Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christophe M. Thomas | -- | 2096 | 2022-07-18 07:52:24 | | | |
| 2 | Sirius Huang | Meta information modification | 2096 | 2022-07-18 08:14:17 | | | | |
| 3 | Sirius Huang | -72 word(s) | 2024 | 2022-07-18 08:59:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Marshall, A.; Jiang, B.; Gauvin, R.M.; Thomas, C.M. Synthesis of 2,5-Furandicarboxylic Acid. Encyclopedia. Available online: https://encyclopedia.pub/entry/25219 (accessed on 07 February 2026).
Marshall A, Jiang B, Gauvin RM, Thomas CM. Synthesis of 2,5-Furandicarboxylic Acid. Encyclopedia. Available at: https://encyclopedia.pub/entry/25219. Accessed February 07, 2026.
Marshall, Adam, Bo Jiang, Régis M. Gauvin, Christophe M. Thomas. "Synthesis of 2,5-Furandicarboxylic Acid" Encyclopedia, https://encyclopedia.pub/entry/25219 (accessed February 07, 2026).
Marshall, A., Jiang, B., Gauvin, R.M., & Thomas, C.M. (2022, July 18). Synthesis of 2,5-Furandicarboxylic Acid. In Encyclopedia. https://encyclopedia.pub/entry/25219
Marshall, Adam, et al. "Synthesis of 2,5-Furandicarboxylic Acid." Encyclopedia. Web. 18 July, 2022.
Copy Citation
The most versatile furanic building block for chemical and polymer applications is 2,5-furandicarboxylic acid. However, the classical 2,5-furandicarboxylic acid production methodology has been found to have significant drawbacks that hinder industrial-scale production. The following content highlights new alternative methods to synthesize 2,5-furandicarboxylic acid that are both more advantageous and attractive than conventional oxidation of 5-hydroxymethylfurfural.
biobased monomers
sustainable polymers
furan-based materials
FDCA
1. Introduction
2,5-furandicarboxylic acid (FDCA) bears a structural resemblance to terephthalic acid, a petroleum-derived monomer used to make plastics for food and beverage packaging (Figure 1). Terephthalic acid is industrially polymerized with ethylene glycol to obtain the commodity polymer poly(ethylene terephthalate) (PET), which is produced on a scale of 50 million tons per year. As PET is widely used in packaging, an FDCA-based polymer alternative must meet the same high standards of stability, mechanical strength, color, transparency, and gas barrier properties, if it is to compete as a renewable alternative [1].
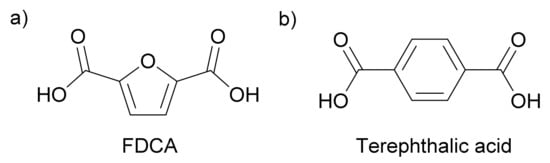
Figure 1. Chemical structures of (a) 2,5-furandicarboxylic acid and (b) terephthalic acid.
Although some companies are taking steps toward commercial FDCA production, conventional synthetic routes still present many drawbacks that hinder industrial applications, as will be detailed below. In addition, the main application of FDCA for the synthesis of poly(ethylene furanoate) (PEF) has not been directly compared to the alternative polyesters that can be produced from the parent diol of FDCA. In the following content, recent developments in the production and use of FDCA will be presented. This includes a review of the current routes and methods of producing FDCA and its products, in order to understand the problems encountered with these approaches and to propose beneficial alternatives. Alternatives to FDCA have also been studied, namely the corresponding diol and dimethyl ester. Methods and products from these derivatives were extensively reviewed to determine whether FDCA is the best choice for the final product.
2. FDCA Synthesis
The most common method for the synthesis of FDCA from lignocellulosic biomass is the catalytic oxidation of HMF [2]. Despite a large number of studies on the process, this route is still not economically feasible on an industrial scale due to the cost of the process along with technical issues such as the low efficiency in the production and isolation of HMF from lignocellulosic biomass and the poor selectivity to FDCA [3][4]. Lignocellulosic biomass comes from waste streams such as agriculture, forestry, and paper, and therefore does not compete with food sources. Lignocellulose itself is a composite of three types of materials: lignin (an aromatic polymer), cellulose, and hemicellulose (hexose and pentose polysaccharides) [5]. The acid-catalyzed hydrolysis of cellulose and hemicellulose produces glucose, fructose, and xylose, which can be dehydrated to produce the platform chemicals HMF and furfural. These compounds are furanic molecules functionalized with formyl- and hydroxymethyl groups that can be converted to a wide range of highly valuable C6-bifunctionalized furanic products.
The classical oxidation of HMF to FDCA occurs via an easy pathway (Scheme 1). Once HMF has been produced from C6 sugars by acid hydrolysis, a series of oxidation steps are performed before the formation of FDCA. If the alcohol function on HMF is oxidized first, 2,5-diformylfuran (DFF) will be produced. If the aldehyde group is oxidized first, the intermediate will instead be 5-hydroxymethylfuran-2-carboxylic acid (HMFCA). Then, either compound is oxidized to 5-formylfuran-2-carboxylic acid (FFCA), and finally to FDCA [5]. There are two main problems in this regard. First, the hydrolysis of cellulose to HMF produces insoluble polymeric by-products called humins, which color the final product and are difficult to remove. Second, the reaction may also involve further oxidation products of FDCA such as CO2 and CO, which means that at the end of the reaction, the selectivity to FDCA is low, as other products have been formed by decarboxylation [6]. This is a challenging reaction that can either achieve complete HMF conversion, resulting in a mixture of FDCA with its oxidation products (e.g., CO2, CO), or stop the reaction before this additional oxidation, resulting in a mixture of all of the intermediates, with low conversion to FDCA. In this section, new methods that can address this problem are discussed, either directly by solving the issues of HMF oxidation, or by presenting alternative routes to FDCA that avoid these issues altogether. As shown in Figure 2, these new pathways can be divided into two sections (to the left and right of the lignocellulosic biomass on the diagram): routes from cellulose (glucose and fructose) and routes from hemicellulose (xylose).
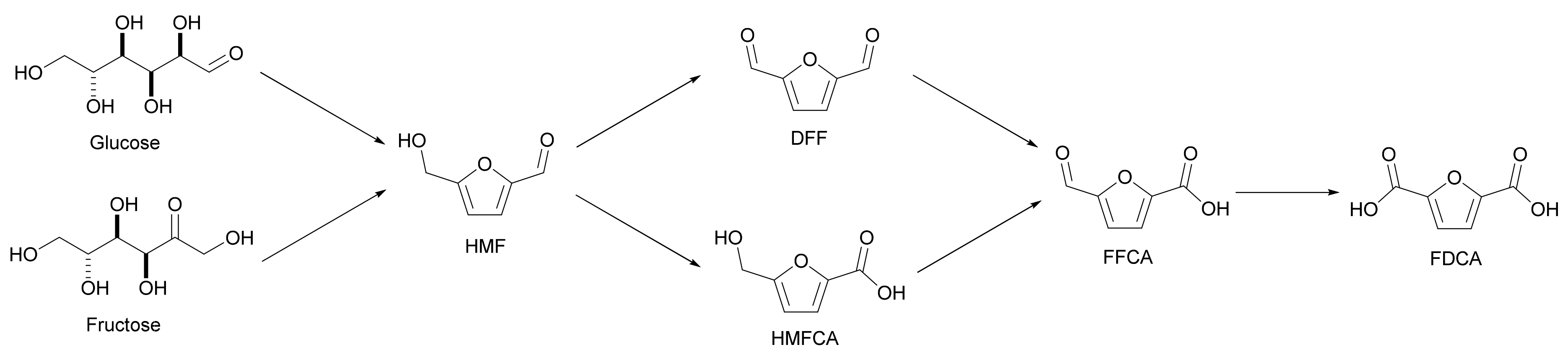
Scheme 1. Conventional synthesis and oxidation of HMF to FDCA.
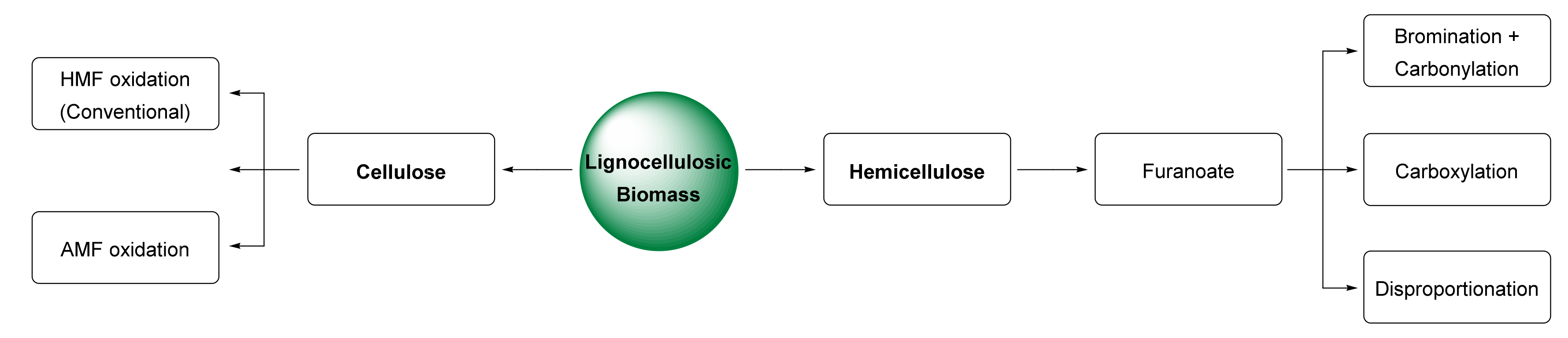
Figure 2. Alternative routes to 2,5-furandicarboxylic acid from lignocellulosic biomass.
2.1. FDCA from Cellulose
Avantium, a Dutch renewable chemistry company, has developed and patented a method for the synthesis of aromatic dicarboxylic acids by electrolytic oxidation of an aqueous electrolyte feedstock containing aromatic aldehydes using non-noble metal electrodes [7]. This method facilitates the oxidation of aromatic aldehyde FFCA to FDCA. Avantium reports that previous studies on the electrolytic oxidation of HMF directly to FDCA showed poor yields and that the conversion of the aldehyde functional group (as in FFCA) to a carboxylic functional group is “easier” than the oxidation of a furfural derivative with a hydroxymethyl group (as in HMF) [7]. The results claim complete conversion of the starting materials to FDCA with this method. Feedstocks containing only the aromatic aldehyde require considerable residence times, making the industrial process uneconomical. The process can be improved by including an aromatic dicarboxylic acid in the feedstock, preferably the same as the product to be generated (i.e., FDCA). When the aromatics in question are furanic derivatives, the process will also oxidize other components of the feedstock, such as HMF, DFF, and HMFCA. The application of this process can then be used for the purification of the products of conventional oxidation. It will oxidize the intermediates of conventional oxidation without the formation of CO2 and CO, thus increasing the yield of FDCA. This negates the high yield requirement in the conventional oxidation process, as all intermediates can be converted to FDCA if the feedstock contains up to 10 wt% FFCA. Furthermore, during conventional oxidation, humin by-products act as colorants that must be removed from the FDCA crude by additional separation methods. Avantium’s electrolytic oxidation process also removes humins, saving money by eliminating the need for more separation processes. In addition, there is no requirement to use expensive electrodes. The cathode can be made from carbon and the anode from non-noble metals or their oxides/hydroxides on a carbon support.
Despite extensive research, the production of the HMF platform chemical from abundant lignocellulosic biomass has been found to be uneconomical on an industrial scale in most cases [4]. Kang et al. proposed 5-acetoxymethylfufural (AMF) as a suitable alternative to HMF, as it can also be derived from cellulose and offers simple pathways to furan derivatives, including FDCA [8]. As discussed earlier, HMF is produced by acidic dehydration of the carbohydrates in lignocellulosic biomass, a reaction that involves many side reactions. By-products, including levulinic acid and insoluble polymeric humins, are formed during the hydrolysis and condensation of HMF, which reduces the selectivity and efficiency of the process [9]. In contrast, AMF can be easily synthesized from lignocellulosic biomass-derived 5-chloromethylfurfural (CMF) and alkylammonium acetates in a process without side reactions and/or by-products (Scheme 2). The acetoxymethyl group in AMF makes the compound less reactive and more hydrophobic than the hydroxymethyl group in HMF. This stability (and hydrophobicity) facilitates the isolation of AMF from the produced mixture allowing purities of up to 99.9% to be achieved.
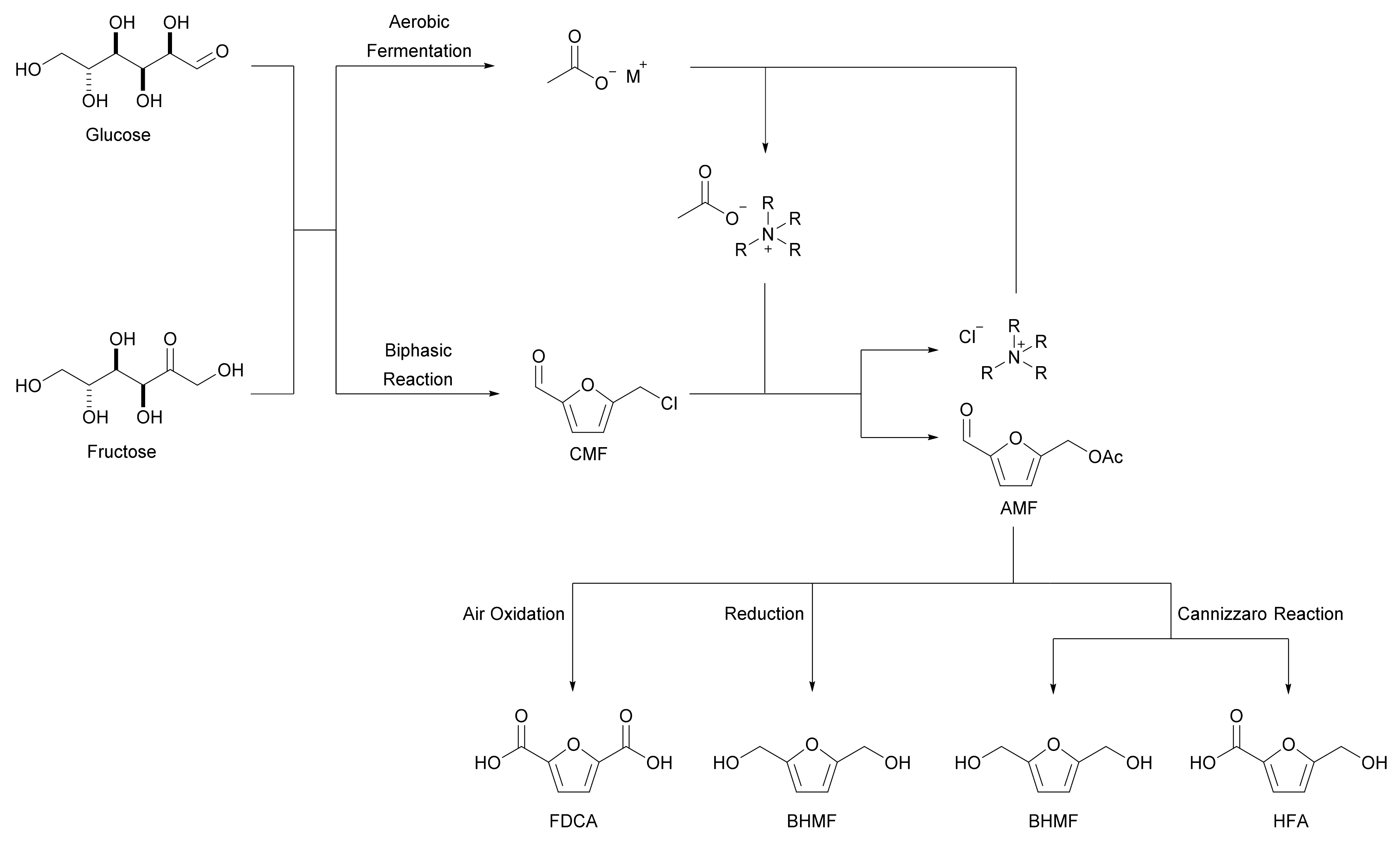
Scheme 2. Formation of biomass-derived 5-acetoxymethylfurfural and its valorization routes.
Both HMF and AMF can be used to produce FDCA, with the advantage of using AMF being that it avoids the aforementioned issues associated with the HMF platform chemical while achieving an 82% yield. AMF also lacks the cytotoxicity and mutagenicity of HMF in humans [10]. This alternative addresses several of the obstacles to industrial HMF production. AMF shares the features of HMF that give it versatility as a platform chemical and can achieve the synthesis of 2,5-bis(hydroxymethyl)furan (BHMF), 5-hydroxymethylfuran-2-carboxylic acid (HFA), and FDCA (Scheme 2).
2.2. FDCA from Hemicellulose
The other important platform chemical which can be obtained by acid-catalyzed hydrolysis of lignocellulosic biomass is furfural [5]. Produced from the xylan or hemicellulose contained in lignocellulose, furfural is a bulk chemical produced on a scale of 280,000 tons per year. Thus, the production of FDCA from furfural as a platform chemical may be more attractive than that from HMF, which has not yet been proven to be produced on a scale comparable to furfural [3]. This section presents three potentially valuable routes to FDCA from furfural.
Carbonylation is an attractive entry into FDCA production. It relies on 5-bromofuroic acid which is produced through the bromination of 2-furancarboxylic acid, a furfural oxidation derivative, which is currently used in the pharmaceutical industry as a process intermediate. It is a suitable platform for producing furfural-derived monomers as it is already well-established and hence more readily available than HMF [11]. Although the homogeneous carbonylation of 5-bromofuroic acid is possible, Shen et al. proposed a heterogeneous carbonylation method using a supported palladium catalyst to avoid the drawbacks of the homogeneous process and achieved a 97% isolated yield (Scheme 3). The activated carbon-supported Pd(Xantphos)Cl2 catalyst that developed showed no significant decrease in performance when tested over 10 cycles [2].
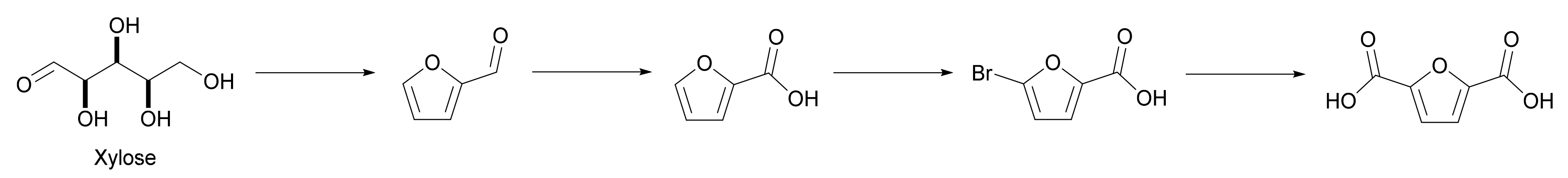
Scheme 3. Bromination of 2-furancarboxylic acid to 5-bromofuroic acid and subsequent reductive carbonylation to 2,5-furandicarboxylic acid.
With only NaBr as a by-product (which can be recycled after accumulation), this process demonstrates another successful alternative method of synthesizing bioderived FDCA, without the drawbacks of conventional HMF oxidation [2].
Interestingly, 2-furancarboxylic acid can also be directly carboxylated to FDCA using CO2 or inorganic carbonates. Dick et al. developed a process of producing FDCA by this method with an isolated yield of 89%, using a molten salt of cesium carbonate (Cs2CO3) (Scheme 4) [12]. Although this method has shown good results on a laboratory scale, the industrial scalability of this process would be economically limited by the relatively high cost of cesium carbonate.
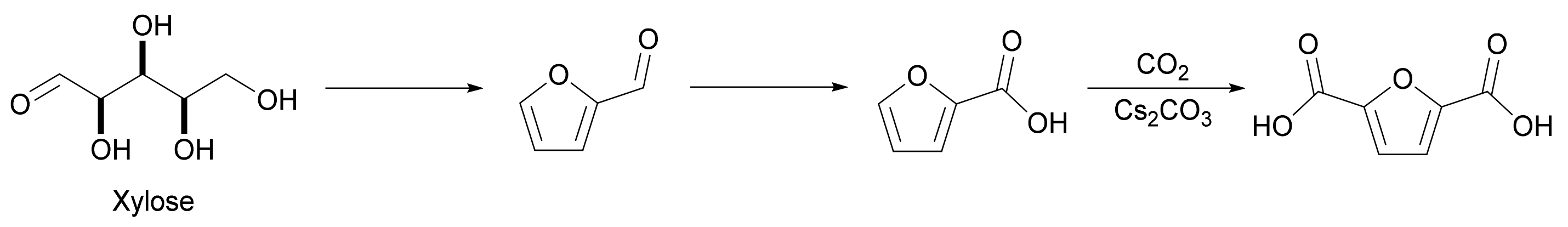
Scheme 4. Carboxylation of 2-furancarboxylic acid to 2,5-furandicarboxylic acid.
Alternatively, Nocito et al. proposed the synthesis and characterization of an alternative intermediate complex, copper-difuroate [13]. This option is more economical, as copper is cheaper than cesium. This new complex also increases the yield of FDCA up to 99%, compared to 76% starting from 2-furanoic acid under the same conditions due to the increased acidity of the proton in the fifth position on the furoic moiety, which allows for greater reactivity with the carbonate anion [13]. This is another potential route for obtaining FDCA from 2-furancarboxylic acid; however, the added complexity of the required expensive catalyst or intermediate complex may outweigh the benefits of eliminating the issues of the much simpler conventional oxidation of HMF. Further work is needed by research groups to determine the industrial feasibility of this method. A further option is the disproportionation of 2-furancarboxylic acid to FDCA and polyester. Polyesters are one of the main applications of FDCA. Pan et al. propose a method of producing FDCA directly from furfural along with 1,4-butanediol (1,4-BDO), which is polymerized with FDCA to produce poly(butylene 2,5-furandicarboxylate) (PBF).
As shown in Scheme 5, this method first involves the oxidation of the furfural feedstock into 2-furancarboxylic acid. Catalytic aerobic oxidation is suggested for this [14]. The monoacid is then catalytically disproportionated into furan and FDCA by a variation of the Henkel reaction. Furan is converted to 1,4-BDO preferably through direct hydrogenation, but also possibly by catalytic oxidation to maleic anhydride and subsequent hydrogenation to 1,4-BDO through butyrolactone. Polycondensation can then be used to produce PBF. This method not only successfully produces FDCA from furfural but also produces an aliphatic diol that can be used to obtain a bio-based furanic polymer. Polymerization with diols is FDCA’s main application. Most polymer synthesis processes utilize an aliphatic diol (such as 1,4-BDO) from one source and FDCA from another. If both components of polymerization can be synthesized from the same source of the platform chemical, it significantly simplifies process feedstock economics and increases the carbon utilization of the lignocellulosic biomass. Although only proven at the lab scale to date, this route has significant potential if it can be scaled up to commercial volumes.
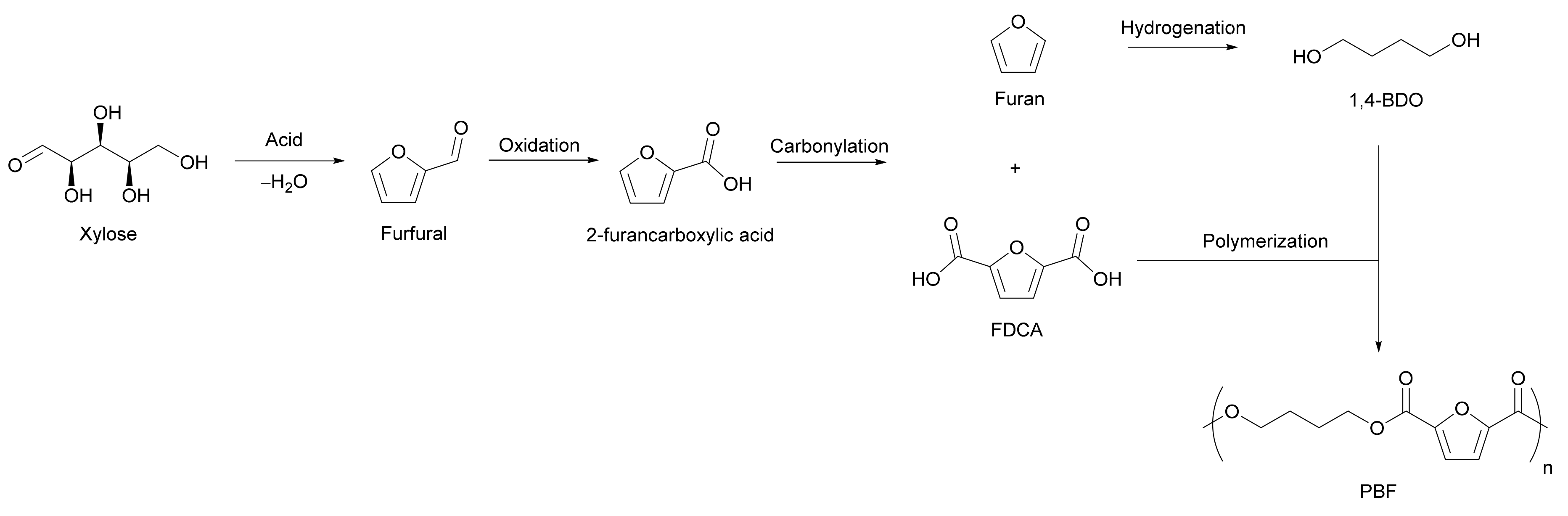
Scheme 5. Disproportionation of 2-furancarboxylic acid into furan and 2,5-furandicarboxylic acid and the subsequent polymerization to poly(butylene furanoate).
References
- Pandey, S.; Dumont, M.-J.; Orsat, V.; Rodrigue, D. Biobased 2,5-furandicarboxylic acid (FDCA) and its emerging copolyesters’ properties for packaging applications. Eur. Polym. J. 2021, 160, 110778.
- Shen, G.; Shi, J.; Lei, Y.; Fu, C.; Chen, Z.; Andrioletti, B.; Yin, G. Aqueous carbonylation of furfural-derived 5-bromofuroic acid to 2,5-furandicarboxylic acid with supported palladium catalyst. Ind. Eng. Chem. Res. 2019, 58, 22951–22957.
- Pan, T.; Deng, J.; Xu, Q.; Zuo, Y.; Guo, Q.; Fu, Y. Catalytic conversion of furfural into a 2,5-furandicarboxylic acid-based polyester with total carbon utilization. ChemSusChem 2013, 6, 47–50.
- Zakrzewska, M.E.; Bogel-Lukasik, E.; Bogel-Lukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural—a promising biomass-derived building block. Chem. Rev. 2011, 111, 397–417.
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative monomers based on lignocellulose and their use for polymer production. Chem. Rev. 2016, 116, 1540–1599.
- Thomás, R.A.F.; Bordado, J.C.M.; Gomes, J.F.P. p-Xylene oxidation to terephthalic acid: A literature review oriented toward process optimization and development. Chem. Rev. 2013, 133, 7421–7469.
- Schouten, K.J.P.; Waal, J.C.V.D.; Varini, M.; Gruter, G.J.M. Process for the Preparation of an Aromatic Dicarboxylic Acid. WO Patent EP3297995B1, 10 July 2019.
- Kang, E.; Hong, Y.; Chae, D.W.; Kim, B.; Kim, B.; Kim, Y.J.; Cho, J.K.; Kim, Y.G. From lignocellulosic biomass to furans via 5-acetoxymethylfurfural as an alternative to 5-hydroxymethylfurfural. ChemSusChem 2015, 8, 1179–1188.
- Lewkowski, J. Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives. Arkivoc 2001, 1, 17–54.
- Janzowski, C.; Glaab, V.; Samimi, E.; Schlatter, J.; Eisenbrand, G. 5-Hydroxymethylfurfural: Assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem. Toxicol. 2000, 38, 801–809.
- Harrisson, R.J.; Moyle, M. 2-Furoic Acid. Org. Synth. 1956, 36, 36.
- Dick, G.R.; Frankhouser, A.D.; Banerjee, A.; Kanan, M. A scalable carboxylation route to furan-2,5-dicarboxylic acid. Green Chem. 2017, 19, 2966–2972.
- Nocito, F.; Ditaranto, N.; Dibenedetto, A. Valorization of C5 polyols by direct carboxylation to FDCA: Synthesis and characterization of a key intermediate and role of carbon dioxide. J. CO2 Util. 2019, 32, 170–177.
- Thiyagarajan, S.; Pukin, A.; van Haveren, J.; Lutz, M.; van Es, D.S. Concurrent formation of furan-2,5- and furan-2,4-dicarboxylic acid: Unexpected aspects of the Henkel reaction. RSC Adv. 2013, 3, 15678–15686.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Revisions:
3 times
(View History)
Update Date:
19 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




