as well as low-risk HPV types 6 and 11. Gardasil9 provides protection against a broad panel of HPV strains, protecting against the seven most common oncogenic HPV types (HPV16, 18, 31, 33, 45, 52 and 58) and the two low risk types (HPV6 and 11) [30][31]. For HSV lesions, the primary therapeutic arsenal consists of anti-herpesviridae antiviral drugs such as aciclovir, valaciclovir, or famciclovir [32]. The use of topical aciclovir still has a strong following. However, new approaches are in the evaluation phase, but do not yet present an established management plan in current practice [33].
For infectious diseases caused by C. trachomatis, the first-line treatment is doxycycline in the European and American guidelines [34]. However, in the event of an allergy to doxycycline or in pregnant women, a single dose of oral azithromycin or erythromycin (500 mg) has been used [35]. Exceptional treatment failure has been reported with this treatment, which could then be controlled with moxifloxacin. Due to the frequent co-infection with gonococcus bacteria, treatment with ceftriaxone is often added to this regimen. Nevertheless, the notable limitations of these antibiotics, highlight the need for further, updated research in this area, particularly for low- and middle-income settings [36]. Furthermore, azithromycin (500 mg) and doxycycline (100 mg) are also recommended for the treatment of Mycoplasma genitalium. The persistence of this infection requires treatment with pristinamycin (1 g) [37]. To date, all findings suggest that doxycycline is inefficient for the eradication of M. genitalium. Although azithromycin was not significantly less efficient than extended dosage doxycycline, it was associated with the selection of macrolide-resistant M. genitalium strains. The monitoring of M. genitalium macrolide resistance should be encouraged.
In the case of gonococcal infections, the WHO Global Gonococcal Antimicrobial Surveillance Program (WHO GASP) reported that between 2009 and 2014, there emerged a persistent and widespread resistance to penicillin, tetracycline, ciprofloxacin, and azithromycin. This was accompanied by a decrease in sensitivity to broad-spectrum cephalosporins, especially cefixime [38]. To date, three strains of gonococci with a high resistance to ceftriaxone have been reported in France, Japan and Spain [39][40]. There is a need to develop and implement a national sentinel program for gonococcal antimicrobial susceptibility and to develop new therapeutic strategies to combat this scourge.
STIs can also be caused by a parasitic infection, particularly Trichomonas vaginalis. The standard treatment for trichomoniasis is metronidazole. This molecule is an antibiotic and an antiparasitic drug. Treatment adherence should be followed closely to avoid re-infection [41]. Therapeutic failure of this protocol sometimes occurs, and then the treatment dose is increased. Because metronidazole can cause leukopenia, an antabuse effect (similar to disulfiram) or a candida secondary infection, there is a relative contraindication in early pregnancy [42].
Drug resistance is increasingly observed in pathogens causing STIs. Clinical trials have used some physical therapy approaches that do not cause resistance. In this research, researchers are particularly interested in the treatment of STIs by phototherapy (PT). PT is a major therapeutic approach based on the controlled administration of light in the visible, near infrared, or UV spectrum, with or without the application of an external photosensitizer. When the light is combined with a photosensitizer, it is called photodynamic therapy (PDT). PDT is a physicochemical method initially developed to treat cancer and tumors [19]. It was first approved in the 1980s, as a new approach intended for cancer patients who could not be treated by surgery or radiotherapy [43]. During phototherapy, the non-toxic photosensitizer agents can be activated by light irradiation to induce cell death without causing much damage to normal tissues [44]. A successful clinical PDT involves complex procedures, but can lead to the eradication of tumor or infected tissue with lower toxicity due to more limited light penetration compared to PT [44]. The three key components of the PDT are photosensitizer, oxygen and light, with light dosimetry being a key factor. The photosensitizer should have some selective affinity for the target cells. Since the 1980s, three generations of photosensitizers have emerged. The first generation derivatives were the first photosensitizers to have been used clinically, hematoporphyrin and its derivatives (HpD) [45]. HpD is difficult to obtain pure and contains oligomeric compounds responsible for photocytotoxicity [44]. Photofrin (first generation) is a purified form of HpD. Photofrin can persist in normal skin for long periods of time [46][47], meaning that patients should be protected from strong light sources for several weeks after Photofrin administration. This problem was minimized when new photosensitizing agents were identified with shorter persistence [46]. The second generation photosensitizers (e.g., Visudyne, Foscan, 5-aminolevulinic acid (5-ALA) …) have been developed to overcome some of these drawbacks [48]. They are pure compounds; mostly absorbing strongly in the red part of the spectrum, and have a high quantum yield of singlet oxygen formation. Rapid elimination of the photosensitizer from the body is also desirable in order to limit residual phototoxicity after treatment. The third generation photosensitizers have been modified by conjugation or by encapsulation (e.g., liposomes and nanoparticles) of the second generation photosensitizers in order to allow passive or active targeting of neoplastic cells, and thus improve their selectivity for the lesion to be treated [49]. Basic studies made it possible to establish the mechanistic principles of PDT, which relies on the absorption of light by a photosensitizer molecule or dye, leading to a photochemical reaction to produce reactive oxygen species that can kill cancer cells or microorganisms [46]. Furthermore, it is only relatively recently that PDT has been studied as a treatment for various types of localized infections. This resurgence of interest has been partly motivated by the alarming increase in drug resistance amongst bacteria and other pathogens. The clinical application of antimicrobial PDT to localized viral infections caused by herpes or papilloma viruses, or non-viral dermatological infections such as acne, yeast, fungal, and bacterial skin infections has been validated. PDT has been used to treat bacterial infections in brain abscesses and non-healing ulcers [50]. Figure 1 schematically illustrates the applications of PT and PDT to treat a variety of STIs.
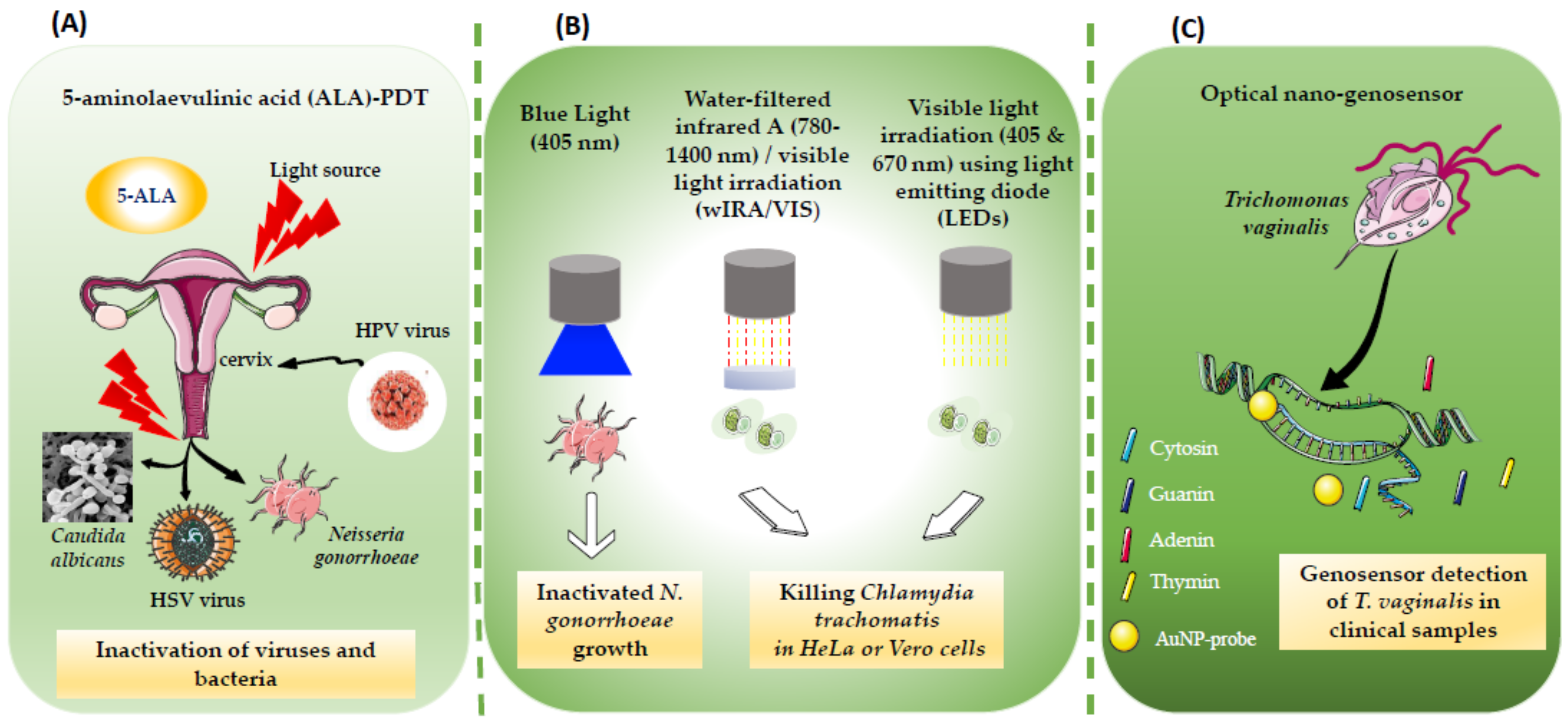 Figure 1. (A) Treatment of multifocal disease based on 5-aminolaevulinic acid (ALA)-PDT, effective in treating HPV lesions. The PDT can be also used for selective and specific destruction of subclinical bacteria and fungui-infected areas, as in the case of N. gonorrhoeae and C. albicans. (B) The combination of water-filtered infrared A (wIRA) with visible light VIS (wIRA/VIS) is used for killing intracellular and extracellular Chlamydia trachomatis strains in Vero and HeLa cell lines. Visible light irradiation (405 and 670 nm) also leeds to dose-dependent inhibitory effect on Chlamydia. (C) Diagnostic approach based on an optical nano-genosensor and gold nanoparticles conjugated to a specific oligonucleotide for T. vaginalis PCR detection.
Figure 1. (A) Treatment of multifocal disease based on 5-aminolaevulinic acid (ALA)-PDT, effective in treating HPV lesions. The PDT can be also used for selective and specific destruction of subclinical bacteria and fungui-infected areas, as in the case of N. gonorrhoeae and C. albicans. (B) The combination of water-filtered infrared A (wIRA) with visible light VIS (wIRA/VIS) is used for killing intracellular and extracellular Chlamydia trachomatis strains in Vero and HeLa cell lines. Visible light irradiation (405 and 670 nm) also leeds to dose-dependent inhibitory effect on Chlamydia. (C) Diagnostic approach based on an optical nano-genosensor and gold nanoparticles conjugated to a specific oligonucleotide for T. vaginalis PCR detection.2. Phototherapy (PT) for Viral Sexually Transmitted Infections (STIs)
Human papilloma virus (HPV) infection, the most common sexually transmitted disease in the world and the main cause of genital warts, infects millions of people worldwide every year [51], with an estimated 291 million HPV-positive women worldwide in 2007 [52]. Among diseases caused by HPV, condyloma acuminata refers to an anogenital infection caused by HPV strains 6 and 11 [53][54]. Successful treatment can still be challenging. Traditional forms of treatment for condyloma acuminata that are effective at removing wart tissue including topical approaches, carbon dioxide laser (CO2 laser), cryotherapy, or electrosurgery [55], or medical treatment with topical agents (imiquimod, podofilox, podophyllin, trichloroacetic acid, sinecatechins ointment) [56][57].
However, these therapies are often ineffective with a high recurrence rate, as they cannot eliminate subclinical latent HPV infection. Multiple reports have shown that 5-aminolaevulinic acid (ALA)-PDT can be effective in treating HPV lesions [16][58]. A real benefit of PDT lies in its ability to treat multifocal disease without tissue loss [59]. Treatment with 5-ALA-PDT can destroy the warts and cause selective and specific destruction of subclinical virus infected areas [58]. The cure rate and viral clearance rate are significantly higher after ALA-PDT therapy compared to pharmacotherapy or physically destructive therapies. However, clinical trials have demonstrated the feasibility of applying topical ALA cream for photodiagnosis (PD) and PDT of condylomas caused by HPV. Women receiving PDT in this clinical trial had often failed with conventional treatment [56]. Conventional treatment can often be painful and sometimes disfiguring, and often results in high recurrence rates, which reinforces the need for new approaches [56][60]. One study evaluated the persistence or clearance of HPV infection after ALA-PDT in patients suffering from genital warts. The data was analyzed between January 2019 and December 2020 at Nanfang Hospital and Dermatology Department, Hospital of Southern Medical University in China, and showed that different variables such as multiple sexual partners, a history of recurrent infection, and severe pain during PDT affected the overall outcomes of PDT treatment. The researchers suggested that the patients may need additional PDT sessions. Interestingly, PDT appears from this research to be effective against single strain HPV infections [61].
In one patient with condyloma acuminata covering the glans penis, a case study reported a patient who had a single large lesion. ALA-PDT was used as a therapeutic approach to reduce the risk of recurrence and minimize the trauma caused by traditional methods such as CO2 laser therapy. The choice of therapy by ALA-PDT is dictated by the fact that ALA accumulates the warts and reduce the rate of recurrence in the surrounding tissue with subclinical infection [55]. ALA-PDT is an effective, safe and curative alternative to the conventional treatment of genital warts [55]. ALA-PDT-based treatment was also effective against urethral condyloma acuminata; in this case, the researchers suggested that the dynamic monitoring of the HPV viral load could objectively demonstrate the effectiveness and guide the treatment of PDT [62]. These results were reinforced by a randomized controlled clinical trial carried out recently. The objective was to compare the use of PDT with the application of trichloracetic acid (TAA) in the treatment of HPV condyloma in the perianal and vulval regions. A total of 16 patients was treated with PDT using the prodrug methyl aminolevulinate incubated for 3 h and irradiation at 630 nm (100 J/cm2). Fifteen patients were treated with TAA, received acid using a cotton swab. The results of these experimentations revealed that the PDT-based treatment appeared to be effective in the treatment of lesions due to the physical destruction of condyloma and subclinical lesions. A complete response rate was evaluated at 63% for PDT versus 60% (10 had a complete response and 6 had a partial response) for TAA (9 patients had a complete response in the elimination of lesions, 3 had a partial response), and a recurrence rate of 0% for PDT versus 33% for TAA. In addition, treatment with PDT led to complete clearance in an area with many warts; the researchers suggested that PDT may be more beneficial for patients with recurrent HPV warts [63].
Another therapeutic strategy based on a combination of CO2 laser and ALA-PDT was tested to treat condyloma acuminata in 98 adult patients (male and female). Firstly, patients were treated by CO2 laser to remove the visible warts. Secondly, the patients had the ALA-PDT treatment immediately after the laser exposition. The ALA surface application was performed for 3 h at light irradiation of 100–150 J/cm2. The ALA-PDT was continued once a week for three weeks. A combination of CO2 laser and ALA-PDT has been shown to be feasible and effective in the treatment of condyloma acuminata. The cure rate was high at 93.8% (92/98) [64]. Shi et al., (2013) tested a different treatment strategy on 361 patients diagnosed with condyloma acuminata. Patients were divided into three groups according to the maximum diameter of their lesion (A < 0.5, B = 0.5–2.0, and C > 2.0–4.0 cm). Five treatments were compared in each group (cryotherapy, CO2 laser, ALA-PDT alone, ALA-PDT plus CO2 laser, ALA-PDT plus cryotherapy). The clinical outcomes evaluated during follow up after each treatment showed that the ALA-PDT was best if the maximum lesion diameter was <0.5 cm, while ALA-PDT plus cryotherapy was better for lesions 0.5–2.0 cm. ALA-PDT treatment, after either cryotherapy or CO2 laser was effective for lesions >2.0–4.0 cm, which should be the first choice. They suggested that all treatments could be effective, but the choice depended on the size of the condyloma lesion [65].
However, a study by Szeimies et al. (2009) reported a different result. CO2 laser ablation followed by ALA-PDT was investigated in a phase III prospective randomized bicenter double-blind clinical trial to prevent recurrence of condyloma acuminata. One hundred seventy-five patients with condyloma acuminata received CO2 laser vaporization plus adjuvant ALA-PDT or adjuvant placebo-PDT. Results showed no statistically significant difference between the groups with regard to recurrence rates up to 12 months after treatment. No major complications were observed [66].
Persistent HPV infection can lead to the development of malignant lesions in the vaginal and cervical epithelium. Indeed, HPV infection represents the main cause of cervical intraepithelial neoplasia [15]. The clinical treatments recommended for cervical intraepithelial neoplasia have already been mentioned, and mainly include the topical application of certain drugs or surgical excision, as well as irradiation by intravaginal radiotherapy or ablation by a laser [67]. PDT is a new therapeutic tool which has been used mainly in HPV infections causing condyloma acuminata, or in the case of non-melanoma skin tumors [58][68]. PDT has become a promising therapeutic method used in the treatment of various tumors including cervical intraepithelial neoplasia, cervical HPV infection, and vaginal intraepithelial neoplasia [69]. One meta-analysis revealed that out of 77 patients with cervical HPV infection included in four randomized controlled trials who received PDT, 48 of them showed complete remission. The complete remission rate ranged from 53.5 to 94.4% [69]. The researchers of this meta-analysis concluded that PDT was effective for HPV clearance, particularly high-risk HPV genotypes. In addition, the study showed that out of 120 patients with cervical intraepithelial neoplasia treated by PDT, 77 patients achieved full primary remission by the end of the 3-month follow-up. Interestingly, the complete remission rate ranged from 31.3 to 100% [69]. Clinically, PDT is becoming increasingly employed to treat malignant HPV viral infection, including cervical intraepithelial neoplasia and cervical HPV infection, and has shown complete remission and local eradication of the virus. Another systematic review also demonstrated the efficacy of PDT for the treatment of cervical intraepithelial neoplasia. Analysis revealed that the complete remission rate of PDT for cervical intraepithelial neoplasia ranged from 0 to 100%, with an HPV eradication rate varying from 53.4 to 80% [70]. These results were based on analysis of the data published in several studies [71][72]. The effectiveness of PDT for the treatment of cervical intraepithelial neoplasia and for the eradication of HPV has also been confirmed in several studies by Li et al. [73], Cang et al. [74], Wu et al. [75], Su et al. [76] and Zang et al. [15]. Furthermore, the efficacy of PDT for the treatment of cervical cancer has also recently been demonstrated. At three months after PDT, complete elimination of HPV was detected in more than 90% of patients with early stage cervical cancer [19].
References
- Sexually Transmitted Infections (STIs). Available online: https://www.who.int/westernpacific/health-topics/sexually-transmitted-infections (accessed on 19 November 2021).
- Williamson, D.A.; Chen, M.Y. Emerging and Reemerging Sexually Transmitted Infections. N. Engl. J. Med. 2020, 382, 2023–2032.
- Smith, L.; Angarone, M.P. Sexually Transmitted Infections. Urol. Clin. N. Am. 2015, 42, 507–518.
- Ciccarese, G.; Herzum, A.; Pastorino, A.; Dezzana, M.; Casazza, S.; Mavilia, M.G.; Copello, F.; Parodi, A.; Drago, F. Prevalence of Genital HPV Infection in STI and Healthy Populations and Risk Factors for Viral Persistence. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 885–888.
- Cole, S. Herpes Simplex Virus: Epidemiology, Diagnosis, and Treatment. Nurs. Clin. N. Am. 2020, 55, 337–345.
- Kardani, K.; Basimi, P.; Fekri, M.; Bolhassani, A. Antiviral Therapy for the Sexually Transmitted Viruses: Recent Updates on Vaccine Development. Expert Rev. Clin. Pharmacol. 2020, 13, 1001–1046.
- Juliana, N.C.A.; Deb, S.; Ouburg, S.; Chauhan, A.; Pleijster, J.; Ali, S.M.; Morré, S.A.; Sazawal, S.; Ambrosino, E. The Prevalence of Chlamydia Trachomatis and Three Other Non-Viral Sexually Transmitted Infections among Pregnant Women in Pemba Island Tanzania. Pathogens 2020, 9, 625.
- Gannon-Loew, K.E.; Holland-Hall, C. A Review of Current Guidelines and Research on the Management of Sexually Transmitted Infections in Adolescents and Young Adults. Ther. Adv. Infect. Dis. 2020, 7, 2049936120960664.
- Doernberg, S.B.; Komarow, L.; Tran, T.T.T.; Sund, Z.; Pandori, M.W.; Jensen, D.; Tsalik, E.L.; Deal, C.D.; Chambers, H.F.; Fowler, V.G.; et al. Simultaneous Evaluation of Diagnostic Assays for Pharyngeal and Rectal Neisseria Gonorrhoeae and Chlamydia Trachomatis Using a Master Protocol. Clin. Infect. Dis. 2020, 71, 2314–2322.
- Yarbrough, M.L.; Burnham, C.-A.D. The ABCs of STIs: An Update on Sexually Transmitted Infections. Clin. Chem. 2016, 62, 811–823.
- Lin, K.-Y.; Sun, H.-Y.; Lee, T.-F.; Chuang, Y.-C.; Wu, U.-I.; Liu, W.-C.; Chang, S.-Y.; Chen, Y.-J.; Hung, C.-C.; Chang, S.-C. High Prevalence of Sexually Transmitted Coinfections among At-Risk People Living with HIV. J. Formos. Med. Assoc. 2021, 120, 1876–1883.
- Tien, V.; Punjabi, C.; Holubar, M.K. Antimicrobial Resistance in Sexually Transmitted Infections. J. Travel. Med. 2020, 27, taz101.
- Williams, E.; Fairley, C.K.; Williamson, D. Novel Strategies for Prevention and Treatment of Antimicrobial Resistance in Sexually-Transmitted Infections. Curr. Opin. Infect. Dis. 2021, 34, 591–598.
- Li, Y.; Yu, T.; Yan, H.; Li, D.; Yu, T.; Yuan, T.; Rahaman, A.; Ali, S.; Abbas, F.; Dian, Z.; et al. Vaginal Microbiota and HPV Infection: Novel Mechanistic Insights and Therapeutic Strategies. Infect. Drug Resist. 2020, 13, 1213–1220.
- Zhang, T.; Hu, R.; Tang, Y.; Zhang, Y.; Qin, L.; Shen, Y.; Wang, B.; Zhang, L.; Cao, L.; Zhou, Y.; et al. The Effect of Local Photodynamic Therapy with 5-Aminolevulinic Acid in the Treatment of Vaginal Intraepithelial Lesions with High-Risk HPV Infection. Photodiagn. Photodyn. Ther. 2022, 37, 102728.
- Fathi, R.; Tsoukas, M.M. Genital Warts and Other HPV Infections: Established and Novel Therapies. Clin. Dermatol. 2014, 32, 299–306.
- Schnürch, H.-G.; Ackermann, S.; Alt-Radtke, C.D.; Angleitner, L.; Barinoff, J.; Beckmann, M.W.; Böing, C.; Dannecker, C.; Fehm, T.; Gaase, R.; et al. Diagnosis, Therapy and Follow-up of Vaginal Cancer and Its Precursors. Guideline of the DGGG and the DKG (S2k-Level, AWMF Registry No. 032/042, October 2018). Geburtshilfe Und Frauenheilkd. 2019, 79, 1060–1078.
- Chumworathayi, B.; Thinkhamrop, J.; Blumenthal, P.D.; Thinkhamrop, B.; Pientong, C.; Ekalaksananan, T. Cryotherapy for HPV Clearance in Women with Biopsy-Confirmed Cervical Low-Grade Squamous Intraepithelial Lesions. Int. J. Gynaecol. Obstet. 2010, 108, 119–122.
- Afanasiev, M.D.D.S.M.S.; Dushkin, M.D.A.D.; Grishacheva, D.S.T.G.; Afanasiev, M.D.D.S.S.S.; Karaulov Academician Ras, M.D.D.S.A.V. Photodynamic Therapy for Early-Stage Cervical Cancer Treatment. Photodiagn. Photodyn. Ther. 2021, 37, 102620.
- Viviano, M.; Kenfack, B.; Catarino, R.; Tincho, E.; Temogne, L.; Benski, A.-C.; Tebeu, P.-M.; Meyer-Hamme, U.; Vassilakos, P.; Petignat, P. Feasibility of Thermocoagulation in a Screen-and-Treat Approach for the Treatment of Cervical Precancerous Lesions in Sub-Saharan Africa. BMC Womens Health 2017, 17, 2.
- Gutierrez, P.; Garza, J.; Gandhi, K.; Voice, A.; Stout, E.; Ventolini, G. Carbon Dioxide (CO2) Laser Ablation Treatment of a Peri-Urethral Genital Wart: A Case Report. Case Rep. Womens Health 2020, 27, e00226.
- Cosper, P.F.; McNair, C.; González, I.; Wong, N.; Knudsen, K.E.; Chen, J.J.; Markovina, S.; Schwarz, J.K.; Grigsby, P.W.; Wang, X. Decreased Local Immune Response and Retained HPV Gene Expression during Chemoradiotherapy Are Associated with Treatment Resistance and Death from Cervical Cancer. Int. J. Cancer 2020, 146, 2047–2058.
- Foster, C.C.; Lee, A.Y.; Furtado, L.V.; Hart, J.; Alpert, L.; Xiao, S.-Y.; Hyman, N.H.; Sharma, M.R.; Liauw, S.L. Treatment Outcomes and HPV Characteristics for an Institutional Cohort of Patients with Anal Cancer Receiving Concurrent Chemotherapy and Intensity-Modulated Radiation Therapy. PLoS ONE 2018, 13, e0194234.
- Chera, B.S.; Amdur, R.J.; Green, R.; Shen, C.; Gupta, G.; Tan, X.; Knowles, M.; Fried, D.; Hayes, N.; Weiss, J.; et al. Phase II Trial of De-Intensified Chemoradiotherapy for Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma. J. Clin. Oncol. 2019, 37, 2661–2669.
- Sindhu, S.K.; Bauman, J.E. Current Concepts in Chemotherapy for Head and Neck Cancer. Oral. Maxillofac. Surg. Clin. N. Am. 2019, 31, 145–154.
- Bonomi, M.; Ahmed, T.; Warner, D.; Waltonen, J.; Sullivan, C.; Porosnicu, M.; Batt, K.; Ruiz, J.; Cappellari, J. Human Papillomavirus-Related Small Cell Carcinoma of the Oropharynx: A Case Report and Literature Review. Cancers Head Neck 2017, 2, 3.
- Perrone, A.M.; Tesei, M.; Ferioli, M.; De Terlizzi, F.; Della Gatta, A.N.; Boussedra, S.; Dondi, G.; Galuppi, A.; Morganti, A.G.; De Iaco, P. Results of a Phase I-II Study on Laser Therapy for Vaginal Side Effects after Radiotherapy for Cancer of Uterine Cervix or Endometrium. Cancers 2020, 12, 1639.
- Bogani, G.; Ditto, A.; Martinelli, F.; Mosca, L.; Chiappa, V.; Rossetti, D.; Leone Roberti Maggiore, U.; Sabatucci, I.; Lorusso, D.; Raspagliesi, F. LASER Treatment for Women with High-Grade Vaginal Intraepithelial Neoplasia: A Propensity-Matched Analysis on the Efficacy of Ablative versus Excisional Procedures. Lasers Surg. Med. 2018, 50, 933–939.
- Boonlikit, S. Recurrence of High-Grade Vaginal Intraepithelial Neoplasia after Various Treatments. Curr. Probl. Cancer 2022, 46, 100792.
- Farmer, E.; Cheng, M.A.; Hung, C.-F.; Wu, T.-C. Vaccination Strategies for the Control and Treatment of HPV Infection and HPV-Associated Cancer. Recent Results Cancer Res. 2021, 217, 157–195.
- Dadar, M.; Chakraborty, S.; Dhama, K.; Prasad, M.; Khandia, R.; Hassan, S.; Munjal, A.; Tiwari, R.; Karthik, K.; Kumar, D.; et al. Advances in Designing and Developing Vaccines, Drugs and Therapeutic Approaches to Counter Human Papilloma Virus. Front. Immunol. 2018, 9, 2478.
- Taylor, M.; Gerriets, V. Acyclovir. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Feng, E.; Balint, E.; Vahedi, F.; Ashkar, A.A. Immunoregulatory Functions of Interferons during Genital HSV-2 Infection. Front. Immunol. 2021, 12, 724618.
- Lau, A.; Kong, F.Y.S.; Fairley, C.K.; Templeton, D.J.; Amin, J.; Phillips, S.; Law, M.; Chen, M.Y.; Bradshaw, C.S.; Donovan, B.; et al. Azithromycin or Doxycycline for Asymptomatic Rectal Chlamydia Trachomatis. N. Engl. J. Med. 2021, 384, 2418–2427.
- Zofkie, A.C.; Fomina, Y.Y.; Roberts, S.W.; McIntire, D.D.; Nelson, D.B.; Adhikari, E.H. Effectiveness of Chlamydia Trachomatis Expedited Partner Therapy in Pregnancy. Am. J. Obstet. Gynecol. 2021, 225, 325.e1–325.e7.
- Adachi, K.N.; Nielsen-Saines, K.; Klausner, J.D. Chlamydia Trachomatis Screening and Treatment in Pregnancy to Reduce Adverse Pregnancy and Neonatal Outcomes: A Review. Front. Public Health 2021, 9, 531073.
- Gnanadurai, R.; Fifer, H. Mycoplasma Genitalium: A Review. Microbiology 2020, 166, 21–29.
- Unemo, M.; Lahra, M.M.; Cole, M.; Galarza, P.; Ndowa, F.; Martin, I.; Dillon, J.-A.R.; Ramon-Pardo, P.; Bolan, G.; Wi, T. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): Review of New Data and Evidence to Inform International Collaborative Actions and Research Efforts. Sex. Health 2019, 16, 412–425.
- Poncin, T.; Fouere, S.; Braille, A.; Camelena, F.; Agsous, M.; Bebear, C.; Kumanski, S.; Lot, F.; Mercier-Delarue, S.; Ngangro, N.N.; et al. Multidrug-Resistant Neisseria Gonorrhoeae Failing Treatment with Ceftriaxone and Doxycycline in France, November 2017. Eurosurveillance 2018, 23, 1800264.
- Salmerón, P.; Viñado, B.; Arando, M.; Alcoceba, E.; Romero, B.; Menéndez, B.; Bernal, S.; Idigoras, P.; Colomina, J.; Martin-Saco, G.; et al. Neisseria Gonorrhoeae Antimicrobial Resistance in Spain: A Prospective Multicentre Study. J. Antimicrob. Chemother. 2021, 76, 1523–1531.
- Bouchemal, K.; Bories, C.; Loiseau, P.M. Strategies for Prevention and Treatment of Trichomonas Vaginalis Infections. Clin. Microbiol. Rev. 2017, 30, 811–825.
- Graves, K.J.; Novak, J.; Secor, W.E.; Kissinger, P.J.; Schwebke, J.R.; Muzny, C.A. A Systematic Review of the Literature on Mechanisms of 5-Nitroimidazole Resistance in Trichomonas Vaginalis. Parasitology 2020, 147, 1383–1391.
- Lam, S. Photodynamic Therapy of Lung Cancer. Semin. Oncol. 1994, 21, 15–19.
- Kessel, D. Photodynamic Therapy: Critical PDT Theory. Photochem. Photobiol. 2022.
- Dougherty, T.J. Hematoporphyrin Derivative for Detection and Treatment of Cancer. J. Surg. Oncol. 1980, 15, 209–210.
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603.
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An Updated Overview on the Development of New Photosensitizers for Anticancer Photodynamic Therapy. Acta. Pharm. Sin. B 2018, 8, 137–146.
- Bellnier, D.A.; Greco, W.R.; Loewen, G.M.; Nava, H.; Oseroff, A.R.; Dougherty, T.J. Clinical Pharmacokinetics of the PDT Photosensitizers Porfimer Sodium (Photofrin), 2--2-Devinyl Pyropheophorbide-a (Photochlor) and 5-ALA-Induced Protoporphyrin IX. Lasers Surg. Med. 2006, 38, 439–444.
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy: Review. Photodiagn. Photodyn. Ther. 2021, 34, 102091.
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.-Y.; Dai, T.; Hamblin, M.R. Photodynamic Therapy for Infections: Clinical Applications. Lasers Surg. Med. 2011, 43, 755–767.
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.-A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2020, 8, 552028.
- De Sanjosé, S.; Diaz, M.; Castellsagué, X.; Clifford, G.; Bruni, L.; Muñoz, N.; Bosch, F.X. Worldwide Prevalence and Genotype Distribution of Cervical Human Papillomavirus DNA in Women with Normal Cytology: A Meta-Analysis. Lancet Infect. Dis. 2007, 7, 453–459.
- Pennycook, K.B.; McCready, T.A. Condyloma Acuminata. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Hu, Z.; Zheng, H.; Zeng, K. Patterns of Multiple Human Papillomavirus Clearance during 5-Aminolevulinic Acid-Based Photodynamic Therapy in Patients with Genital Warts. Photodiagn. Photodyn. Ther. 2021, 35, 102454.
- Yin, G.; Zhang, Y.; Geng, M.; Cai, B.; Zheng, Y. Cure of Condyloma Acuminata Covering the Glans Penis Using Aminolevulinic Acid/Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2020, 30, 101658.
- Inada, N.M.; Buzza, H.H.; Carbinatto, F.M.; Blanco, K.C.; de Andrade, C.T.; Vollet-Filho, J.D.; Bagnato, V.S.; Allison, R.R. Optical Techniques for the Diagnosis and Treatment of Lesions Induced by the Human Papillomavirus—A Resource Letter. Photodiagn. Photodyn. Ther. 2018, 23, 106–110.
- Owczarek, W.; Slowinska, M.; Walecka, I.; Ciazynska, M.; Nowicka, D.; Walczak, L.; Paluchowska, E. Correlation of the ALA-PDT Treatment Efficacy and the HPV Genotype Profile of Genital Warts after Cryotherapy Failure and Podophyllotoxin Therapy in Male Patients. Life 2021, 11, 146.
- Kechichian, E.; Helou, E.; Sarkis, J.; Hayek, C.; Labaki, C.; Nemr, E.; Tomb, R. The Place of 5-Aminolaevulinic Acid-Photodynamic Therapy in the Treatment Landscape of Urethral Warts: A Systematic Review. Photodiagn. Photodyn. Ther. 2021, 33, 102204.
- Stern, P.L.; van der Burg, S.H.; Hampson, I.N.; Broker, T.R.; Fiander, A.; Lacey, C.J.; Kitchener, H.C.; Einstein, M.H. Therapy of Human Papillomavirus-Related Disease. Vaccine 2012, 30 (Suppl. S5), F71–F82.
- Mistrangelo, M.; Dal Conte, I.; Volpatto, S.; DI Benedetto, G.; Testa, V.; Currado, F.; Morino, M. Current Treatments for Anal Condylomata Acuminata. Minerva Chir. 2018, 73, 100–106.
- Hu, Z.; Zheng, H.; Zeng, K. Predictors of Human Papillomavirus Persistence or Clearance after 5-Aminolevulinic Acid-Based Photodynamic Therapy in Patients with Genital Warts. Photodiagn. Photodyn. Ther. 2021, 35, 102431.
- Xie, J.; Ao, C.; Li, J.; Jiang, L.; Liu, H.; Zeng, K. 5-Aminolevulinic Acid Photodynamic Therapy for Condyloma Acuminatum of Urethral Meatus. J. Dermatolog. Treat. 2019, 30, 714–717.
- Buzzá, H.H.; Stringasci, M.D.; de Arruda, S.S.; Crestana, R.H.S.; de Castro, C.A.; Bagnato, V.S.; Inada, N.M. HPV-Induced Condylomata Acuminata Treated by Photodynamic Therapy in Comparison with Trichloroacetic Acid: A Randomized Clinical Trial. Photodiagn. Photodyn. Ther. 2021, 35, 102465.
- Hu, S.; Yang, Y.; Jiang, B.; Su, D.; Zhang, L.; Huang, Z.; Zhang, F. Treatment of Condyloma Acuminatum Using the Combination of Laser Ablation and ALA-PDT. Photodiagn. Photodyn. Ther. 2019, 25, 193–196.
- Shi, H.; Zhang, X.; Ma, C.; Yu, N.; Wang, J.; Xia, L.; Ge, X.; Liu, M.; Duan, A. Clinical Analysis of Five Methods Used to Treat Condylomata Acuminata. Dermatology 2013, 227, 338–345.
- Szeimies, R.-M.; Schleyer, V.; Moll, I.; Stocker, M.; Landthaler, M.; Karrer, S. Adjuvant Photodynamic Therapy Does Not Prevent Recurrence of Condylomata Acuminata after Carbon Dioxide Laser Ablation-A Phase III, Prospective, Randomized, Bicentric, Double-Blind Study. Dermatol. Surg. 2009, 35, 757–764.
- Kim, J.-H.; Kim, J.; Kim, K.; No, J.H.; Kim, Y.B.; Suh, D.H. Risk Factor and Treatment of Vaginal Intraepithelial Neoplasia After Hysterectomy for Cervical Intraepithelial Neoplasia. J. Low Genit. Tract. Dis. 2022, 26, 147–151.
- Hampson, L.; Martin-Hirsch, P.; Hampson, I.N. An Overview of Early Investigational Drugs for the Treatment of Human Papilloma Virus Infection and Associated Dysplasia. Expert. Opin. Investig. Drugs 2015, 24, 1529–1537.
- Zhang, W.; Zhang, A.; Sun, W.; Yue, Y.; Li, H. Efficacy and Safety of Photodynamic Therapy for Cervical Intraepithelial Neoplasia and Human Papilloma Virus Infection. Medicine 2018, 97, e10864.
- Tao, X.H.; Guan, Y.; Shao, D.; Xue, W.; Ye, F.S.; Wang, M.; He, M.H. Efficacy and Safety of Photodynamic Therapy for Cervical Intraepithelial Neoplasia: A Systemic Review. Photodiagn. Photodyn. Ther. 2014, 11, 104–112.
- Bodner, K.; Bodner-Adler, B.; Wierrani, F.; Kubin, A.; Szölts-Szölts, J.; Spängler, B.; Grünberger, W. Cold-Knife Conization versus Photodynamic Therapy with Topical 5-Aminolevulinic Acid (5-ALA) in Cervical Intraepithelial Neoplasia (CIN) II with Associated Human Papillomavirus Infection: A Comparison of Preliminary Results. Anticancer. Res. 2003, 23, 1785–1788.
- Wang, J.; Xu, J.; Chen, J.; He, Q.; Xiang, L.; Huang, X.; Ding, G.; Xu, S. Successful Photodynamic Therapy with Topical 5-Aminolevulinic Acid for Five Cases of Cervical Intraepithelial Neoplasia. Arch. Gynecol. Obstet. 2010, 282, 307–312.
- Li, D.; Zhang, F.; Shi, L.; Lin, L.; Cai, Q.; Xu, Y. Treatment of HPV Infection-Associated Low Grade Cervical Intraepithelial Neoplasia with 5-Aminolevulinic Acid-Mediated Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2020, 32, 101974.
- Cang, W.; Gu, L.; Hong, Z.; Wu, A.; Di, W.; Qiu, L. Effectiveness of Photodynamic Therapy with 5-Aminolevulinic Acid on HPV Clearance in Women without Cervical Lesions. Photodiagn. Photodyn. Ther. 2021, 34, 102293.
- Wu, A.; Li, Q.; Ling, J.; Gu, L.; Hong, Z.; Di, W.; Qiu, L. Effectiveness of Photodynamic Therapy in Women of Reproductive Age with Cervical High-Grade Squamous Intraepithelial Lesions (HSIL/CIN2). Photodiagn. Photodyn. Ther. 2021, 36, 102517.
- Su, Y.; Zhang, Y.; Tong, Y.; Zhang, L.; Li, P.; Zhang, H.; Zhang, X.; Tang, Y.; Qin, L.; Shen, Y.; et al. Effect and Rational Application of Topical Photodynamic Therapy (PDT) with 5-Aminolevulinic Acid (5-ALA) for Treatment of Cervical Intraepithelial Neoplasia with Vaginal Intraepithelial Neoplasia. Photodiagn. Photodyn. Ther. 2022, 37, 102634.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
703
Revisions:
2 times
(View History)
Update Date:
20 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




