Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tharuka Gunathilake | -- | 2860 | 2022-07-19 00:56:19 | | | |
| 2 | Camila Xu | Meta information modification | 2860 | 2022-07-19 03:53:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gunathilake, T.; Akanbi, T.O.; Suleria, H.A.R.; Nalder, T.D.; Francis, D.S.; Barrow, C.J. Seaweed Phenolics. Encyclopedia. Available online: https://encyclopedia.pub/entry/25253 (accessed on 07 February 2026).
Gunathilake T, Akanbi TO, Suleria HAR, Nalder TD, Francis DS, Barrow CJ. Seaweed Phenolics. Encyclopedia. Available at: https://encyclopedia.pub/entry/25253. Accessed February 07, 2026.
Gunathilake, Tharuka, Taiwo O. Akanbi, Hafiz A. R. Suleria, Tim D. Nalder, David S. Francis, Colin J. Barrow. "Seaweed Phenolics" Encyclopedia, https://encyclopedia.pub/entry/25253 (accessed February 07, 2026).
Gunathilake, T., Akanbi, T.O., Suleria, H.A.R., Nalder, T.D., Francis, D.S., & Barrow, C.J. (2022, July 18). Seaweed Phenolics. In Encyclopedia. https://encyclopedia.pub/entry/25253
Gunathilake, Tharuka, et al. "Seaweed Phenolics." Encyclopedia. Web. 18 July, 2022.
Copy Citation
Seaweed, also referred to as macroalgae, have been studied as potential aquafeed ingredients since the late 1970s but have been implemented as a poultry feed supplement since the 1950s. Seaweed phenolics provide alternative ingredients that are complementary to synthetic additives used in aquaculture, possessing a broad spectrum of bioactive properties such as antimicrobial, antiviral, antifungal, anti-stress, antioxidant, anti-inflammatory, immunostimulant, and appetite stimulation. Also, their antioxidant properties retard lipid oxidation and preserve feed quality improving shelf life.
aquafeed
phenolics
seaweed
veterinary drug
1. Seaweed Phenolics as Sustainable Aquafeed Additives
Acceptability and commercialization of novel aquafeed ingredients depend on the nutritional profile, cultivation cost, seasonality, and economies of scale [1][2]. Seaweed, also referred to as macroalgae, have been studied as potential aquafeed ingredients since the late 1970s [3] but have been implemented as a poultry feed supplement since the 1950s [4]. Some species of seaweed grow rapidly with no requirement for freshwater, arable land, fertiliser or pesticides, and are usually available year-round with some seasonal variations in growth [5]. The constant supply of biomass coupled with a high nutritional profile makes seaweed a sustainable choice for aquaculture and food security [6]. Seaweeds, along with microalgae, form the base of the aquatic food web, where nutrients pass through the food chain toward top-end predators such as sharks, dolphins, and whales [7]. As such, seaweeds contribute substantially to aquatic life, either directly or indirectly, by providing essential nutrients. However, to date, seaweed remain underutilised in aquaculture.
Previous studies suggest that most seaweeds can be fed to an aquatic animal as an additive or supplement [1][2], providing their dietary inclusion levels are not too high. The inclusion of seaweed in aquafeed has been attempted as either seaweed meal or seaweed extract [1]. This has been shown to provide general physiological improvements in animals, such as growth performance [8], feed utilisation [9], disease resistance, stress response [9], fillet quality, natural pigmentation, protein retention during winter, and increased long-chain polyunsaturated fatty acid concentration in fillets [9][10]. Therefore, preparing aquafeed formulations containing targeted seaweed-derived ingredients could promote fish health at a low cost. It has been reported that when the inclusion of seaweed in aquafeed is beyond 10% w/w it impacts negatively on most species [11]. Mechanistically it has been suggested that non-starch polysaccharides (cellulose and hemicellulose) and anti-nutritional factors (tannins, phytic acid, lectins, amylase, and trypsin inhibitors) present in seaweeds can reduce nutrient accessibility [12]. Extraction of bioactive compounds from seaweeds for inclusion into aquafeeds would help overcome the problems caused by the inclusion of the whole biomass, of which carbohydrates are generally the major component.
In addition to basic nutrients, seaweeds are relatively unexplored sources of numerous bioactive compounds (polyphenols, pigments, essential fatty acids, vitamins and amino acids). These are categorised as secondary metabolites, which are synthesised for a range of reasons, such as protective mechanisms against infections and environmental stress conditions [13]. With advances in separation science, a vast range of these compounds can now be isolated and characterised [14]. Recently, interest has grown considerably in using seaweed as a source of functional ingredients rather than whole macronutrient sources. Seaweed phenolics provide alternative ingredients that are complementary to synthetic additives used in aquaculture, possessing a broad spectrum of bioactive properties such as antimicrobial, antiviral, antifungal, anti-stress, antioxidant, anti-inflammatory, immunostimulant, and appetite stimulation [15]. Also, their antioxidant properties retard lipid oxidation and preserve feed quality improving shelf life. In addition, low molecular weight (LMW) phenolic compounds such as bromophenols (BP) (2-BP, 4-BP, 2,4-BP, 2,6-BP, 2,4,6-BP) are natural flavour compounds that can enhance fish fillet flavour and increase the market value [16]. According to Ma et al. [16], silver seabream-fed diets supplemented with seaweed showed significant deposition of bromophenol in fish flesh and gut, which imparted a “sea-like flavour” in the fillets.
This demonstrates that seaweed phenolics can be safe, sustainable and natural additives in aquatic health management, as well as having the added benefit of consumer appeal regarding the demand for natural products in food labelling.
2. Overview of Seaweeds
For centuries, seaweeds have been widely used in Asia (particularly in China, Japan, and Korea) as a traditional food source. The majority of global seaweed production (more than 80%) is consumed by humans as fresh or dried whole seaweed or used to produce food hydrocolloids (mainly agar, alginate and carrageenan). The remaining less than 20% are used for a range of industrial applications, such as feed ingredients (animal and fish feed), cosmetics, bioplastics and fertilisers [17]. Seaweeds fall into three taxonomic groups based on their thallus pigmentation: brown seaweed (Ochrophyta), red seaweed (Rhodophyta) and green seaweed (Chlorophyta) [18]. Brown seaweeds are long, thick, and leather-like species and can reach up to 45 m long [19]. Red seaweeds are relatively small (up to ~1 m) species with different shades, including red, purple, or brownish-red [18]. Green seaweeds are closely related to red seaweeds and are similar in size [18]. The basic thallus structures of brown, green and red seaweeds are shown in Figure 1. Seaweeds are typically found in estuarine intertidal and subtidal habitats and coastal areas, with some kelps being among the fastest-growing plants on earth [2].
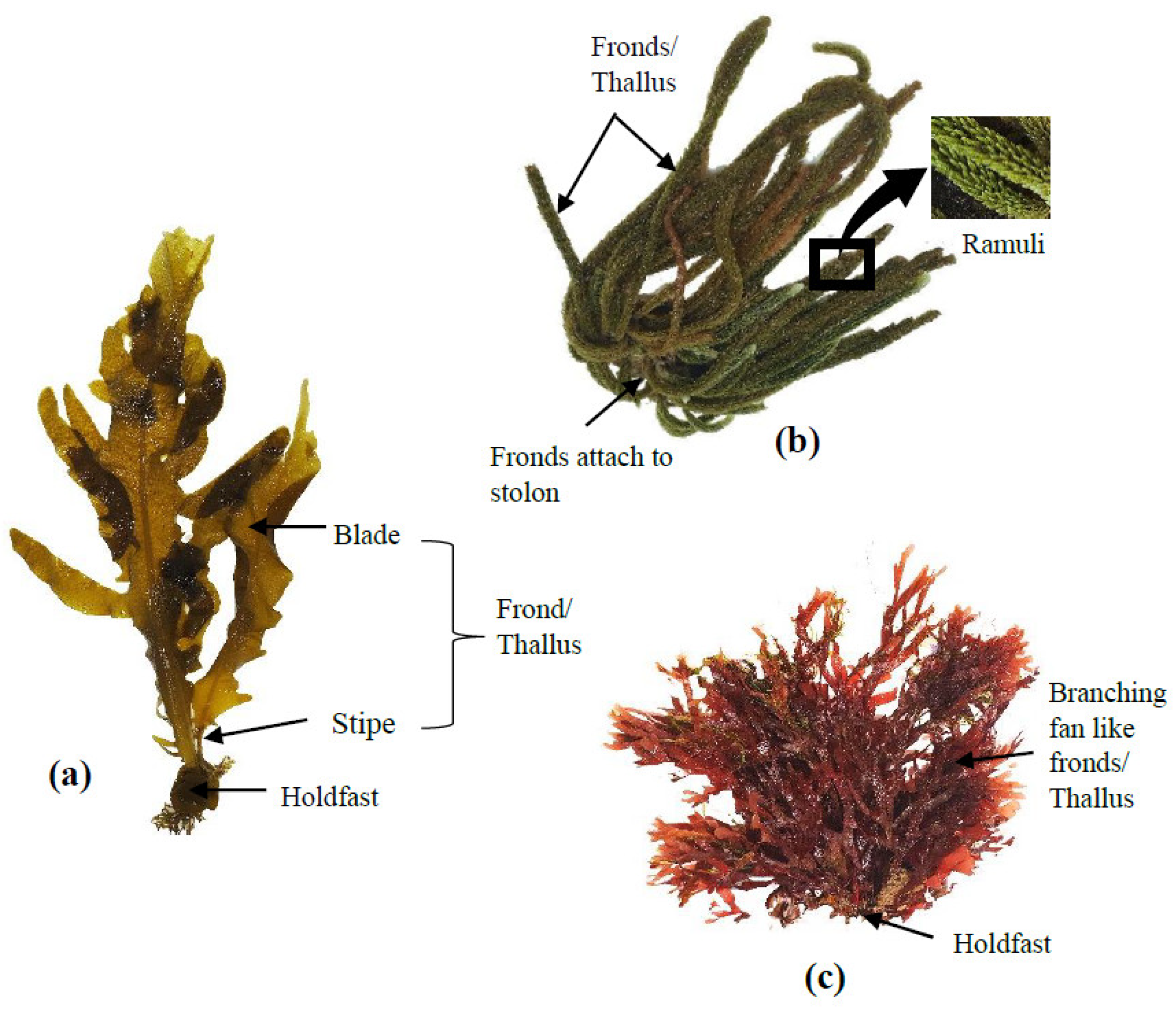 Figure 1. Seaweed thallus structures for (a) brown seaweed (e.g., Lessoniaceae), (b) green seaweed (e.g., Caulerpaceae) and (c) red seaweed (e.g., Galaxauraceae).
Figure 1. Seaweed thallus structures for (a) brown seaweed (e.g., Lessoniaceae), (b) green seaweed (e.g., Caulerpaceae) and (c) red seaweed (e.g., Galaxauraceae).Approximately 25,000–30,000 species of seaweed are known to exist worldwide [20]. Global seaweed production is currently worth over USD $6 billion per annum, with 85% of production for human consumption [21]. There are about 50 countries involved in commercial seaweed farming, with the most production in Asia (China, Indonesia, Philippines, Republic of Korea, Malaysia and Japan), South America (Chile), Europe (Denmark) and Africa (Tanzania) [21][22].
Seaweeds are also an attractive resource for integrated multi-trophic aquaculture systems (IMTA) as a food source for aquaculture species (e.g., sea urchins) that can provide a buffer against ocean movement and actively bioremediate organic waste produced from intensive fish farming practices [23]. IMTA is a viable approach in both sea-based aquaculture (salmon, bluefin tuna) and land-based farms (prawns, abalone, and finfish aquaculture) [23]. IMTA shows numerous benefits over monoculture systems, including multiple harvests, lowered production costs, large-scale viability and minimisation of environmental impact [2].
3. Seaweed Phenolics Compounds
3.1. Classification of Phenolic Compounds
Phenolic compounds are a highly heterogeneous group of compounds found in terrestrial plants and marine seaweeds [24]. These bioactive molecules contain at least one aromatic ring with a single hydroxyl group (–OH) [25]. So far, over 8000 phenolic compounds have been identified with structural diversity ranging from low molecular weight single aromatic ring monomers to highly complex polymerised structures [25]. In the literature, phenolics are divided into different categories based on their origin, structure, and functionality. To simplify, this research categorises phenolic compounds into two groups based on their chemical structures: flavonoids and non-flavonoids. Flavonoids are the most widely distributed group and account for nearly two-thirds of all known phenolics [26]. These molecules are made up of two phenyl rings attached to a heterocyclic pyran ring (Figure 2) [26]. By changing the structural properties of the pyran ring, flavonoids are further divided into six groups: flavones, isoflavones, flavanols, flavanones, flavonols (flavan-3-ol), anthocyanidins [26][27]. However, individual compounds in each of these groups vary by the methylation and hydroxylation patterns of the two phenyl rings [26].
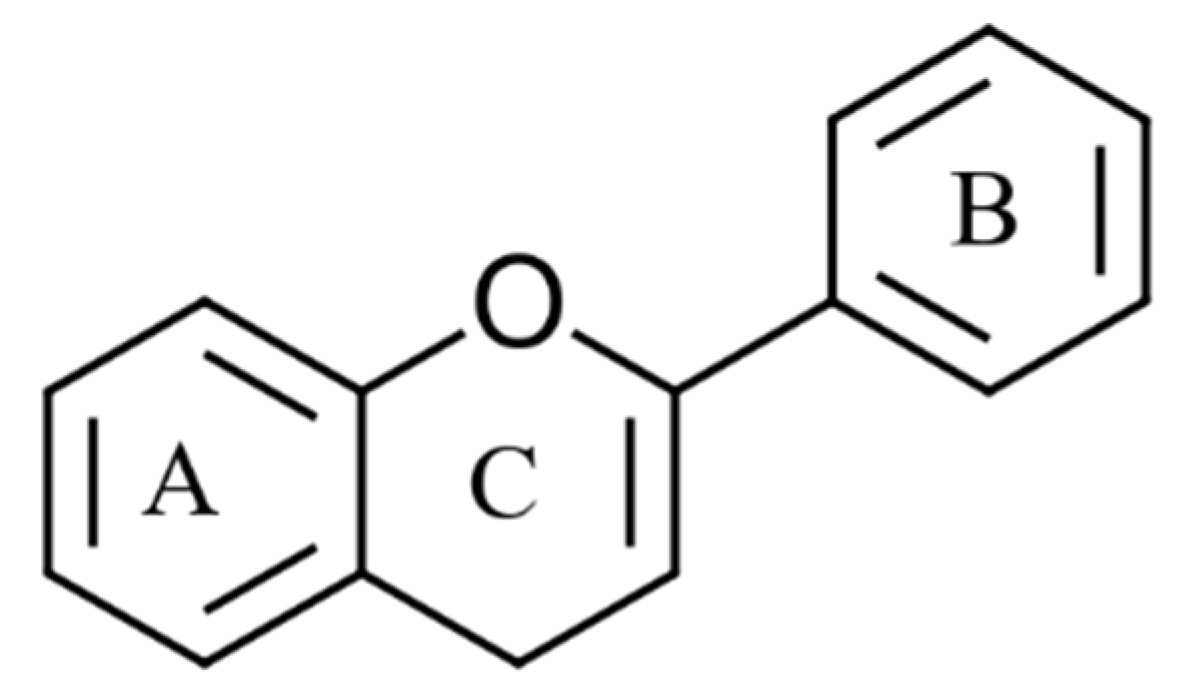 Figure 2. The basic skeleton of flavonoid compounds. A & B; phenyl rings and C; heterocyclic pyran ring.
Figure 2. The basic skeleton of flavonoid compounds. A & B; phenyl rings and C; heterocyclic pyran ring.Non-flavonoids are phenolic compounds consisting of a single phenyl group through to high molecular weight polymerised complexes [26]. Most are found in fruits and vegetables and are referred to as phenolic acids, commonly containing a phenyl ring bound to one or more functional groups [26]. Based on their derivatives, either benzoic or cinnamic acid, most phenolic acids are further divided as hydroxybenzoic or hydroxycinnamic acids, respectively (Figure 3) [27]. Additionally, other phenolic acids exist as hydroxyphenyl acids (acetic, propanoic and pentaenoic) [28]. Lignans, stilbenes and tannins are different groups of non-flavonoid compounds found in the plant kingdom [27]. The major flavonoids and non-flavonoid compounds found in seaweeds are discussed below.
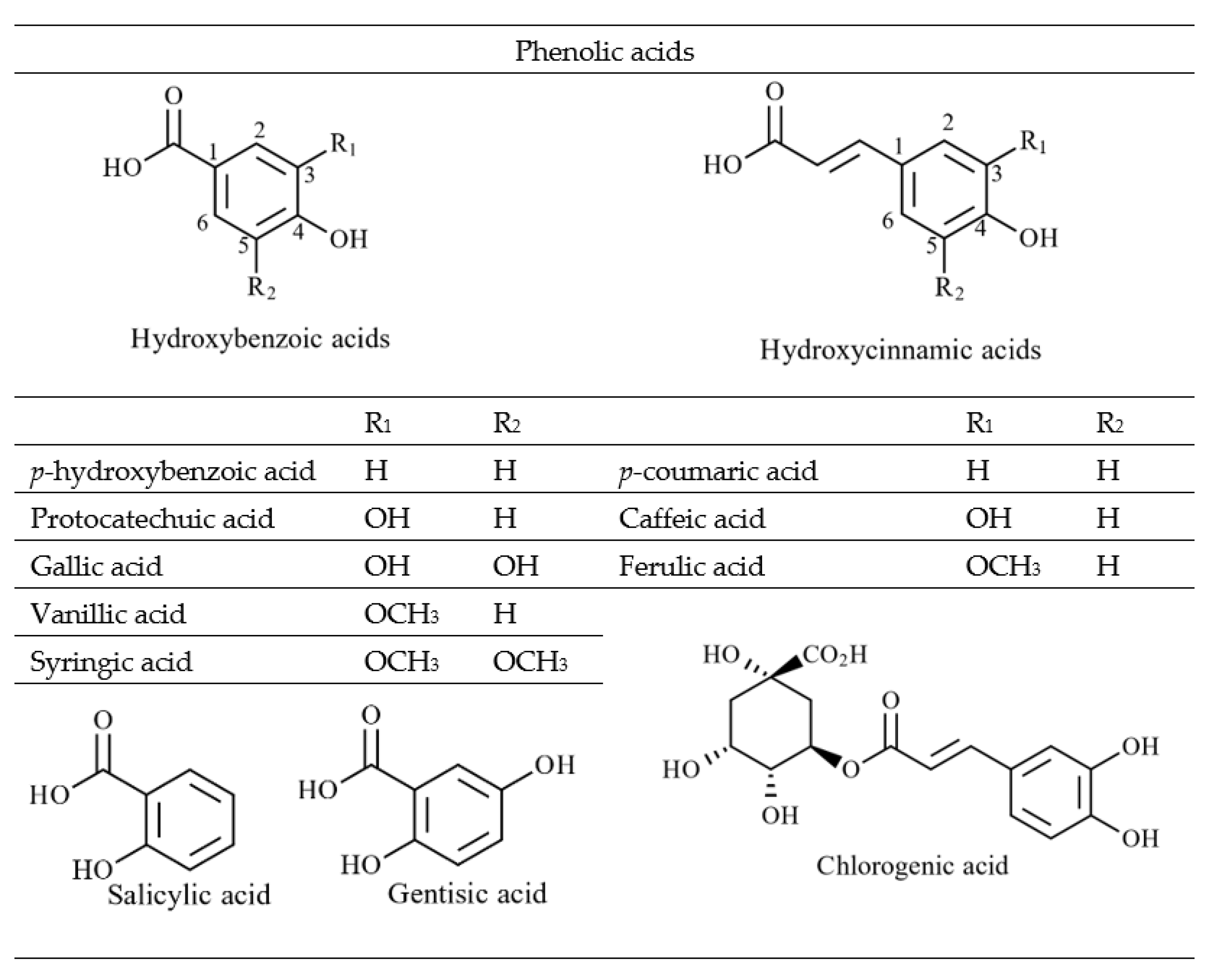 Figure 3. The basic skeleton of hydroxybenzoic acids and hydroxycinnamic acids found in seaweed.
Figure 3. The basic skeleton of hydroxybenzoic acids and hydroxycinnamic acids found in seaweed.3.2. Phenolic Compounds Found in Seaweeds
3.2.1. Phenolic Acids and Flavonoids
To date, numerous polymeric structures have been identified in red, green and brown seaweed species [14]. As reported previously, the major phenolic constituent of red and green seaweeds are flavonoids (Figure 4) and phenolic acids [14]. However, bromophenols (halogenated phenolics) mainly exist in red seaweeds, while phlorotannins are found exclusively in brown seaweeds [27]. Some studies report that phlorotannins are the only phenolic compound found in brown seaweeds [29], whilst others report the presence of flavonoids and phenolic acids [30][31][32][33]. Quantification analysis of S. scoparium (brown seaweeds) aqueous extract reported the presence of significant concentrations of phenolic acids and flavonoids; 90 mg/100 g dry weight (DW) of gallic acid followed by catechin and epicatechin (6–7 mg/100 g DW) [31]. Yoshie-Stark et al. studied flavonoid distribution in methanolic extracts of 27 Japanese seaweed species (6 green, 11 brown and 10 red seaweeds), revealing a high abundance of flavonoids in red seaweeds compared to green and brown seaweeds [33]. Hesperidin was found in all red seaweeds (626–119,000 µg/g) and some green and brown seaweeds [33]. Catechol was common in all green and red seaweeds (1660–77,700 µg/g) as well as most brown seaweeds [33]. Rutin and caffeic acids were distributed amongst all three groups but were most prominent in red seaweeds (23,200–4,000 µg/g) [33]. Quercitrin and myricetin were present in low concentrations in brown and red seaweeds (202–466 µg/g), whereas morin was detected in all seaweed samples in small quantities (257–3730 µg/g) [33].
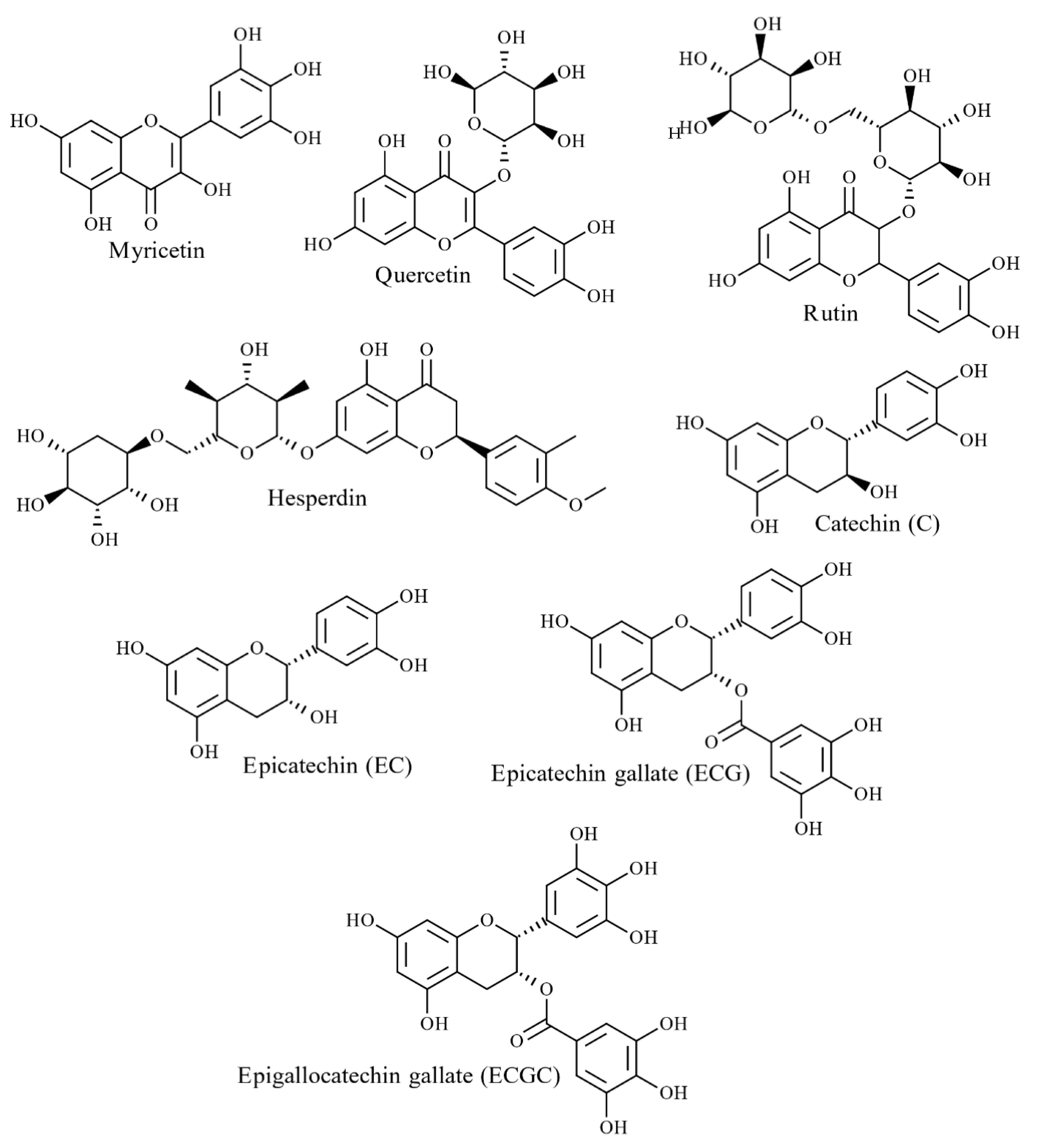 Figure 4. Structures of major flavonoids identified in seaweeds.
Figure 4. Structures of major flavonoids identified in seaweeds.Furthermore, twelve phenolic acids were reported in red and brown seaweed extracts: Porphyra tenera (nori) and Undaria pinnatifida (wakame), namely hydroxybenzoic acid (salicylic acid, 2,3-dihydroxybenzoic, p-hydroxybenzoic, protocatechuic), hydroxycinnamic acid (p-coumaric, caffeic, chlorogenic) and hydroxybenzaldehydes (3,4-dihydroxybenzaldehyde, p-hydroxybenzaldehyde) [34]. Further, Klejdus et al. [35] identified eight isoflavones (daidzin, daidzein, genistein, formononetin, sissotrin, biochanin A and ononin) in seven red and brown seaweeds, with the highest concentrations present in Chondrus crispus (red seaweed 86–229 ng/g) followed by Halopytis incurvus (red seaweed 7–50 ng/g) and Sargassum muticum (brown seaweed 7–144 ng/g) suggesting that these isoflavone compounds are restricted to specific seaweed species [14]. In addition to halogenated phenolics, sulphate metabolites have occasionally been observed in red, green and brown seaweed [14][36]. For instance, sulphated coumaric acids, benzoic acids and phenylacetic acid have been reported in the green seaweed Dasycladus vermicularis [37].
3.2.2. Phlorotannin
Phlorotannin, like tannin in terrestrial plants, is a heterogeneous group of polymeric compounds found only in brown seaweeds [13]. They are biosynthesised through dehydro-oligomerisation and dehydro-polymerisation of phloroglucinol (1,3,5-trihydroxybenzene) units (PGU) via aryl-aryl (C-C) bonds and/or diaryl-ether (C-O) bonds, which produce molecules with masses ranging from 126–650 kDa [24][32]. Based on the position of polymerisation, different isomers exist [38] that create difficulty in the structural elucidation of seaweed extracts. Phlorotannins with 12 PGU in F. vesiculosus lead to 61 isomers demonstrating the complex nature of these compounds [39]. Unlike other phenolic compounds, this group is believed to have over 150 molecular structures due to different molecular sizes and linkages [40].
The presence of many phenolic hydroxyl groups makes them more hydrophilic. Furthermore, it contributes to the different roles they play in seaweeds, such as chelate divalent metals, integral structural constituents that bind with polysaccharides, protein and other biopolymers [38]. Concentrations of phlorotannins upwards of 15% in dry weight (DW) have been reported in brown seaweeds [41], with 12% DW reported in Fucus sp. and 14% DW in A. nodosum [13].
The type of linkages between monomers can be used to classify phlorotannins into four groups: phlorethols and fuhalols with an ether linkage, fucols with a phenyl linkage, fucophlorethols with ether and a phenyl linkage, and eckols and carmalols with a benzodioxin linkage [42]. Compounds in all four groups can be further divided into linear (bound only to two phloroglucinol units) and branched phlorotannins (bound to three/more phloroglucinol units) [38]. Figure 5 depicts the linear and branched arrangements of tetrafucol in Fucus vesiculosus [43], while Figure 6 shows major classes of phlortannins.
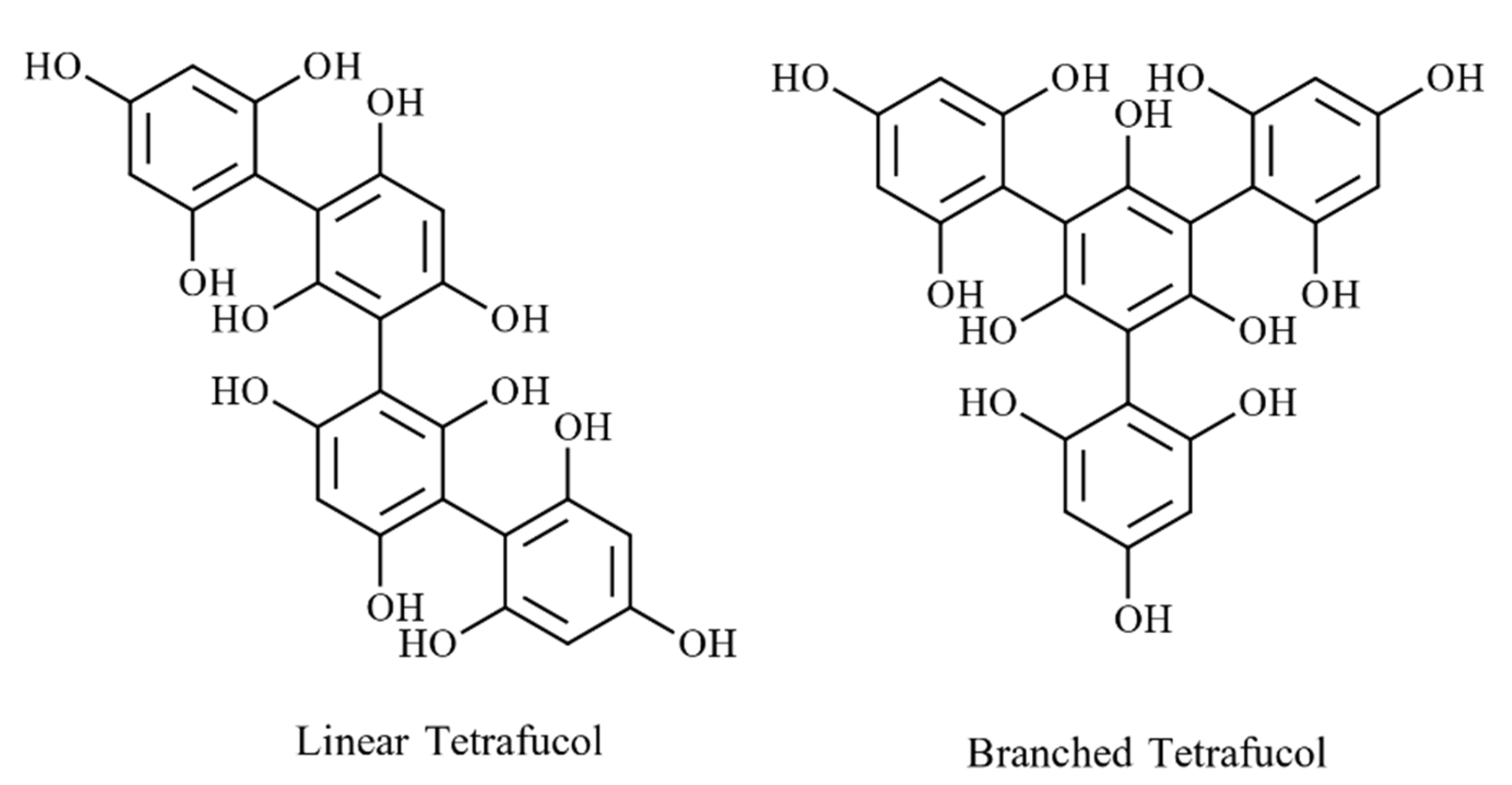 Figure 5. Examples for linear and branched phlorotannin (e.g., Tetrafucol).
Figure 5. Examples for linear and branched phlorotannin (e.g., Tetrafucol).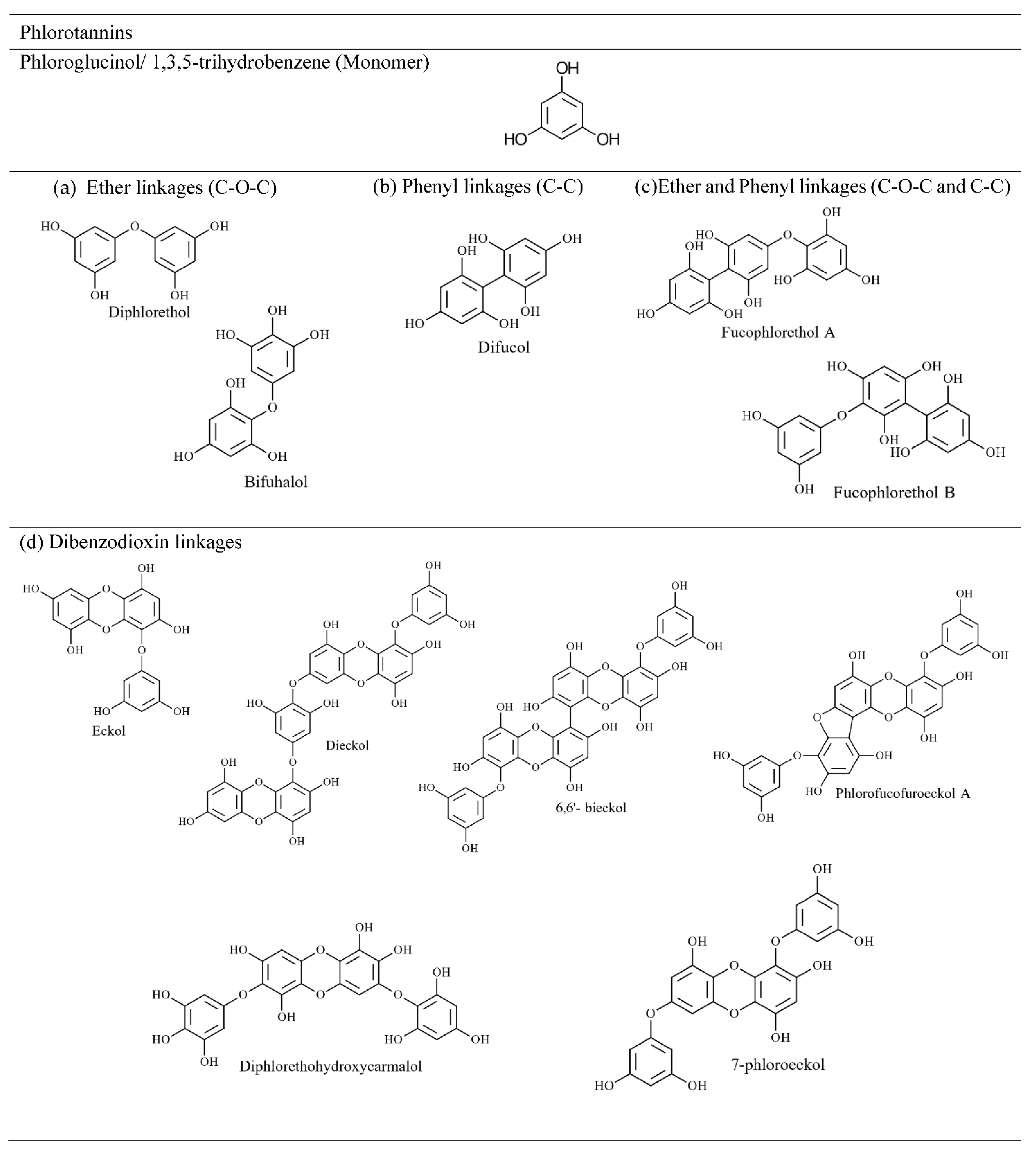 Figure 6. Phlorotannins classification based on type of linkage between aromatic units, (a) phlorethol and fuhalol, (b) fucol, (c) fucophlorethol, (d) eckol and carmalol.
Figure 6. Phlorotannins classification based on type of linkage between aromatic units, (a) phlorethol and fuhalol, (b) fucol, (c) fucophlorethol, (d) eckol and carmalol.Significant amounts of phlorotannins have been recorded in Laminariales (Ecklonia spp. and Eisenia spp.), Fucaceae (A. nodosum and F. vesiculosus) and Sargassaceae families [32][44]. Previously, phlorotannins found in brown seaweeds have been reported by compound type rather than subclass [24], with some researchers claiming that fucols and fucophlorethols are prominent among the Fucaceae family [44]. Accordingly, phloroglucinol, eckol (including carmalol derivatives), dieckol, 6,6′-bieckol, fuhalol, 7-phloroeckol and phlorofucofuroeckol, and fucophloroethol, have been frequently identified in brown seaweed species [45][46]. Using conventional molecular characterisation techniques, the size of phlorotannin compounds was reported to be between 2 and 8 PGU [47]. However, using rapid phenolic profiling chromatographic and mass spectrometric techniques, larger phlorotannin compounds of 16 PGU [39] and 17 and 27 PGU [48] repeating units have been identified. Steevensz et al. [49] successfully investigated rapid phlorotannins profiling methods using ultra-high-performance liquid chromatography-high-resolution mass spectrometry (UHPLC-HRMS), enabling the characterisation of phlorotannins with 3–49 PGU in P. canaliculata, which is by far the highest level of polymerisation recorded in brown seaweed. However, low molecular weight phlorotannins preferentially have 4–12 PGU according to Ultra Performance Liquid Chromatography–Triple-Quadrupole Mass Spectrometry (UPLC-QQQ-MS) data in Fucaceae and Himanthaliaceae species [39].
3.3. Occurrence and Biosynthesis
Phenolic compounds in seaweeds originate as secondary metabolites and are available in two forms [50]. Soluble forms protect against stress conditions, herbivory, heavy metal exposure and oxidative damage emanating from UV radiation and nutrient deficiency [51]. Insoluble forms, also known as cell wall-bound polyphenols (mostly phlorotannins), usually bind with alginic acid and protein via ester covalent bonds [52]. Phlorotannin with structural polysaccharides plays a vital role in the structural development of brown seaweed cell walls [53]. However, quantification studies of brown seaweed phlorotannin from F. vesiculosus show that their soluble form in the cytoplasm was more abundant than the cell wall-bound form [54].
Phenolics accumulate in membrane-bound vesicles called physodes in the cytoplasm of seaweed cells [51]. Physodes are produced in the endoplasmic reticulum and Golgi apparatus before being transferred to fuse with the cell membrane and begin phenolic secretion [51]. Baardseth, E. [55] reported that physodes represent 3–11% v/v of the seaweed body of F. vesiculosus, A. nodosum and F. serratus. Based on reports from Laminaria hyperborea and Fucus serratus species, the size of physodes is relatively uniform at ~2,500 μm [56]. The study between three brown seaweed species revealed phlorotannin is exclusively produced in the peripheric vegetative cells of thalli [57]. Phlorotannins are synthesised due to polymerisation of phloroglucinol (1,3,5-trihydroxybenzene) monomers [40]. The biosynthesis of phloroglucinol happens via the acetate-malonate (polyketide) pathway utilising the type III polyketide synthase enzyme followed by oxidative polymerisation process to form phlorotannins [58].
3.4. Variation of Phenolic Content
The phenolic content of seaweeds varies and is dependent on many factors. The bioactive properties of these compounds help seaweeds survive under the harsh conditions of the marine environment [24]. Phenolic compositions vary interspecies and/or intraspecies, and sometimes even within a single species thallus [24]. Intraspecies phenolic composition varies according to specific intrinsic (thalli size, reproductive state, age, tissue type) and extrinsic factors (temperature, light, contaminants, nutrients availability, salinity and geography) [59]. Additionally, harvest season, location and extraction methods are known to affect the phenolic profile. Due to the high variability of these factors, it becomes difficult to compare interspecies and/or intraspecies differences in phenolic composition.
Compared to red and green seaweeds, brown seaweeds possess more phenolic compounds, particularly phlorotannins [60]. The phenolic content in brown seaweed is influenced by species, location, and season. Connan et al. [61] studied the variation among eight brown seaweed species across 14 months, revealing greater phenolic content in Fucales sp. (>2% DW) in comparison to Laminaria digitata as a result of genetic adaptation to environmental factors. They also found that species occupying the middle intertidal zone exhibited the highest phenol concentration (~5.8% DW) compared to lower and upper levels of the shore (0.03–1.40%) [61]. Seaweeds in different intertidal zones are subject to different environmental conditions such as light intensity, salinity, temperature, and periods of dehydration [61]. Regarding seasonal variation, there was a taxonomic difference among the species studied. The highest phenolic content was observed in Fucaleans in summer, given the need for photo-protective mechanisms, whereas Laminariales demonstrated the maximum amount of these compounds in winter [61]. Similar results demonstrated the highest level of phlorotannin excretion in Ascophyllum nodosum (member of Fucaceae family) in summer [62]. The correlation between phenolic excretion and light intensity was also observed by Abdala-Díaz et al. [63]. Accordingly, the phenolic concentration of brown seaweed (Cystoseira tamariscifolia) was the highest in the apical section of the thallus. It decreased towards the middle and basal sections, given they were tightly packed against each other and shaded from UV and photosynthetically active radiation [63]. The variation of phlorotannin content with respect to reproductive status had been studied for Ecklonia cava, having phlorotannin levels 1.5 times higher in mature thalli than in younger samples [38]. Salinity also appears to have a positive relationship with phenolic excretion [64]. For instance, studies of F. vesiculosus and A. nodosum showed an increase in phenolic content as the salinity of surrounding water increased [65].
The extraction method employed for analysis is also a factor that significantly impacts the total phenolic content (TPC) of seaweed samples. According to Dang et al. [66], novel phenolic extraction methods, such as ultrasonication-assisted extraction, gave higher yields than conventional extraction methods. However, the vast range of inter- and intra-species phenolic variation is problematic for the use of these products in aquafeed formulations, as difficulties arise in obtaining standard phenolic composition in the final products, particularly when harvested from natural habitats [67]. On the other hand, this presents an opportunity for seaweed farmers to change and maximise phenolic composition based on their intended requirements through stimulating growth conditions and selective breeding programs [2][68].
References
- Teves, J.F.C.; Ragaza, J.A. The quest for indigenous aquafeed ingredients: A review. Rev. Aquac. 2016, 8, 154–171.
- Wan, A.H.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2019, 11, 458–492.
- Stanley, J.G.; Jones, J.B. Feeding algae to fish. Aquaculture 1976, 7, 219–223.
- Black, W. Seaweed as a poultry food. World’s Poult. Sci. J. 1954, 10, 33–35.
- Lorbeer, A.; Tham, R.; Zhang, W. Potential products from the highly diverse and endemic macroalgae of Southern Australia and pathways for their sustainable production. J. Appl. Phycol. 2013, 25, 717–732.
- Ragaza, J.A.; Koshio, S.; Mamauag, R.E.; Ishikawa, M.; Yokoyama, S.; Villamor, S.S. Dietary supplemental effects of red seaweed Eucheuma denticulatum on growth performance, carcass composition and blood chemistry of juvenile Japanese flounder, Paralichthys olivaceus. Aquac. Res. 2015, 46, 647–657.
- Thorp, J.H.; Rogers, D.C. Chapter 4—A Primer on Ecological Relationships among Freshwater Invertebrates. In Field Guide to Freshwater Invertebrates of North America; Thorp, J.H., Rogers, D.C., Eds.; Academic Press: Boston, MA, USA, 2011; pp. 37–46.
- Omont, A.; Quiroz-Guzman, E.; Tovar-Ramirez, D.; Peña-Rodríguez, A. Effect of diets supplemented with different seaweed extracts on growth performance and digestive enzyme activities of juvenile white shrimp Litopenaeus vannamei. J. Appl. Phycol. 2019, 31, 1433–1442.
- Kamunde, C.; Sappal, R.; Melegy, T.M. Brown seaweed (AquaArom) supplementation increases food intake and improves growth, antioxidant status and resistance to temperature stress in Atlantic salmon, Salmo salar. PLoS ONE 2019, 14, e0219792.
- Wilke, T.; Faulkner, S.; Murphy, L.; Kealy, L.; Kraan, S.; Brouns, F. Seaweed enrichment of feed supplied to farm-raised Atlantic salmon (Salmo salar) is associated with higher total fatty acid and LC n-3 PUFA concentrations in fish flesh. Eur. J. Lipid Sci. Technol. 2015, 117, 767–772.
- Moutinho, S.; Linares, F.; Rodríguez, J.; Sousa, V.; Valente, L. Inclusion of 10% seaweed meal in diets for juvenile and on-growing life stages of Senegalese sole (Solea senegalensis). J. Appl. Phycol. 2018, 30, 3589–3601.
- Fernandes, H.; Salgado, J.M.; Martins, N.; Peres, H.; Oliva-Teles, A.; Belo, I. Sequential bioprocessing of Ulva rigida to produce lignocellulolytic enzymes and to improve its nutritional value as aquaculture feed. Bioresour. Technol. 2019, 281, 277–285.
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597.
- Santos, S.A.; Félix, R.; Pais, A.; Rocha, S.M.; Silvestre, A.J. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847.
- Freitas, A.C.; Pereira, L.; Rodrigues, D.; Carvalho, A.P.; Panteleitchouk, T.; Gomes, A.M.; Duarte, A.C. Marine Functional Foods. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 969–994.
- Ma, W.C.J.; Chung, H.Y.; Ang, P.O.; Kim, J.-S. Enhancement of bromophenol levels in aquacultured silver seabream (Sparus sarba). J. Agric. Food Chem. 2005, 53, 2133–2139.
- ABARES. Australian Seaweed Production. Available online: https://www.agriculture.gov.au/abares/research-topics/fisheries/fisheries-and-aquaculture-statistics/australian-seaweed-production (accessed on 12 August 2020).
- McHugh, D. A Guide to the Seaweed Industry FAO Fisheries Technical Paper 441; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; Available online: http://www.fao.org/3/y4765e/y4765e.pdf (accessed on 12 August 2020).
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2022. Available online: http://www.algaebase.org (accessed on 16 May 2022).
- Santos, S.A.; Vilela, C.; Freire, C.S.; Abreu, M.H.; Rocha, S.M.; Silvestre, A.J. Chlorophyta and Rhodophyta macroalgae: A source of health promoting phytochemicals. Food Chem. 2015, 183, 122–128.
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murúa, P.; Yang, Z. The global status of seaweed production, trade and utilization. Globefish Res. Programme 2018, 124, I. Available online: https://www.proquest.com/openview/63a9872d1ea30c63f92d5d8acfcd6e35/1?pq-origsite=gscholar&cbl=237312 (accessed on 13 August 2020).
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406.
- RIRDC. Cultivated Seaweed. Available online: https://www.agrifutures.com.au/farm-diversity/cultivated-seaweed/#:~:text=Growing%20regions,Victoria%20and%20New%20South%20Wales. (accessed on 13 August 2020).
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. An emerging trend in functional foods for the prevention of cardiovascular disease and diabetes: Marine algal polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1342–1358.
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043.
- Laura, A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Cambridge, UK, 2019; pp. 253–271.
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250.
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024.
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105.
- Agregan, R.; Munekata, P.E.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985.
- López, A.; Rico, M.; Rivero, A.; de Tangil, M.S. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109.
- Ragan, M.A. Phlorotannins, brown algal polyphenols. Prog. Phycol. Res. 1986, 4, 177–241.
- Yoshie-Stark, Y.; Hsieh, Y.-P.; Suzuki, T. Distribution of flavonoids and related compounds from seaweeds in Japan. J.-Tokyo Univ. Fish. 2003, 89, 1–6.
- Onofrejová, L.; Vašíčková, J.; Klejdus, B.; Stratil, P.; Mišurcová, L.; Kráčmar, S.; Kopecký, J.; Vacek, J. Bioactive phenols in algae: The application of pressurized-liquid and solid-phase extraction techniques. J. Pharm. Biomed. Anal. 2010, 51, 464–470.
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štěrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965.
- Jones, B.; Smullen, R.; Carton, A. Flavour enhancement of freshwater farmed barramundi (Lates calcarifer), through dietary enrichment with cultivated sea lettuce, Ulva ohnoi. Aquaculture 2016, 454, 192–198.
- Hartmann, A.; Ganzera, M.; Karsten, U.; Skhirtladze, A.; Stuppner, H. Phytochemical and analytical characterization of novel sulfated coumarins in the marine green macroalga Dasycladus vermicularis (Scopoli) Krasser. Molecules 2018, 23, 2735.
- Imbs, T.; Zvyagintseva, T. Phlorotannins are polyphenolic metabolites of brown algae. Russ. J. Mar. Biol. 2018, 44, 263–273.
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528.
- Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Marine algae as a potential pharmaceutical source for anti-allergic therapeutics. Process Biochem. 2012, 47, 386–394.
- Kim, S.-K. Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons: Hoboken, NJ, USA, 2011.
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591.
- Glombitza, K.-W.; Rauwald, H.-W.; Eckhardt, G. Fucole, polyhydroxyoligophenyle aus Fucus vesiculosus. Phytochemistry 1975, 14, 1403–1405.
- Catarino, M.D.; Silva, A.; Cardoso, S.M. Fucaceae: A source of bioactive phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327.
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49.
- Olate-Gallegos, C.; Barriga, A.; Vergara, C.; Fredes, C.; García, P.; Giménez, B.; Robert, P. Identification of Polyphenols from Chilean Brown Seaweeds Extracts by LC-DAD-ESI-MS/MS. J. Aquat. Food Prod. Technol. 2019, 28, 375–391.
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709.
- Montero, L.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Separation and characterization of phlorotannins from brown algae Cystoseira abies-marina by comprehensive two-dimensional liquid chromatography. Electrophoresis 2014, 35, 1644–1651.
- Steevensz, A.J.; MacKinnon, S.L.; Hankinson, R.; Craft, C.; Connan, S.; Stengel, D.B.; Melanson, J.E. Profiling phlorotannins in brown macroalgae by liquid chromatography–high resolution mass spectrometry. Phytochem. Anal. 2012, 23, 547–553.
- Leyton, A.; Pezoa-Conte, R.; Barriga, A.; Buschmann, A.; Mäki-Arvela, P.; Mikkola, J.-P.; Lienqueo, M. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016, 16, 201–208.
- Schoenwaelder, M.E. The occurrence and cellular significance of physodes in brown algae. Phycologia 2002, 41, 125–139.
- Singh, I.P.; Sidana, J. 5-Phlorotannins. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 181–204.
- Schoenwaelder, M.E.; Clayton, M.N. The presence of phenolic compounds in isolated cell walls of brown algae. Phycologia 1999, 38, 161–166.
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212.
- Baardseth, E. A method of estimating the physode content in brown algae. Rep. Norw. Inst. Seaweed Res. 1958, 20, 1–16.
- Jacobsen, C.; Sørensen, A.-D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, extraction, characterization, and applications of novel antioxidants from seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568.
- Shibata, T.; Kawaguchi, S.; Hama, Y.; Inagaki, M.; Yamaguchi, K.; Nakamura, T. Local and chemical distribution of phlorotannins in brown algae. J. Appl. Phycol. 2004, 16, 291–296.
- Rugiu, L.; Panova, M.; Pereyra, R.T.; Jormalainen, V. Gene regulatory response to hyposalinity in the brown seaweed Fucus vesiculosus. BMC Genom. 2020, 21, 42.
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501.
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107.
- Connan, S.; Goulard, F.; Stiger, V.; Deslandes, E.; Gall, E.A. Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot. Mar. 2004, 47, 410–416.
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Seasonal variation of chemical composition and biomethane production from the brown seaweed Ascophyllum nodosum. Bioresour. Technol. 2016, 216, 219–226.
- Abdala-Díaz, R.T.; Cabello-Pasini, A.; Márquez-Garrido, E.; López-Figueroa, F. Intra-thallus variation of phenolic compounds, antioxidant activity, and phenolsulphatase activity in Cystoseira tamariscifolia (Phaeophyceae) from southern Spain. Cienc. Mar. 2014, 40, 1–10.
- Van Hees, D.H.; Olsen, Y.S.; Wernberg, T.; Van Alstyne, K.L.; Kendrick, G.A. Phenolic concentrations of brown seaweeds and relationships to nearshore environmental gradients in Western Australia. Mar. Biol. 2017, 164, 74.
- Pedersen, A. Studies on Phenol Content and Heavy Metal Uptake in Fucoids; Bird, C.J., Ragan, M.A., Eds.; Springer: Dordrecht, The Netherlands, 1984; pp. 498–504.
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksii using response surface methodology. J. Appl. Phycol. 2017, 29, 3161–3173.
- Tanniou, A.; Vandanjon, L.; Incera, M.; Leon, E.S.; Husa, V.; Le Grand, J.; Nicolas, J.-L.; Poupart, N.; Kervarec, N.; Engelen, A. Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. J. Appl. Phycol. 2014, 26, 1215–1230.
- Mukherjee, P.; Gorain, P.C.; Paul, I.; Bose, R.; Bhadoria, P.; Pal, R. Investigation on the effects of nitrate and salinity stress on the antioxidant properties of green algae with special reference to the use of processed biomass as potent fish feed ingredient. Aquac. Int. 2020, 28, 211–234.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Revisions:
2 times
(View History)
Update Date:
19 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




