Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Margarida Abrantes | -- | 1356 | 2022-07-14 12:22:18 | | | |
| 2 | Catherine Yang | Meta information modification | 1356 | 2022-07-15 07:59:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gonçalves, D.; Pires, A.S.; Marques, I.A.; Gomes, I.; Sousa, G.; Botelho, M.F.; Abrantes, A.M. Radiation Sensitivity in Hereditary Breast and Ovarian Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/25144 (accessed on 01 March 2026).
Gonçalves D, Pires AS, Marques IA, Gomes I, Sousa G, Botelho MF, et al. Radiation Sensitivity in Hereditary Breast and Ovarian Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/25144. Accessed March 01, 2026.
Gonçalves, Diana, Ana Salomé Pires, Inês A. Marques, Inês Gomes, Gabriela Sousa, Maria Filomena Botelho, Ana Margarida Abrantes. "Radiation Sensitivity in Hereditary Breast and Ovarian Cancer" Encyclopedia, https://encyclopedia.pub/entry/25144 (accessed March 01, 2026).
Gonçalves, D., Pires, A.S., Marques, I.A., Gomes, I., Sousa, G., Botelho, M.F., & Abrantes, A.M. (2022, July 14). Radiation Sensitivity in Hereditary Breast and Ovarian Cancer. In Encyclopedia. https://encyclopedia.pub/entry/25144
Gonçalves, Diana, et al. "Radiation Sensitivity in Hereditary Breast and Ovarian Cancer." Encyclopedia. Web. 14 July, 2022.
Copy Citation
Hereditary breast and ovarian cancer (HBOC) syndrome is a condition in which individuals have an increased risk of developing different types of cancer when compared to the general population. BRCA1 repair associated (BRCA1) and BRCA2 repair associated (BRCA2) genes are tumor suppressor genes that play a crucial role in cell, by repairing DNA damage.
hereditary breast and ovarian cancer
BRCA1
BRCA2
ionizing radiation
1. Hereditary Breast and Ovarian Cancer Syndrome
Cancer is a heterogeneous and mostly non-hereditary disease. Considering breast and ovarian cancers, most occur sporadically, resulting from mutations that occur in somatic cells. However, there are cases where genetic alterations are transmitted to offspring. Hereditary breast and ovarian cancer (HBOC) is an autosomal dominant inherited syndrome characterized by a high risk of breast cancer, in both genders, associated or not with ovarian cancer, including fallopian tube cancer and primary peritoneal cancer, in females [1][2].
HBOC is mostly caused by germline deleterious mutations in BRCA1 DNA repair associated (BRCA1) and BRCA2 DNA repair associated (BRCA2) genes and they affect all ethnic groups and races. In the general population, excluding the Ashkenazi Jewish population, mutations in BRCA1 and BRCA2 genes are estimated to have a frequency between 1:400 and 1:500. However, in the Ashkenazi Jewish population, the frequency of causal variants is 1 in 40 [1][2]. These genes are tumor suppressor genes that play a crucial role in the cell, so when mutated they lead to the development of dysfunctional proteins, unable to exert the DNA repair function. Non-repaired DNA leads to genetic instability with an increased risk of malignancy [2][3]. Affected individuals tend to develop these types of cancer earlier, usually before 50 years of age [1][2][4].
BRCA1 and BRCA2 Genes
DNA, which harbors genetic information, is the most important structure at the cellular level and is constantly suffering damage caused by internal and external factors. Repairing these injuries is crucial for the proper cell function [2]. Tumor suppressor genes are important for the normal functioning and development of the organism, constituting the key to the regulation of cell division. From these genes, specific proteins are encoded with several functions in the cell [5].
The BRCA1 and BRCA2 genes were first linked to breast and ovarian cancer susceptibility by Mick and colleagues in 1994 (BRCA1) and by Wooster et al., in 1995 (BRCA2) [6].
These are tumor suppressor genes responsible for repairing DNA double-strand breaks, allowing genomic stability to be maintained. In addition to the repair function, these genes control centrosome dynamics, chromosomal segregation, and cytokinesis, and stabilize the genome temporally and spatially in the cell cycle. Thus, an interruption in these cellular functions leads to genomic instability as well as the emergence of a carcinogenic environment. In addition to the different roles that these genes play, BRCA1 is involved in other functions, such as in onset of breast and ovarian cancer, healthy embryonic development, centrosome replication, and brain size (Figure 1) [2].
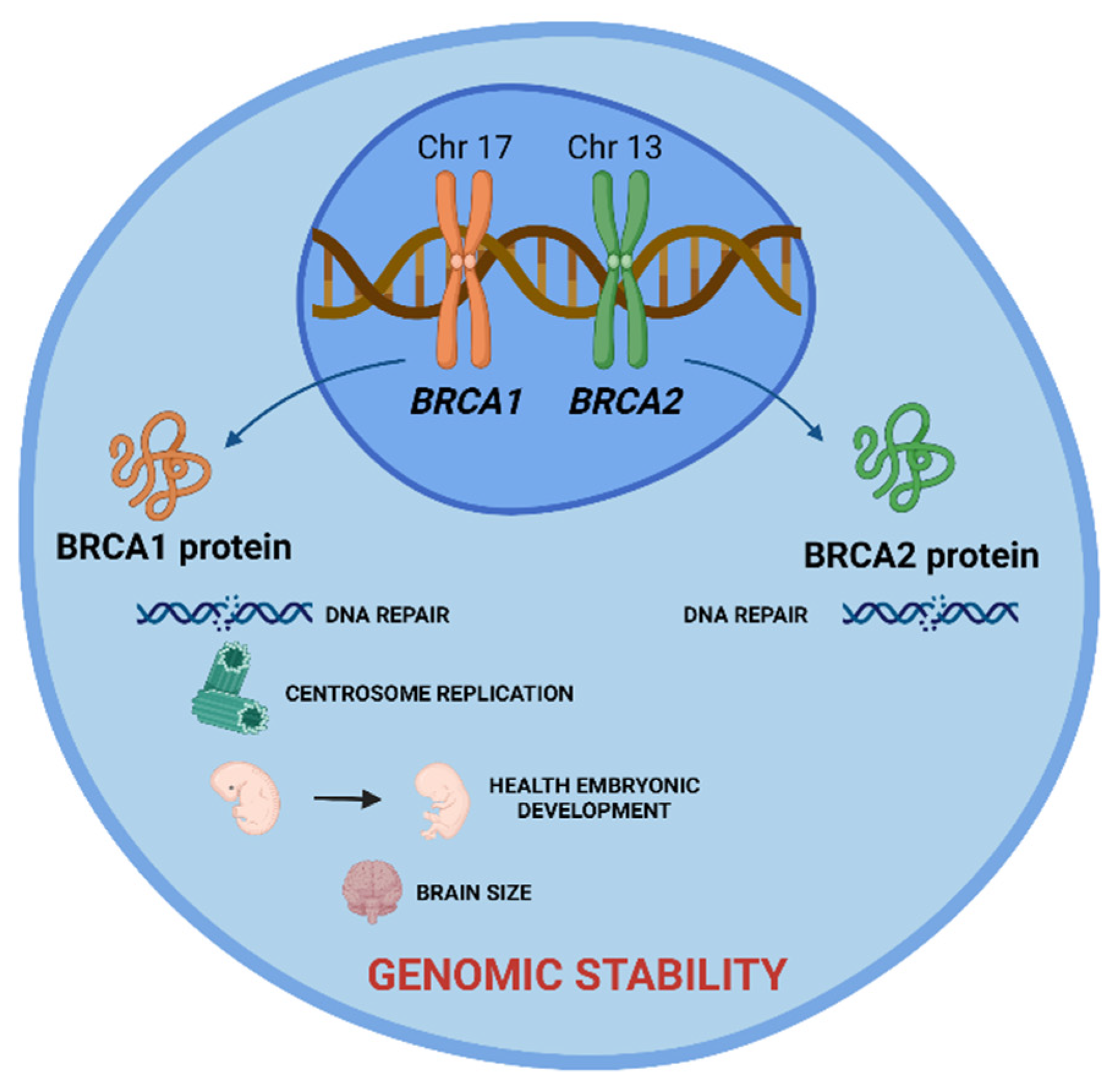
Figure 1. BRCA1 and BRCA2 tumor suppressor genes and the role of their proteins in cell. BRCA 1 and BRCA2 proteins are encoded by these genes and play a crucial role in DNA damage repair, allowing the maintenance of genomic stability. Additionally, BRCA1 protein is involved in centrosome replication, health embryonic development, and brain size. Created with BioRender.com.
The BRCA1 gene is located in region 21.31 of the long arm of chromosome 17 (17q21.31). It is composed of 24 exons, 22 of which are coding, spread over 100 kb of genomic DNA. More than 1700 mutations in this gene have been described, with more than 800 being associated with tumor incidence susceptibility. This gene is responsible for encoding and synthesizing the BRCA1 protein (Figure 1) [6][7][8].
The BRCA1 protein consists of three essential domains for its multiple functions: an amino terminal region (N-terminal), a central region, and a carboxyl terminal (C-terminal). The N-terminal region contains the RING finger domain, which is essential for the association with the BRCA1-associated RING domain protein 1 (BARD1) and formation of the BRCA1 complex with the E3 ubiquitin ligase. The central part of BRCA1 contains two nuclear localization signals (NLS) and a CHEK2 phosphorylation site. Finally, the C-terminal is constituted by the coiled-coil domain, which associates with PALB2, and by two BRCA1 C-terminal (BRCT) domains that mediate the interaction with important proteins, such as BRCA1-A complex subunit (ABRAXAS), BRCA1-interacting protein C-terminal helicase 1 (BRIP1) and C-terminal binding protein 1 interacting protein (CtIP). Several inherited cancer-associated BRCA1 mutations have been found within the RING domain and BRCT domains, which indicates that both domains are involved in the suppression of breast and ovarian cancer (Figure 2) [6][9].
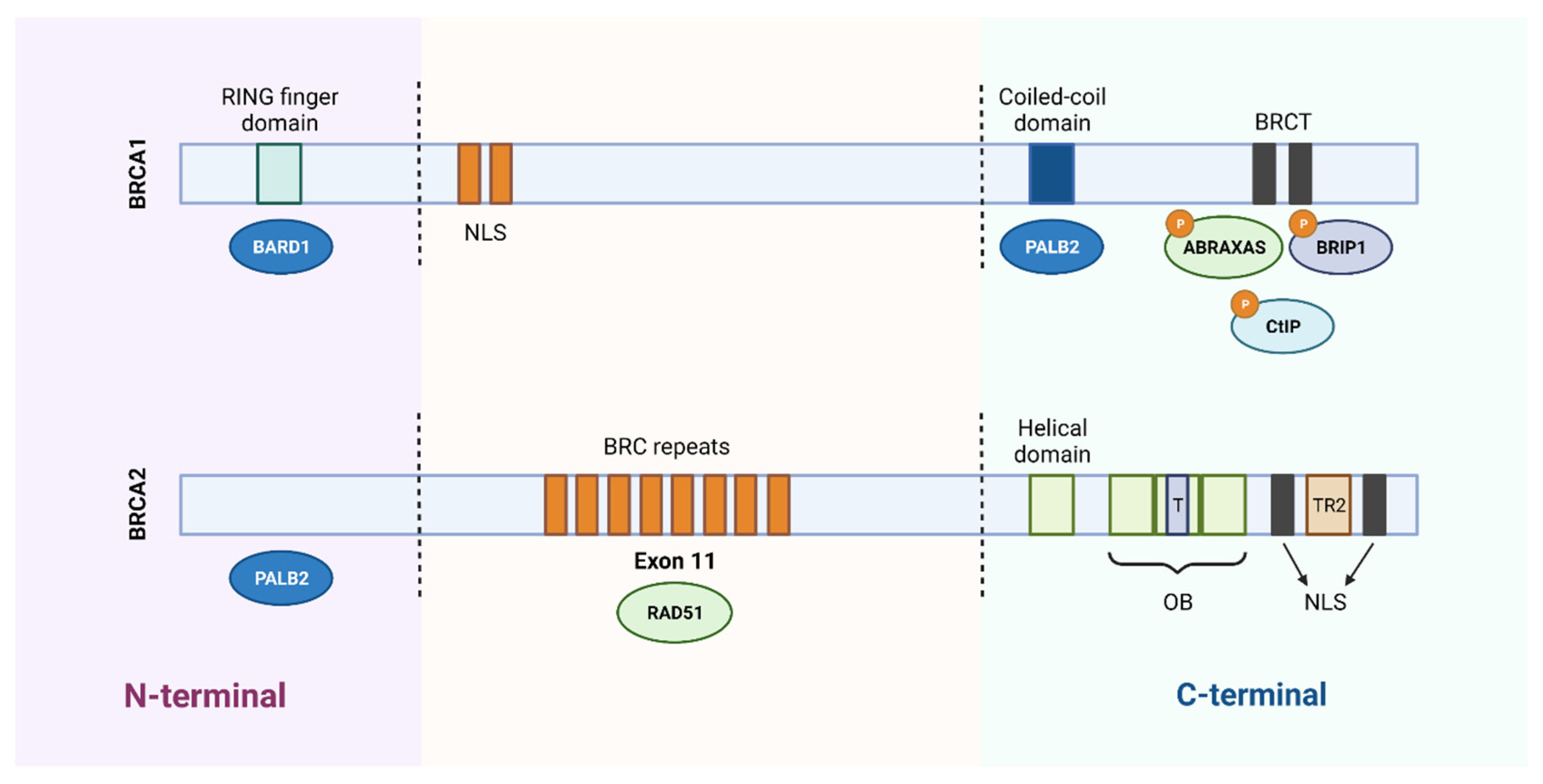
Figure 2. Schematic representation of the functional domains of BRCA1 and BRCA2 proteins. BRCA1 protein consists of 1863 amino acids and BRCA2 protein of 3418 amino acids. The N-terminal region of BRCA1 contains a RING domain that associates with the BARD1 protein. The central region of the protein contains two nuclear localization signals (NLS) and the C-terminal of BRCA1 contains a coiled-coil domain that associates with PALB2 and two BRCT domains that mediate interaction with different proteins. Regarding BRCA2, it binds to PALB2 through the N-terminal region and contains 8 BRC repeats, in the central region, responsible for its association with the RAD51 protein. In the C-terminal region, it contains a DNA-binding domain, consisting of a helical domain, a tower domain (T) and three oligonucleotide binding (OB) domains, which facilitates the binding of BRCA2 to DNA lesions. Furthermore, the carboxy terminal contains two NLS domains and a TR2 domain. Created with BioRender.com.
The BRCA2 gene is located on the long arm of chromosome 13 in region 12.3 (13q12.3). It is composed of 27 exons, of which 26 are coding, distributed over 384 kDa. More than 1800 mutations associated with this gene are described [6][10][11].
2. Ionizing Radiation and BRCA1 and BRCA2 Mutations
Ionizing radiation is responsible for causing dose-dependent DNA damage and, thus, induces the activation of relevant signaling pathways such as DNA repair, cell cycle control, and cell death. When considering exposure to low doses (<100 mSv), used during diagnostic medical imaging techniques such as mammography and chest x-rays, cells show a certain adaptative response and the damage to critical cell structures is low. The adaptive response, described by Olivieri et al., is defined as the induction of cellular resistance to genotoxic effects caused by subsequent exposure to high-dose radiation. Additionally, damage that occurs at the DNA level can, in general, be repaired, with recovery of function [12][13][14]. For exposure to high doses, used in radiotherapy as an oncological treatment, they exhibit a high potential to induce cellular damage in healthy cells, such as SSBs and DSBs. Since DSBs are very severe injuries, they are difficult to repair and can eventually result in the development of a new cancer [15]. However, although in the literature the effects of radiation seem to be well defined, in individuals with mutations in the BRCA1 and BRCA2 genes, there is still a great deal of controversy regarding the effects of low and high doses of radiation.
BRCA1 and BRCA2 genes play a key role in repairing DNA damage, which can arise because of ionizing radiation, and in regulating the cell cycle, leading to genomic stability and tumor suppression. Ionizing radiation can cause DNA damage, either directly through ionization or indirectly through radiolysis of water. Changes at the DNA level, such as DNA base changes, cross-linking, and DNA SSBs or DSB, are known to cause cancer.
Radiation-induced carcinogenesis may occur in individuals who have mutations in the BRCA1 and BRCA2 genes, as proteins encoded by them are essential in repairing DNA damage. A loss of function in one of the proteins encoded leads to inefficient DNA repair and an increase in genomic instability, sometimes culminating in cancer (Figure 3) [2][16].

Figure 3. Effect of internal and external factors on BRCA1 and BRCA2 genes. Mutations in these genes lead to inefficient DNA repair, contributing to genomic instability. This instability can lead to cancer. Created with BioRender.com.
This explains the fact that individuals who have mutations in the BRCA1 and BRCA2 genes have an increased risk of developing different types of cancer, such as breast and ovarian cancers. Individuals with HBOC are often subjected to diagnostic exams and therapeutic options that use ionizing radiation. For example, women initiate mammography at very early ages, employing a diagnostic tool that uses ionizing radiation. Thus, it is important to understand the role of diagnostic and therapeutic doses in patients with HBOC and whether the capacity to repair DNA damage is different between carriers and non-carriers of mutations in the BRCA genes.
References
- Petrucelli, N.; Daly, M.B.; Pal, T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer; GeneReviews; University of Washington: Seattle, WA, USA, 1993.
- Yoshida, R. Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer 2021, 28, 1167–1180.
- Hodgson, A.; Turashvili, G. Pathology of Hereditary Breast and Ovarian Cancer. Front. Oncol. 2020, 10, 531790.
- Okano, M.; Nomizu, T.; Tachibana, K.; Nagatsuka, M.; Matsuzaki, M.; Katagata, N.; Ohtake, T.; Yokoyama, S.; Arai, M.; Nakamura, S. The relationship between BRCA-associated breast cancer and age factors: An analysis of the Japanese HBOC consortium database. J. Hum. Genet. 2021, 66, 307–314.
- Lee, E.Y.H.P.; Muller, W.J. Oncogenes and tumor suppressor genes. Cold Spring Harb. Perspect. Biol. 2010, 2, a003236.
- Gorodetska, I.; Kozeretska, I.; Dubrovska, A. BRCA Genes: The Role in Genome Stability, Cancer Stemness and Therapy Resistance. J. Cancer 2019, 10, 2109–2127.
- Clark, S.L.; Rodriguez, A.M.; Snyder, R.R.; Hankins, G.D.; Boehning, D. STRUCTURE-FUNCTION OF THE TUMOR SUPPRESSOR BRCA1. Comput. Struct. Biotechnol. J. 2012, 1.
- National Center for Biotechnology Information. BRCA1 BRCA1 DNA Repair Associated . 2022. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=672 (accessed on 8 June 2022).
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2011, 12, 68–78.
- National Center for Biotechnology Information. BRCA2 BRCA2 DNA Repair Associated . 2022. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=675 (accessed on 8 June 2022).
- Vidarsson, H.; Mikaelsdottir, E.; Eyfjörd, J.; Ogmundsdottir, H.; Rafnar, T.; Valgeirsdottir, S. BRCA2 interacting proteins. Breast Cancer Res. 2000, 2.
- Cai, L. Research of the adaptive response induced by low-dose radiation: Where have we been and where should we go? Hum. Exp. Toxicol. 1999, 18, 419–425.
- Short, S.C.; Bourne, S.; Martindale, C.; Woodcock, M.; Jackson, S.P. DNA Damage Responses at Low Radiation Doses. Radiat. Res. 2005, 164, 292–302.
- Sakane, H.; Ishida, M.; Shi, L.; Fukumoto, W.; Sakai, C.; Miyata, Y.; Ishida, T.; Akita, T.; Okada, M.; Awai, K.; et al. Biological Effects of Low-Dose Chest CT on Chromosomal DNA. Radiology 2020, 295, 439–445.
- Jia, C.; Wang, Q.; Yao, X.; Yang, J. The Role of DNA Damage Induced by Low/High Dose Ionizing Radiation in Cell Carcinogenesis. Explor. Res. Hypothesis Med. 2021, 6, 177–184.
- Paul, A.; Paul, S. The breast cancer susceptibility genes (BRCA) in breast and ovarian cancers. Front. Biosci. Landmark Ed. 2014, 19, 605.
More
Information
Subjects:
Psychology, Social; Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.2K
Revisions:
2 times
(View History)
Update Date:
15 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





