You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Connor Cole | -- | 1676 | 2022-07-15 18:32:26 | | | |
| 2 | Rita Xu | -3 word(s) | 1673 | 2022-07-18 05:14:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cole, C.; Borradori, L.; Amber, K.T. BP230 Autoantibodies in Bullous Pemphigoid. Encyclopedia. Available online: https://encyclopedia.pub/entry/25190 (accessed on 30 December 2025).
Cole C, Borradori L, Amber KT. BP230 Autoantibodies in Bullous Pemphigoid. Encyclopedia. Available at: https://encyclopedia.pub/entry/25190. Accessed December 30, 2025.
Cole, Connor, Luca Borradori, Kyle T. Amber. "BP230 Autoantibodies in Bullous Pemphigoid" Encyclopedia, https://encyclopedia.pub/entry/25190 (accessed December 30, 2025).
Cole, C., Borradori, L., & Amber, K.T. (2022, July 15). BP230 Autoantibodies in Bullous Pemphigoid. In Encyclopedia. https://encyclopedia.pub/entry/25190
Cole, Connor, et al. "BP230 Autoantibodies in Bullous Pemphigoid." Encyclopedia. Web. 15 July, 2022.
Copy Citation
Bullous pemphigoid (BP) is a subepidermal autoimmune blistering disease predominantly affecting elderly patients and carries significant morbidity and mortality. Patients typically suffer from severe itch with eczematous lesions, urticarial plaques, and/or tense blisters. BP is characterized by the presence of circulating autoantibodies against two components of the hemidesmosome, BP180 and BP230.
bullous pemphigoid
BP230
autoimmune blistering disease
1. Introduction
Bullous pemphigoid (BP) is the most common autoimmune subepidermal blistering disease and is generally seen in elderly patients. The typical clinical manifestations of BP include eczematous lesions and urticarial plaques in variable combination with tense blisters which predominantly develop on the trunk and the proximal region of the upper and lower limbs. Atypical forms without obvious blistering are observed in up to 20% of cases [1][2]. Patients also typically experience severe pruritus. Mucosal involvement, almost invariably limited to the oral cavity, can be seen in nearly one-fifth of patients [2][3][4].
Immunologically, the disease is characterized by the presence of circulating and tissue-bound IgG autoantibodies directed against BP180 and BP230. Autoantibodies of the IgE and IgA class are also less frequently detectable. The two target antigens, BP180 (a transmembrane collagenous protein, also called type XVII collagen or BPAG2) and BP230 (a cytoplasmic protein of the plakin family) are important components of hemidesmosomes, which promote dermo-epidermal adhesion and epidermal integrity.
The diagnosis of BP relies on clinical and immunopathological findings. While light microscopy studies of blistered skin specimens characteristically demonstrate subepidermal blister formation with an infiltrate rich in eosinophils, histopathological findings may be often nonspecific and are invariably not sufficient to diagnose BP [1]. Direct immunofluorescence microscopy studies, which typically demonstrate linear deposits of IgG and/or C3 along the basement membrane zone, as well as immunoserological tests, are usually required and necessary for its diagnosis. ELISAs to search for circulating autoantibodies against BP180 and/or BP230 or indirect immunofluorescence studies using NaCl-separated normal human skin are very useful for the proper classification and diagnosis of patients with suspected BP. Systemic oral corticosteroids or high potency topical corticosteroids are regarded as the first-line of therapy for both moderate and severe disease. The disease has significant mortality and well-documented associations with several neurological conditions [5].
There are ample data indicating that BP180 plays a primary role in the pathogenicity of BP, with nearly 90% of BP patients having IgG autoantibodies targeting the extracellular membrane proximal region of BP180, termed the NC16A domain. However, as many as 80% and 68% of BP patients also have circulating anti-BP230 IgG and IgE autoantibodies, respectively [6][7][8][9][10][11][12][13][14][15][16][17].
Although the exact pathogenic role of the cytoplasmic protein BP230 in BP pathogenesis has not yet been fully elucidated, results obtained from several mouse models of BP strongly suggest that autoantibodies against BP230 alone directly contribute to tissue damage and blister formation [18][19][20][21][22]. In addition, it is likely that the serological profile affects the clinical features observed in BP [23].
2. BP230 Structure and Expression
BP230, also known as bullous pemphigoid antigen 1 is a 230 kDa intracellular protein [24]. It was the first protein that was identified as a target by circulating autoantibodies from BP sera as assessed by immunoprecipitation studies. BP230/BPAG1e represents the epithelial protein isoform encoded by the dystonin (DST) gene (hence referred to as BPAG1e). The other major isoforms BPAG1a and BPAG1b are predominantly expressed in the brain and skeletal muscle, respectively.
BP230/BPAG1e is an important component of hemidesmosomes, junctional adhesion complexes present in the epidermis and other stratified squamous epithelia, including the cornea. BP230/BPAG1e is a member of the plakin family of cytolinkers, such as desmoplakin and plectin [25][26][27]. It contains an N-terminus followed by a spectrin repeat, a plakin domain, a coiled-coil rod domain, two plakin repeat domains, and a C-terminal extremity [28]. The N-terminal portion of the protein interacts with the α6β4 integrin and with BP180 (Figure 1). The C-terminal regions encompassing the plakin repeat domain and the C-extremity mediate the binding of BPAG1e to the epidermal specific intermediate filament K5/K14 and K6/K17. These interactions are critical in promoting stable adhesion between the epidermal cells and the basement membrane as well as cytoskeletal architecture [29][30]. DST-knockout mice develop discrete signs of skin blistering as a result of basal keratinocyte fragility with cytoskeletal disruption [31]. In addition, these mice demonstrate degeneration of sensory nerves with severe dystonia as well as skeletal muscle defects. These observations confirm the important role of the various BPAG1 isoforms in the maintenance of keratinocyte, skeletal muscle, and neuronal cell homeostasis and resilience.
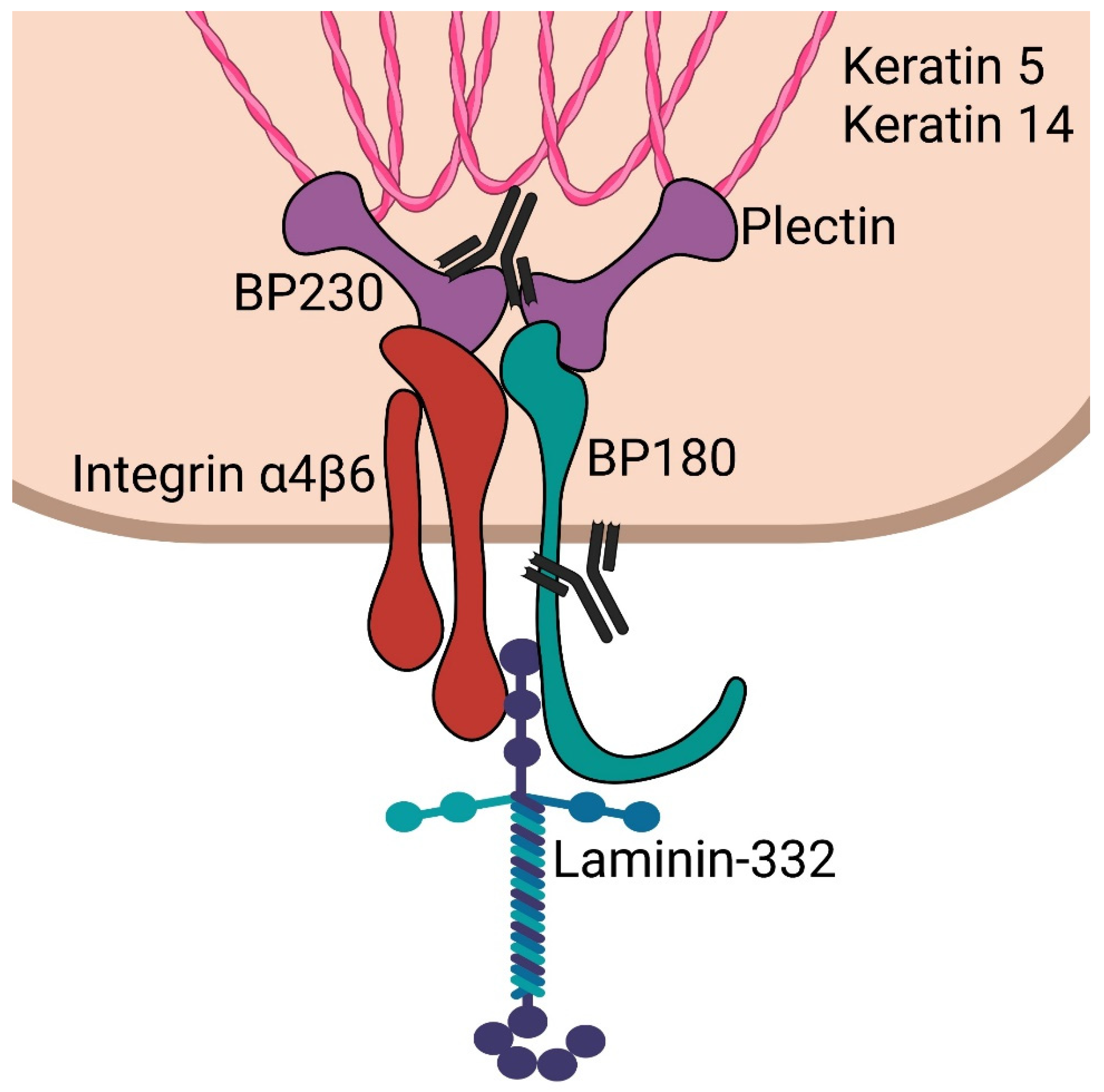
Figure 1. Schematic of epidermal hemidesmosome. BP230 and plectin bind to keratin intermediate filaments. These subsequently complex with integrin α4β6 and BP180 (collagen 17). These then interact with laminin-332 in the lamina lucida. Antibodies are shown targeting the C-terminus of BP230 and the NC16a domain of BP180, which are most often targeted in bullous pemphigoid.
BPAG1a and BPAG1b constitute the two other major isoforms encoded by the DST gene, showing a similar domain organization. BPAG1a has a predicted molecular mass of 625 kDa. In mice, it has been found to be predominantly expressed in the brain, as well as the lung, liver, kidney, ovary, spleen, and testis. BPAG1b is 834 kDa and shares a very similar structure with BPAG1a with the exception of one region between the plakin domain and spectrin repeats which contains two plakin repeat domains and two spectrin repeats. This isoform is expressed in the myocardium and skeletal muscle of mice, as well as the liver, ovary, testis, spleen, vertebral cartilage, and tongue epithelium [32][33]. It is important to note that no data for specific isoform expression in humans have been obtained as of yet. The functions of BPAG1a/b on the cellular level include interactions with microtubules, vesicular transport, effects on Golgi positioning, and regulation of the actin network [34][35]. Pathogenic variants in DST which selectively affect the BPAG1a/b isoforms in humans can result in severe neurological diseases including encephalopathy, delayed visual maturation, and mental retardation [36].
3. The Pathogenic Contribution of BP230 Autoantibodies
There are compelling data that support the role of BP180 in the pathogenesis of BP. Several in vitro and in vivo studies have shown ample evidence that the binding of anti-BP180 autoantibodies to their target antigen results in the activation of the complement cascade and granulocytes [37][38][39][40][41]. Although circulating autoantibodies recognize several antigenic regions throughout the BP180 molecule, the NC16A domain, located in the extracellular cell membrane proximal portion of BP180, appears to be the immunodominant region. The latter is recognized by 85% of patients’ sera [7][42][43][44].
In contrast to the transmembrane BP180, the intracellular BP230 antigen was originally thought to play a minor pathogenic role. However, anti-BP230 antibodies are found in the majority of BP patients [13][35]. Although several studies have investigated their contribution to the BP disease process, the pathogenic relevance of anti-BP230 antibodies has been a matter of debate for decades [18][19][45]. In 2003, Kiss et al. found that passive transfer of anti-BP230 IgG can induce clinical and immunopathological features of BP in neonatal mice [19]. Another study demonstrated that rabbits immunized with BP230-derived peptides developed antibodies that could deposit on the BMZ and trigger an enhanced inflammatory response following UVB-induced epithelial injury [18]. Two recent studies by Haeberle et al. and Muramatsu et al. provide additional evidence supporting the pathogenicity of BP230. They demonstrated the production of anti-BP230 IgG autoantibodies in mice with dysfunctional regulatory T cells [20][21]. Haeberle et al. also reported that the monoclonal antibody 20B12 against BP230 is capable of inducing subepidermal blistering in neonatal mice [20]. However, this model lacked eosinophilic infiltrate in the BMZ and thus did not replicate the characteristic histology of BP. This is, however, generally the case in passive transfer murine models of BP.
A novel tissue-specific conditional knockout mouse model has been very useful to gain significant insight into the effect of autoimmunity against BP230 [46]. To investigate the role of BPAG1 antibodies in the pathogenicity of BP, Makita et al. generated a mouse model with the conditional knockout of BPAG1 confined to keratin-5 expressing stratified epithelial cells. These mice were then immunized against the C-terminal portion of BP230. The latter is thought to contain the immunodominant antigenic regions that are recognized by anti-BP230 antibodies in BP. Splenocytes from these mice were then transferred into immunodeficient mice. These recipient mice developed a BP-like phenotype displaying scaling and erosions on multiple areas of skin as well as histologically, subepidermal blistering. In addition, direct immunofluorescence studies showed IgG deposition along the dermo-epidermal junction. The study additionally found that skin wounding resulted in increased blistering in these mice. Overall, these findings provided strong evidence that antibodies to BP230, and specifically autoreactivity of the C-terminal domain, may have a pathogenic role in the development of tissue damage and blister formation in BP.
On the other hand, Feldrihan et al. reported that rabbit polyclonal anti-BP230 IgG antibodies subcutaneously transferred into neonatal and adult mice did not induce experimental BP [45]. However, the transferred anti-BP230 antibodies were not able to activate the complement system in vivo, an observation most likely explaining the lack of obvious clinical effect. The latter study has additional important limitations. For example, since the employed mice expressed their own native BP230 it is conceivable that they developed immune tolerance to the injected antibodies. Furthermore, the autoantibodies may require a separate trigger or tissue damage resulting in the exposure of the intracellular BP230 antigen to either cause or aggravate disease in the used mouse strains. Finally, longer administration and observation times than those used by the authors may be needed to reach the disease threshold. Feldrihan et al. suggested that BP230 could support ongoing disease initiated by the granulocyte and complement activation of BP180 antibodies, or that BP230 could trigger disease via non-inflammatory mechanisms.
As previously noted, IgE autoantibodies against BP230 are also present in a significant number of BP patients. While anti-BP180 IgE antibodies have more robust mechanistic data [47][48], the presence of anti-BP230 IgE autoantibodies has been associated with increased local eosinophil recruitment. Additionally, the presence of BP230 IgE autoantibodies has been associated with increased resistance to topical corticosteroids [49]. Given its intracellular localization, it remains unclear whether anti-BP230 IgE autoantibodies drive similar immune activation and granulocyte mediated blistering as anti-BP180 IgE.
References
- Cozzani, E.; Gasparini, G.; Burlando, M.; Drago, F.; Parodi, A. Atypical presentations of bullous pemphigoid: Clinical and immunopathological aspects. Autoimmun. Rev. 2015, 14, 438–445.
- Di Zenzo, G.; Thoma-Uszynski, S.; Fontao, L.; Calabresi, V.; Hofmann, S.C.; Hellmark, T.; Sebbag, N.; Pedicelli, C.; Sera, F.; Lacour, J.-P.; et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin. Immunol. 2008, 128, 415–426.
- Kridin, K.; Bergman, R. Assessment of the Prevalence of Mucosal Involvement in Bullous Pemphigoid. JAMA Dermatol. 2019, 155, 166–171.
- Chen, X.; Zhao, W.; Jin, H.; Li, L. Risk Factors for Mucosal Involvement in Bullous Pemphigoid and the Possible Mechanism: A Review. Front. Med. 2021, 8, 680871.
- Miyamoto, D.; Santi, C.G.; Aoki, V.; Maruta, C.W. Bullous pemphigoid. An. Bras. Dermatol. 2019, 94, 133–146.
- Ishiura, N.; Fujimoto, M.; Watanabe, R.; Nakashima, H.; Kuwano, Y.; Yazawa, N.; Echigo, T.; Okochi, H.; Tamaki, K. Serum levels of IgE anti-BP180 and anti-BP230 autoantibodies in patients with bullous pemphigoid. J. Dermatol. Sci. 2008, 49, 153–161.
- Matsumura, K.; Amagai, M.; Nishikawa, T.; Hashimoto, T. The majority of bullous pemphigoid and herpes gestationis serum samples react with the NC16a domain of the 180-kDa bullous pemphigoid antigen. Arch. Dermatol. Res. 1996, 288, 507–509.
- Skaria, M.; Jaunin, F.; Riou, S.; Saurat, J.-H.; Favre, B.; Borradori, L.; Hunziker, T.; Schumann, H.; Bruckner-Tuderman, L.; Hertl, M.; et al. IgG Autoantibodies from Bullous Pemphigoid Patients Recognize Multiple Antigenic Reactive Sites Located Predominantly Within the B and C Subdomains of the COOH-Terminus of BP230. J. Investig. Dermatol. 2000, 114, 998–1004.
- Hamada, T.; Nagata, Y.; Tomita, M.; Salmhofer, W.; Hashimoto, T. Bullous pemphigoid sera react specifically with various domains of BP230, most frequently with C-terminal domain, by immunoblot analyses using bacterial recombinant proteins covering the entire molecule. Exp. Dermatol. 2001, 10, 256–263.
- Blöcker, I.; Dähnrich, C.; Probst, C.; Komorowski, L.; Saschenbrecker, S.; Schlumberger, W.; Stöcker, W.; Zillikens, D.; Schmidt, E. Epitope mapping of BP230 leading to a novel enzyme-linked immunosorbent assay for autoantibodies in bullous pemphigoid. Br. J. Dermatol. 2012, 166, 964–970.
- Kromminga, A.; Sitaru, C.; Hagel, C.; Herzog, S.; Zillikens, D. Development of an ELISA for the detection of autoantibodies to BP230. Clin. Immunol. 2004, 111, 146–152.
- Thoma-Uszynski, S.; Uter, W.; Schwietzke, S.; Hofmann, S.C.; Hunziker, T.; Bernard, P.; Treudler, R.; Zouboulis, C.C.; Schuler, G.; Borradori, L.; et al. BP230- and BP180-specific Auto-Antibodies in Bullous Pemphigoid. J. Investig. Dermatol. 2004, 122, 1413–1422.
- Yoshida, M.; Hamada, T.; Amagai, M.; Hashimoto, K.; Uehara, R.; Yamaguchi, K.; Imamura, K.; Okamoto, E.; Yasumoto, S.; Hashimoto, T. Enzyme-linked immunosorbent assay using bacterial recombinant proteins of human BP230 as a diagnostic tool for bullous pemphigoid. J. Dermatol. Sci. 2006, 41, 21–30.
- Tampoia, M.; Lattanzi, V.; Zucano, A.; Villalta, D.; Filotico, R.; Fontana, A.; Vena, G.A.; Di Serio, F. Evaluation of a New ELISA Assay for Detection of BP230 Autoantibodies in Bullous Pemphigoid. Ann. N. Y. Acad. Sci. 2009, 1173, 15–20.
- Charneux, J.; Lorin, J.; Vitry, F.; Antonicelli, F.; Reguiai, Z.; Barbe, C.; Tabary, T.; Grange, F.; Bernard, P. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid: A retrospective study of 138 patients. Arch. Dermatol. 2011, 147, 286–291.
- Fania, L.; Caldarola, G.; Müller, R.; Brandt, O.; Pellicano, R.; Feliciani, C.; Hertl, M. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin. Immunol. 2012, 143, 236–245.
- Ghohestani, R.F.; Cozzani, E.; Delaporte, E.; Nicolas, J.F.; Parodi, A.; Claudy, A. IgE antibodies in sera from patients with bullous pemphigoid are autoantibodies preferentially directed against the 230-kDa epidermal antigen (BP230). J. Clin. Immunol. 1998, 18, 202–209.
- Hall, R.P., 3rd; Murray, J.C.; McCord, M.M.; Rico, M.J.; Streilein, R.D. Rabbits immunized with a peptide encoded for by the 230-kD bullous pemphigoid antigen cDNA develop an enhanced inflammatory response to UVB irradiation: A potential animal model for bullous pemphigoid. J. Investig. Dermatol. 1993, 101, 9–14.
- Kiss, M.; Husz, S.; Jánossy, T.; Marczinovits, I.; Molnár, J.; Korom, I.; Dobozy, A. Experimental bullous pemphigoid generated in mice with an antigenic epitope of the human hemidesmosomal protein BP230. J. Autoimmun. 2005, 24, 1–10.
- Haeberle, S.; Wei, X.; Bieber, K.; Goletz, S.; Ludwig, R.J.; Schmidt, E.; Enk, A.H.; Hadaschik, E.N. Regulatory T-cell deficiency leads to pathogenic bullous pemphigoid antigen 230 autoantibody and autoimmune bullous disease. J. Allergy Clin. Immunol. 2018, 142, 1831–1842.e7.
- Muramatsu, K.; Ujiie, H.; Kobayashi, I.; Nishie, W.; Izumi, K.; Ito, T.; Yoshimoto, N.; Natsuga, K.; Iwata, H.; Shimizu, H. Regulatory T-cell dysfunction induces autoantibodies to bullous pemphigoid antigens in mice and human subjects. J. Allergy Clin. Immunol. 2018, 142, 1818–1830.e6.
- Iwata, H.; Kamio, N.; Aoyama, Y.; Yamamoto, Y.; Hirako, Y.; Owaribe, K.; Kitajima, Y. IgG from Patients with Bullous Pemphigoid Depletes Cultured Keratinocytes of the 180-kDa Bullous Pemphigoid Antigen (Type XVII Collagen) and Weakens Cell Attachment. J. Investig. Dermatol. 2009, 129, 919–926.
- Hayakawa, T.; Teye, K.; Hachiya, T.; Uehara, R.; Hashiguchi, M.; Kawakami, T.; Li, X.; Tsuchisaka, A.; Ohara, K.; Sogame, R.; et al. Clinical and immunological profiles of anti-BP230-type bullous pemphigoid: Restriction of epitopes to the C-terminal domain of BP230, shown by novel ELISAs of BP230-domain specific recombinant proteins. Eur. J. Dermatol. 2016, 26, 155–163.
- Sawamura, D.; Li, K.; Chu, M.L.; Uitto, J. Human bullous pemphigoid antigen (BPAG1). Amino acid sequences deduced from cloned cDNAs predict biologically important peptide segments and protein domains. J. Biol. Chem. 1991, 266, 17784–17790.
- Green, K.J.; Parry, D.A.; Steinert, P.M.; Virata, M.L.; Wagner, R.M.; Angst, B.D.; Nilles, L.A. Structure of the human desmoplakins. Implications for function in the desmosomal plaque. J. Biol. Chem. 1990, 265, 2603–2612.
- Wiche, G.; Becker, B.; Luber, K.; Weitzer, G.; Castañon, M.J.; Hauptmann, R.; Stratowa, C.; Stewart, M. Cloning and sequencing of rat plectin indicates a 466-kD polypeptide chain with a three-domain structure based on a central alpha-helical coiled coil. J. Cell Biol. 1991, 114, 83–99.
- Ruhrberg, C.; Watt, F. The plakin family: Versatile organizers of cytoskeletal architecture. Curr. Opin. Genet. Dev. 1997, 7, 392–397.
- Fontao, L.; Favre, B.; Riou, S.; Geerts, D.; Jaunin, F.; Saurat, J.-H.; Green, K.J.; Sonnenberg, A.; Borradori, L. Interaction of the Bullous Pemphigoid Antigen 1 (BP230) and Desmoplakin with Intermediate Filaments Is Mediated by Distinct Sequences within Their COOH Terminus. Mol. Biol. Cell 2003, 14, 1978–1992.
- Borradori, L.; Chavanas, S.; Schaapveld, R.Q.J.; Gagnoux-Palacios, L.; Calafat, J.; Meneguzzi, G.; Sonnenberg, A. Role of the bullous pemphigoid antigen 180 (BP180) in the assembly of hemidesmosomes and cell adhesion--reexpression of BP180 in generalized atrophic benign epidermolysis bullosa keratinocytes. Exp. Cell Res. 1998, 239, 463–476.
- Schaapveld, R.Q.; Borradori, L.; Geerts, D.; van Leusden, M.R.; Kuikman, I.; Nievers, M.G.; Niessen, C.M.; Steenbergen, R.D.; Snijiders, P.J.; Sonnenberg, A. Hemidesmosome formation is initiated by the beta4 integrin subunit, requires complex formation of beta4 and HD1/plectin, and involves a direct interaction between beta4 and the bullous pemphigoid antigen 180. J. Cell Biol. 1998, 142, 271–284.
- Guo, L.; Degenstein, L.; Dowling, J.; Yu, Q.-C.; Wollmann, R.; Perman, B.; Fuchs, E. Gene targeting of BPAG1: Abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell 1995, 81, 233–243.
- Leung, C.L.; Zheng, M.; Prater, S.M.; Liem, R.K.H. The BPAG1 locus: Alternative splicing produces multiple isoforms with distinct cytoskeletal linker domains, including predominant isoforms in neurons and muscles. J. Cell Biol. 2001, 154, 691–697.
- Jefferson, J.J.; Leung, C.L.; Liem, R.K.H. Dissecting the sequence specific functions of alternative N-terminal isoforms of mouse bullous pemphigoid antigen 1. Exp. Cell Res. 2006, 312, 2712–2725.
- Poliakova, K.; Adebola, A.; Leung, C.L.; Favre, B.; Liem, R.K.H.; Schepens, I.; Borradori, L. BPAG1a and b associate with EB1 and EB3 and modulate vesicular transport, Golgi apparatus structure, and cell migration in C2.7 myoblasts. PLoS ONE 2014, 9, e107535.
- Slep, K.C.; Rogers, S.L.; Elliott, S.L.; Ohkura, H.; Kolodziej, P.A.; Vale, R.D. Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the microtubule plus end. J. Cell Biol. 2005, 168, 587–598.
- Kunzli, K.; Favre, B.; Chofflon, M.; Borradori, L. One gene but different proteins and diseases: The complexity of dystonin and bullous pemphigoid antigen 1. Exp. Dermatol. 2016, 25, 10–16.
- Sitaru, C. Bullous Pemphigoid: A Prototypical Antibody-Mediated Organ-Specific Autoimmune Disease. J. Investig. Dermatol. 2009, 129, 822–824.
- Liu, Z.; Sui, W.; Zhao, M.; Li, Z.; Thresher, R.; Guidice, G.J.; Fairley, J.A.; Sitaru, C.; Zillikens, D.; Ning, G.; et al. Subepidermal blistering induced by human autoantibodies to BP180 requires innate immune players in a humanized bullous pemphigoid mouse model. J. Autoimmun. 2008, 31, 331–338.
- Liu, Z.; Diaz, L.A.; Troy, J.L.; Taylor, A.F.; Emery, D.J.; Fairley, J.; Giudice, G.J. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J. Clin. Investig. 1993, 92, 2480–2488.
- Liu, Z.; Giudice, G.J.; Swartz, S.J.; Fairley, J.; Till, G.O.; Troy, J.L.; Diaz, L.A. The role of complement in experimental bullous pemphigoid. J. Clin. Investig. 1995, 95, 1539–1544.
- Liu, Z.; Giudice, G.J.; Zhou, X.; Swartz, S.J.; Troy, J.L.; Fairley, J.; Till, G.O.; Diaz, L.A. A major role for neutrophils in experimental bullous pemphigoid. J. Clin. Investig. 1997, 100, 1256–1263.
- Giudice, G.J.; Emery, D.J.; Zelickson, B.D.; Anhalt, G.J.; Liu, Z.; Diaz, L.A. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. J. Immunol. 1993, 151, 5742–5750.
- Zillikens, D.; Rose, P.A.; Balding, S.D.; Liu, Z.; Olague-Marchan, M.; Diaz, L.A.; Giudice, G.J. Tight Clustering of Extracellular BP180 Epitopes Recognized by Bullous Pemphigoid Autoantibodies. J. Investig. Dermatol. 1997, 109, 573–579.
- Egan, C.A.; Taylor, T.B.; Petersen, M.J.; Meyer, L.J.; Zone, J.J. Bullous Pemphigoid Sera that Contain Antibodies to BPAg2 also Contain Antibodies to LABD97 that Recognize Epitopes Distal to the NC16A Domain. J. Investig. Dermatol. 1999, 112, 148–152.
- Feldrihan, V.; Licarete, E.; Florea, F.; Cristea, V.; Popescu, O.; Sitaru, C.; Chiriac, M.T. IgG antibodies against immunodominant C-terminal epitopes of BP230 do not induce skin blistering in mice. Hum. Immunol. 2014, 75, 354–363.
- Makita, E.; Matsuzaki, Y.; Fukui, T.; Matsui, A.; Minakawa, S.; Nakano, H.; Ito, K.; Kijima, H.; Sawamura, D. Autoantibodies to BPAG1e Trigger Experimental Bullous Pemphigoid in Mice. J. Investig. Dermatol. 2020, 141, 1167–1176.e3.
- Freire, P.C.; Muñoz, C.H.; Stingl, G. IgE autoreactivity in bullous pemphigoid: Eosinophils and mast cells as major targets of pathogenic immune reactants. Br. J. Dermatol. 2017, 177, 1644–1653.
- Lin, L.; Hwang, B.-J.; Culton, D.A.; Li, N.; Burette, S.; Koller, B.H.; Messingham, K.A.; Fairley, J.A.; Lee, J.J.; Hall, R.P.; et al. Eosinophils Mediate Tissue Injury in the Autoimmune Skin Disease Bullous Pemphigoid. J. Investig. Dermatol. 2018, 138, 1032–1043.
- Shih, Y.C.; Yuan, H.; Shen, J.; Zheng, J.; Pan, M. BP230 IgE autoantibodies in topical-steroid-resistant bullous pemphigoid. J. Dermatol. 2021, 48, 1372–1380.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
18 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




