Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | xiancui zhang | -- | 2167 | 2022-07-14 03:55:30 | | | |
| 2 | Jessie Wu | Meta information modification | 2167 | 2022-07-14 07:47:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, X.; Zhang, F.; Lu, X. Functional Roles of the Lepidopteran Gut Microbiota. Encyclopedia. Available online: https://encyclopedia.pub/entry/25121 (accessed on 07 February 2026).
Zhang X, Zhang F, Lu X. Functional Roles of the Lepidopteran Gut Microbiota. Encyclopedia. Available at: https://encyclopedia.pub/entry/25121. Accessed February 07, 2026.
Zhang, Xiancui, Fan Zhang, Xingmeng Lu. "Functional Roles of the Lepidopteran Gut Microbiota" Encyclopedia, https://encyclopedia.pub/entry/25121 (accessed February 07, 2026).
Zhang, X., Zhang, F., & Lu, X. (2022, July 14). Functional Roles of the Lepidopteran Gut Microbiota. In Encyclopedia. https://encyclopedia.pub/entry/25121
Zhang, Xiancui, et al. "Functional Roles of the Lepidopteran Gut Microbiota." Encyclopedia. Web. 14 July, 2022.
Copy Citation
Lepidopteran insects are one of the most widespread and speciose lineages on Earth, with many common pests and beneficial insect species. The evolutionary success of their diversification depends on the essential functions of gut microorganisms.
lepidopteran insect
gut microbiota
diversity
1. Host Nutrition and Metabolism
Insects provide stable environments and nutrition for symbionts, and in return, symbionts can offer the host necessary enzymes for food digestion, thereby expanding the host’s diet options and even changing the host’s eating habits [1]. The symbionts of the gut can contribute to the nitrogen cycle and can also produce nutrients that are essential to the development of the host organisms but are lacking in natural food, including amino acids, B vitamins, and sterols [2]. For instance, 118 culturable bacterial strains were isolated from the intestine of Diatraea saccharalis larvae. Among them, Klebsiella, Stenotrophomonas, Microbacterium, Bacillus, and Enterococcus were found to possess cellulolytic activity. All bacterial strains were cultured using soluble carboxymethyl cellulose (CMC) for degradation assays, and Bacillus and Klebsiella showed the highest degradation activity [3]. In addition, ten gut bacteria were isolated from the lepidopteran insect gut by in vitro culture, including gram-positive and gram-negative bacteria. Klebsiella can hydrolyse starch, whereas Proteus vulgaris, Erwinia sp., and Serratia liquefaciens can utilize xylanolytic, pectinolytic, and polysaccharides, respectively [4]. The main components of mulberry leaves are cellulose (19% to 25%) and xylan (10% to 40%), which shows the importance of the intestinal microbes of silkworms for food digestion [5][6].
Gut symbionts (Bacillus cereus, Enterococcus gallinarum, E. mundtii, Staphylococcus xylosus) are the pivotal species in soybean pests and are abundant in the caterpillar host. They exhibit a high tolerance for serine-proteinase inhibitors [7]. Enterobacter asburiae YT1 and Bacillus YP1 from the larvae of Plodia interpunctella were capable of degrading polyethylene films [8]. In addition, vitamins are the fundamental micronutrients that are normally found as precursors of various enzymes that are necessary for vital biochemical reactions during insect growth and development [9]. Hassan et al. tested the hypothesis that two actinobacterial gut symbionts provide Dysdercus fasciatus with B vitamins [10]. Insects actively harvest vitamins from bacterial symbionts by using specific enzymes that burst open the bacterial cell walls and thereby ensure host metabolic homeostasis [11].
2. Pathogen and Immune Defences
Under normal living conditions, the gut is the first line of defence because ingestion is the most likely route by which organisms come in contact with pathogens, including bacteria, fungi, viruses, and parasites [12][13]. Insects lack adaptive immune components, such as B cells and T cells, and rely on innate immune responses against infection. To combat infection, insects rely on multiple innate defence mechanisms, including the use of immune responses together with resource competition [14]. Lepidopteran insect guts use a battery of strategies, such as the generation of reactive oxygen species (ROS), to defend against harmful bacteria through cellular immune responses. Moreover, antimicrobial peptides (AMPs) and other immune effector molecules exhibit a broader spectrum of antimicrobial activity (Figure 1). In addition, the peritrophic membrane is a semipermeable barrier that can prevent most pathogens from damaging gut tissue via per os infection [15][16][17].
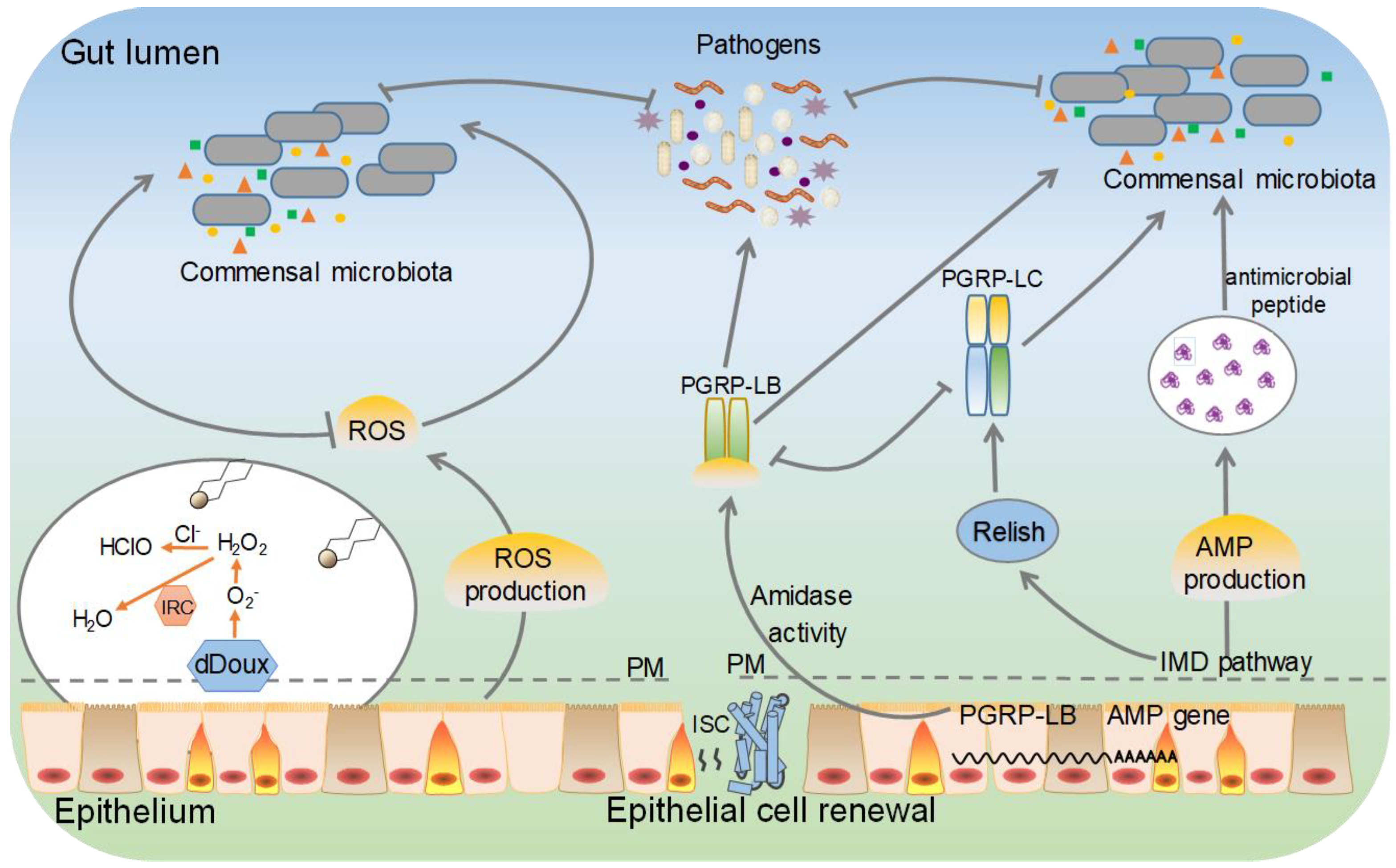
Figure 1. Putative immune signalling pathways are involved in the defences against pathogenic microbial infections in the gut of lepidopteran insects. This model is based on the local production of reactive oxygen species (ROS), and antimicrobial peptide (AMP) of Drosophila and findings in lepidopteran insects. The immune deficiency (IMD) includes the major signalling pathways inducing AMP production, and AMP genes provide inducible defense mechanisms in the gut. PM, peritrophic matrix.
Bacteria are important pathogens of lepidopteran insects [18]. Bacterial peptidoglycans (PGs) and proteases may disrupt the host’s cellular and biochemical processes [19][20]. The recognition of pathogens by lepidopteran insects relies on the interaction between pathogen-associated molecular patterns (PAMPs) and pattern-recognition receptors (PRRs) [21][22]. Lys (lysine) and lipopolysaccharide (LPS), as immune stimulators in insects, are major components of bacterial cell walls. They can trigger strong host immune responses in multitudinous insects [23][24][25]. In the midgut of lepidopteran insects, the immune reaction is primarily mediated by regulating the expression level of key immune components in the dual oxidase (DUOX) system and the immune deficiency (IMD) pathway, thus obtaining immune tolerance to beneficial gut microorganisms. Larvae carrying a Duox deletion are more susceptible to bacterial infection. Similar to the immune response, the local systemic response is regulated via the recognition of gram-negative proteoglycans (PGNs) by peptidoglycan recognition protein LC (PGRP-LC). Injection of pathogenic bacteria induces transient expression of AMP genes, suggesting the existence of a mechanism to downregulate the host immune response (Figure 1) [26][27]. For example, the expression of BmDuox was significantly upregulated in the midgut of B. mori fed Escherichia coli. Microbial proliferation in the midgut was increased after BmDuox knockout, suggesting that BmDuox has an important role in maintaining gut microbial homeostasis [28]. Peroxiredoxins (Prxs), as antioxidant enzymes in the lepidopteran insect gut, are notably enriched upon Pseudomonas aeruginosa and Bacillus bombyseptieus infection, and increased ROS levels can be induced by bacterial infection and proliferation [19][29]. In addition, the immune system of Hyalophora cecropia and Galleria mellonella was found to contain P9A and P9B antibacterial proteins, which are active against several Gram-negative bacteria (i.e., Escherichia coli and P. aeruginosa) [30]. In some lepidopteran insects, such as Choristoneura fumiferana, depletion of the gut microbiota increases the susceptibility of hosts to pathogenic infection [31]. Intriguingly, some lepidopteran gut microbes are universal opportunistic pathogens [32]. A commensal-to-pathogen switch is observed under multifactorial conditions, which depends on the pathogens and immune status of the host. This poses the question of how the immune system in the gut distinguishes between symbiotic microorganisms and pathogenic bacteria [33].
Fungi, such as Beauveria bassiana, Metarhizium anisopliae, and Microsporidia, are another group of important pathogens of lepidopteran insects [34]. Transcriptomic analyses revealed that infection by the B. bassiana strain upregulated the expression of immunity-related genes in G. mellonella, including hydrolytic enzymes, β-1,3-glucan recognition proteins, and spätzle genes [35]. A significant increase in the expression pattern of prophenoloxidase cascade (PPO) genes was found in Chilo suppressalis after treatment with B. bassiana, M. anisopliae, Isaria fumosoroseus and Lecanicilium lecanii, suggesting that host immune responses are critical against fungal infections [36]. Another study predicted serine proteases (SPs) and pattern recognition receptors (PRRs) as upstream components of the Toll pathway in Manduca sexta and Spodoptera exigua infected with Metarhizium rileyi [37]. In addition, Microsporidia, which are pathogens of lepidopteran insects, are a group of obligate intracellular parasites related to fungi. N. bombycis mainly infects B. mori through oral infection, and cuticle infection occasionally occurs [34]. Virulence studies showed that per os infection of silkworm larvae by microsporidia led to stimulation of the JAK/STAT and Toll signalling pathways in the midgut, which possibly induced the upregulation of AMPs to defend against the invading N. bombycis. The subtilisin-like serine protease NbSLP1 was activated after infection of N. bombycis in the midgut [38]. NbSLP1 is localized at the two poles of the spore and is likely involved in the polar tube extrusion process [39]. Two studies have also shown that feeding with Enterococcus faecalis LX10 or Lactobacillus could reduce the spore germination rate or increase the survival rate of silkworm larvae challenged by N. bombycis [40][41].
Viruses are significant natural pathogens of lepidopteran insects, and horizontal transmission of viruses is common in these species [42]. In addition, viruses infecting beneficial insects such as silkworms or bees can cause significant economic losses [43]. Host responses to viral infections include immunoreactions as well as mechanical barriers that prevent viruses from establishing infection [44]. Agata et al. observed that baculovirus infection leads to decreased expression of immune genes in the S. exigua larval gut. The expression of immune genes affects the diversity of gut microorganisms, many of which are responsible for growth and development functions [44]. In addition, several immune-related genes were found to be implicated in the midgut’s response against BmCPV infection of B. mori larval, including proteolytic enzymes, hormonal signaling, and heat-shock proteins [45]. In Drosophila, RNAi is a powerful method for defending against viruses, and activation of the Toll pathway inhibited Drosophila virus growth [46]. In the midgut of B. mori, alkaline trypsin protein and serine protease-2 showed strong antiviral activity, while immunoglobulin proteins, including Hemolin, a lepidopteran plasma protein produced after viral injection, demonstrated antiviral activity in oak silkworm, M. sexta and the Samia cecropia [47][48]. These studies indicate that lepidopterans circulate key proteins that serve as potent antiviral factors in the midgut.
Recent studies have shown that gut microorganisms can protect insects from propagating pathogens by accommodating host metabolism and repairing gut wall integrity, stimulating the host immune system and serving as essential probiotics for insect growth and development [49][50].
3. Potential Mechanism of Detoxificationby Lepidopteran Gut Bacteria
Gut symbiotic bacteria can also assist the host in degrading toxic or harmful substances, including insecticides, secondary plant compounds, and microplastics.
It has been reported that bacteria can directly degrade organic insecticides, such as ethoprophos, dimethoate, and chlorpyrifos, and these bacteria are often ingested from sources in the environment and food sources by agricultural pests [51][52][53]. Moreover, the gut microbiota may also enhance detoxification by influencing host fitness and the immune system [54]. For example, some soil Burkholderia strains degrading fenitrothion establish symbiosis with Riptortus pedestris and enhance host resistance to fenitrothion [55]. Indoxacarb is a highly effective insecticide widely used in the production of fruits and vegetables. B. cereus from the P. xylostella (Linnaeus) gut microbiota degraded indoxacarb by up to 20% and could use insecticides as an energy substance for growth and metabolism [56]. In addition, monoassociation of B. mori with gut bacteria of the genus Stenotrophomonas enhanced host resistance to organophosphate insecticides (chlorpyrifos), as confirmed by gut metabolomic analysis [57].
The majority of plants produce a wide variety of secondary metabolites that are toxic to pathogens and herbivores [58]. Recent studies have shown that gut microorganisms can assist the host in degrading toxic secondary metabolites. For instance, the gut bacteria Acinetobacter sp. R7-1 of Lymantria dispar has already been confirmed to metabolize aspen foliage secretion (phenolic glycosides) [59]. In particular, Klebsiella sp. and Corynebacterium have been isolated from the polyphagous pest larvae of Brithys crini, which participate in the degradation of alkaloids [60]. Gut bacteria protect Trichoplusia ni and Spodoptera eridania from the host plant toxin hydrogen cyanide (HCN) [61]. Some gut bacteria of Trichoplusia ni and S. eridania are capable of detoxifying toxic HCN, producing β-cyanoalanine (nontoxic product) and cysteine [62]. In addition, the E. casseliflavus strain was isolated from the gut and exhibited the ability to tolerate natural latex under laboratory conditions [60]. Xia et al. revealed an important role of Enterobacter cloacae, E. asburiae, and Carnobacterium maltaromaticum in the breakdown of plant cell walls, detoxification of plant phenolics, and synthesis of amino acids of the P. xylostella gut [63]. Members of the genera Pseudomonas, Burkholderia, and Cupriavidus were selected from the moth Retinia resinella and exhibited the ability to degrade specific resin acids such as dehydroabietic or isopimaric acid (diterpenes) [64].
In addition, several polyethylene (PE)-degrading bacteria and fungi have been reported, such as Aspergillus, Acremonium, Fusarium, E. asburiae, and Bacillus [65]. PE is one of the polymer materials that are remarkably resistant to degradation [66]. A fungal strain, Aspergillus flavus, was isolated as a potential microplastic particle-degrading microorganism from the gut contents of wax moth G. mellonella larvae by producing extracellular enzymes [67]. E. asburiae and Bacillus strains isolated from the gut of P. interpunctella can degrade polyethylene by forming biofilms that reduce the hydrophobicity of PE [8]. Subsequently, Ren et al. isolated the Enterobacter sp. strain D1, with the ability to degrade PE films, from the gut of G. mellonella (Ren et al., 2019). Recently, B. mori has also been applied in nanotoxicology studies to assess the potential effects of TiO2 nanoparticles on intestinal microbes [68]. These results suggested that the gut of insects might serve as a potential source for selecting PE-degrading microorganisms. It may also be possible to develop new strategies to reduce the toxic effects of xenobiotics on insects by leveraging their microbial symbionts.
4. Potential Application of Gut Symbionts in Controlling Lepidopteran Pests
The frequent application of insecticides has led to the ongoing development of high resistance in the past two decades, enhancing the urgent need for environmentally friendly long-term alternative strategies to control them [69][70]. Recent studies have shown that bacterial symbionts constitute promising microbial control agents (MCAs) with potential applications in controlling major lepidopteran agricultural pests, such as P. xylostella, S. littoralis, and C. fumiferana [71]. B. thuringiensis (Bt) strains have been developed as commercial biopesticides for more than a decade. Xia et al. found that the abundance of some bacteria in the larval midgut was related to the insecticide resistance of P. xylostella. Inoculating larvae with culturable gut microbes (Enterobacter sp. Mn2) reduced larval mortality after infection with B. thuringiensis in other studies, indicating that the gut microbiota can protect taxonomically diverse hosts from pathogen attack [72]. Xenorhabdus nematophila is another entomopathogenic bacterium that is symbiotically associated with parasitic nematodes (Steinernema). It is very effective against lepidopterans, such as the beet armyworm and diamondback moth [73][74].
In addition, Wolbachia species are widespread endosymbionts of lepidopteran insects. Wolbachia species, which are naturally occurring endosymbiotic bacteria found inside the cells of arthropods and filarial nematodes, can manipulate the host reproduction system [75] and are generally known as potential environmentally friendly biopesticides for the control of disease vectors and pests [76]. Recent studies found that Wolbachia species infect approximately 40% of terrestrial arthropod species, such as Lepidoptera, Hymenoptera, and Diptera species [77][78]. Cytoplasmic incompatibility (CI) is one of the most common phenotypes of reproductive manipulation in Wolbachia [79]. For instance, infection of Homona magnanima by multiple Wolbachia strains causes CI in the host, and Wolbachia increases the H. magnanima pupal weight and shortens the host development time [80]. Interestingly, Wolbachia spreads vertically in insects and is inherited maternally due to its presence in the cytoplasm of female gametes. Fukui et al.’s inhibition study of Ostrinia moths found that Wolbachia targets the population masculinization gene of the host to accomplish male killing by a failure of dosage compensation through unproductive mating [81].
These studies suggest that bacterial symbionts are essential in the evolution of insects, and thus, elucidating the role of bacterial symbionts of lepidopterans might help in the development of improved methods of biological control.
References
- Douglas, A.E. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009, 23, 38–47.
- Zhang, X.C.; Zhang, F. The Potential Control Strategies Based on the Interaction between Indoor Cockroaches and Their Symbionts in China. Adv. Insect Physiol. 2018, 55, 55–122.
- Dantur, K.I.; Enrique, R.; Welin, B.; Castagnaro, A. Isolation of cellulolytic bacteria from the intestine of Diatraea saccharalis larvae and evaluation of their capacity to degrade sugarcane biomass. AMB Express 2015, 5, 15.
- Prem Anand, A.A.; Vennison, S.J.; Sankar, S.G.; Gilwax Prabhu, D.I.; Vasan, P.T.; Raghuraman, T.; Jerome Geoffrey, C.; Vendan, S.E. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. J. Insect Sci. 2010, 10, 107.
- Lohan, O.P. Cell wall constituents and in vitro dry matter digestibility of some fodder trees in Himachal Pradesh. Forage Res. 1980, 6, 21–28.
- Chen, J.; Chen, C.; Liang, G.; Xu, X.; Hao, Q.; Sun, D. In situ preparation of bacterial cellulose with antimicrobial properties from bioconversion of mulberry leaves. Carbohydr. Polym. 2019, 220, 170–175.
- Pilon, F.; Visôtto, L.; Guedes, R.; Oliveira, M. Proteolytic activity of gut bacteria isolated from the velvet bean caterpillar Anticarsia gemmatalis. J. Comp. Physiol. B Biochem. 2013, 183, 735–747.
- Yang, J.; Yang, Y.; Wu, W.-M.; Zhao, J.; Jiang, L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784.
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M.J. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168.
- Chen, B.; Xie, S.; Zhang, X.; Zhang, N.; Feng, H.; Sun, C.; Lu, X.; Shao, Y. Gut microbiota metabolic potential correlates with body size between mulberry-feeding lepidopteran pest species. Pest. Manag. Sci. 2020, 76, 1313–1323.
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34.
- Vesga, P.; Flury, P.; Vacheron, J.; Keel, C.; Croll, D.; Maurhofer, M. Transcriptome plasticity underlying plant root colonization and insect invasion by Pseudomonas protegens. ISME J. 2020, 14, 2766–2782.
- Senger, K.; Harris, K.; Levine, M. GATA factors participate in tissue-specific immune responses in Drosophila larvae. Proc. Natl. Acad. Sci. USA 2006, 103, 15957–15962.
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743.
- Konno, K.; Shimura, S.; Ueno, C.; Arakawa, T.; Nakamura, M. Abnormal swelling of the peritrophic membrane in Eri silkworm gut caused by MLX56 family defense proteins with chitin-binding and extensin domains. Phytochemistry 2018, 147, 211–219.
- Yang, B.; Huang, W.; Zhang, J.; Xu, Q.; Zhu, S.; Zhang, Q.; Beerntsen, B.T.; Song, H.; Ling, E. Analysis of gene expression in the midgut of Bombyx mori during the larval molting stage. BMC Genom. 2016, 17, 866.
- Konno, K.; Mitsuhashi, W. The peritrophic membrane as a target of proteins that play important roles in plant defense and microbial attack. J. Insect Physiol. 2019, 117, 103912.
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41.
- Chen, K.; Lu, Z.J.D.; Immunology, C. Immune responses to bacterial and fungal infections in the silkworm, Bombyx mori. Dev. Comp. Immunol. 2018, 83, 3–11.
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605.
- Wang, X.; Zhang, Y.; Zhang, R.; Zhang, J. The diversity of pattern recognition receptors (PRRs) involved with insect defence against pathogens. Curr. Opin. Insect Sci. 2019, 33, 105–110.
- Yang, P.J.; Zhan, M.Y.; Ye, C.; Yu, X.Q.; Rao, X.J. Molecular cloning and characterization of a short peptidoglycan recognition protein from silkworm Bombyx mori. Insect Mol. Biol. 2017, 26, 665–676.
- Meng, X.; Zhu, F.; Chen, K. Silkworm: A promising model organism in life science. J. Insect Sci. 2017, 17, 97.
- Zhang, K.; Pan, G.; Zhao, Y.; Hao, X.; Li, C.; Shen, L.; Zhang, R.; Su, J.; Cui, H. A novel immune-related gene HDD1 of silkworm Bombyx mori is involved in bacterial response. Mol. Immunol. 2017, 88, 106–115.
- Motokawa, Y.; Kokubo, M.; Kuwabara, N.; Tatematsu, K.I.; Sezutsu, H.; Takahashi, H.; Sakakura, K.; Chikamatsu, K.; Takeda, S. Melanoma antigen family A4 protein produced by transgenic silkworms induces antitumor immune responses. Exp. Ther. Med. 2018, 15, 2512–2518.
- Kaneko, T.; Goldman, W.E.; Mellroth, P.; Steiner, H.; Fukase, K.; Kusumoto, S.; Harley, W.; Fox, A.; Golenbock, D.; Silverman, N. Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity 2004, 20, 637–649.
- Costa, A.; Jan, E.; Sarnow, P.; Schneider, D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS ONE 2009, 4, e7436.
- Hu, X.; Yang, R.; Zhang, X.; Chen, L.; Xiang, X.; Gong, C.; Wu, X. Molecular cloning and functional characterization of the dual oxidase (BmDuox) gene from the silkworm Bombyx mori. PLoS ONE 2013, 8, e70118.
- Kausar, S.; Abbas, M.; Zhao, Y.; Cui, H. Immune strategies of silkworm, Bombyx mori against microbial infections. Invertebr. Surviv. J. 2019, 16, 130–140.
- Hoffmann, D.; Hultmark, D.; Boman, H.G. Insect immunity: Galleria mellonella and other lepidoptera have cecropia-P9-like factors active against gram negative bacteria. Insect Biochem. Mol. Biol. 2019, 11, 537–548.
- Landry, M.; Comeau, A.M.; Derome, N.; Cusson, M.; Levesque, R.C. Composition of the spruce budworm (Choristoneura fumiferana) midgut microbiota as affected by rearing conditions. PLoS ONE 2015, 10, e0144077.
- Paniagua Voirol, L.R.; Frago, E.; Kaltenpoth, M.; Hilker, M.; Fatouros, N.E. Bacterial Symbionts in Lepidoptera: Their Diversity, Transmission, and Impact on the Host. Front. Microbiol. 2018, 9, 556.
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313.
- Senthil-Nathan, S. A review of biopesticides and their mode of action against insect pests. In Environmental Sustainability; Springer: Berlin/Heidelberg, Germany, 2015; pp. 49–63.
- Ding, J.L.; Hou, J.; Feng, M.G.; Ying, S.H. Transcriptomic analyses reveal comprehensive responses of insect hemocytes to mycopathogen Beauveria bassiana, and fungal virulence-related cell wall protein assists pathogen to evade host cellular defense. Virulence 2020, 11, 1352–1365.
- Malagoli, A.Z.a.D. Immune response of Chilo suppressalis Walker (Lepidoptera: Crambidae) larvae to different entomopathogenic fungi. Bull. Entomol. Res. 2014, 104, 155–163.
- Miltan Chandra Roy, Y.K. Toll signal pathway activating eicosanoid biosynthesis shares its conserved upstream recognition components in a lepidopteran Spodoptera exigua upon infection by Metarhizium rileyi, an entomopathogenic fungus. J. Invertebr. Pathol. 2022, 188, 107707.
- Li, G.; Zhou, Q.; Qiu, L.; Yao, Q.; Chen, K.; Tang, Q.; Hu, Z.J. Serine protease Bm-SP142 was differentially expressed in resistant and susceptible Bombyx mori strains, involving in the defence response to viral infection. PLoS ONE 2017, 12, e0175518.
- Liu, F.; Ma, Q.; Dang, X.; Wang, Y.; Song, Y.; Meng, X.; Bao, J.; Chen, J.; Pan, G.; Zhou, Z.J. Identification of a new subtilisin-like protease NbSLP2 interacting with cytoskeletal protein septin in Microsporidia Nosema bombycis. J. Invertebr. Pathol. 2017, 148, 110–117.
- Zhang, X.; Feng, H.; He, J.; Liang, X.; Zhang, N.; Shao, Y.; Zhang, F.; Lu, X. The gut commensal bacterium Enterococcus faecalis LX10 contributes to defending against Nosema bombycis infection in Bombyx mori. Pest. Manag. Sci. 2022, 78, 2215–2227.
- Suraporn, S.; Terenius, O. Supplementation of Lactobacillus casei reduces the mortality of Bombyx mori larvae challenged by Nosema bombycis. BMC Res. Notes 2021, 14, 398.
- Drezen, J.M.; Josse, T.; Bezier, A.; Gauthier, J.; Huguet, E.; Herniou, E.A. Impact of Lateral Transfers on the Genomes of Lepidoptera. Genes 2017, 8, 315.
- Mondotte, J.A.; Saleh, M.C. Antiviral Immune Response and the Route of Infection in Drosophila melanogaster. Adv. Virus Res. 2018, 100, 247–278.
- Jakubowska, A.K.; Vogel, H.; Herrero, S. Increase in gut microbiota after immune suppression in baculovirus-infected larvae. PLoS Pathog. 2013, 9, e1003379.
- Kolliopoulou, A.; Van Nieuwerburgh, F.; Stravopodis, D.J.; Deforce, D.; Swevers, L.; Smagghe, G. Transcriptome analysis of Bombyx mori larval midgut during persistent and pathogenic cytoplasmic polyhedrosis virus infection. PLoS ONE 2015, 10, e0121447.
- Sabin, L.R.; Hanna, S.L.; Cherry, S. Innate antiviral immunity in Drosophila. Curr. Opin. Immunol. 2010, 22, 4–9.
- Cheng, Y.; Wang, X.Y.; Du, C.; Gao, J.; Xu, J.P. Expression analysis of several antiviral related genes to BmNPV in different resistant strains of silkworm, Bombyx mori. J. Insect Sci. 2014, 14, 76.
- Ponnuvel, K.M.; Nithya, K.; Sirigineedi, S.; Awasthi, A.K.; Yamakawa, M. In vitro antiviral activity of an alkaline trypsin from the digestive juice of Bombyx mori larvae against nucleopolyhedrovirus. Arch. Insect Biochem. Physiol. 2012, 81, 90–104.
- Van Arnam, E.B.; Currie, C.R.; Clardy, J.J. Defense contracts: Molecular protection in insect-microbe symbioses. Chem Soc. Rev. 2018, 47, 1638–1651.
- Hajek, A.E.; Morris, E.E.; Hendry, T.A. Context dependent interactions of insects and defensive symbionts: Insights from a novel system in siricid woodwasps. Curr. Opin. Insect Sci. 2019, 33, 77–83.
- Li, X.; He, J.; Li, S. Isolation of a chlorpyrifos-degrading bacterium, Sphingomonas sp. strain Dsp-2, and cloning of the mpd gene. Res. Microbiol. 2007, 158, 143–149.
- Singh, B.K.; Walker, A.; Morgan, J.A.; Wright, D.J. Effects of soil pH on the biodegradation of chlorpyrifos and isolation of a chlorpyrifos-degrading bacterium. Appl. Environ. Microbiol. 2003, 69, 5198–5206.
- Rayu, S.; Nielsen, U.N.; Nazaries, L.; Singh, B.K. Isolation and Molecular Characterization of Novel Chlorpyrifos and 3,5,6-trichloro-2-pyridinol-degrading Bacteria from Sugarcane Farm Soils. Front. Microbiol. 2017, 8, 518.
- Xia, X.; Sun, B.; Gurr, G.M.; Vasseur, L.; Xue, M.; You, M. Gut Microbiota Mediate Insecticide Resistance in the Diamondback Moth, Plutella xylostella (L.). Front. Microbiol. 2018, 9, 25.
- Tago, K.; Kikuchi, Y.; Nakaoka, S.; Katsuyama, C.; Hayatsu, M. Insecticide applications to soil contribute to the development of B urkholderia mediating insecticide resistance in stinkbugs. Mol. Ecol. 2015, 24, 3766–3778.
- Ramya, S.L.; Venkatesan, T.; Srinivasa Murthy, K.; Jalali, S.K.; Verghese, A. Detection of carboxylesterase and esterase activity in culturable gut bacterial flora isolated from diamondback moth, Plutella xylostella (Linnaeus), from India and its possible role in indoxacarb degradation. Braz. J. Microbiol. 2016, 47, 327–336.
- Chen, B.; Zhang, N.; Xie, S.; Zhang, X.; He, J.; Muhammad, A.; Sun, C.; Lu, X.; Shao, Y. Gut bacteria of the silkworm Bombyx mori facilitate host resistance against the toxic effects of organophosphate insecticides. Environ. Int. 2020, 143, 105886.
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690.
- Mason, C.J.; Lowe-Power, T.M.; Rubert-Nason, K.F.; Lindroth, R.L.; Raffa, K.F. Interactions between bacteria and aspen defense chemicals at the phyllosphere–herbivore interface. J. Chem. Ecol. 2016, 42, 193–201.
- Vilanova, C.; Baixeras, J.; Latorre, A.; Porcar, M. The Generalist Inside the Specialist: Gut Bacterial Communities of Two Insect Species Feeding on Toxic Plants Are Dominated by Enterococcus sp. Front. Microbiol. 2016, 7, 1005.
- Wybouw, N.; Dermauw, W.; Tirry, L.; Stevens, C.; Grbić, M.; Feyereisen, R.; Van Leeuwen, T.J.E. A gene horizontally transferred from bacteria protects arthropods from host plant cyanide poisoning. Elife 2014, 3, e02365.
- Wybouw, N.; Van Leeuwen, T.; Dermauw, W. A massive incorporation of microbial genes into the genome ofTetranychus urticae, a polyphagous arthropod herbivore. Insect Mol. Biol. 2018, 27, 333–351.
- Xia, X.; Gurr, G.M.; Vasseur, L.; Zheng, D.; Zhong, H.; Qin, B.; Lin, J.; Wang, Y.; Song, F.; Li, Y.; et al. Metagenomic Sequencing of Diamondback Moth Gut Microbiome Unveils Key Holobiont Adaptations for Herbivory. Front. Microbiol. 2017, 8, 663.
- Vilanova, C.; Marin, M.; Baixeras, J.; Latorre, A.; Porcar, M. Selecting microbial strains from pine tree resin: Biotechnological applications from a terpene world. PLoS ONE 2014, 9, e100740.
- Restrepo-Flórez, J.-M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene-A review. Int. Biodeterior. Biodegrad. 2014, 88, 83–90.
- Muhammad, A.; Zhou, X.; He, J.; Zhang, N.; Shen, X.; Sun, C.; Yan, B.; Shao, Y. Toxic effects of acute exposure to polystyrene microplastics and nanoplastics on the model insect, silkworm Bombyx mori. Environ. Pollut. 2021, 285, 117255.
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931.
- Li, M.; Li, F.; Lu, Z.; Fang, Y.; Qu, J.; Mao, T.; Wang, H.; Chen, J.; Li, B. Effects of TiO2 nanoparticles on intestinal microbial composition of silkworm, Bombyx mori. Sci. Total Environ. 2020, 704, 135273.
- Xie, S.; Lan, Y.; Sun, C.; Shao, Y. Insect microbial symbionts as a novel source for biotechnology. World J. Microbiol. Biotechnol. 2019, 35, 25.
- van den Bosch, T.J.; Welte, C.U. Detoxifying symbionts in agriculturally important pest insects. Microb. Biotechnol. 2017, 10, 531–540.
- Ferreira, C.M.; Soares, H.M.; Soares, E.V. Promising bacterial genera for agricultural practices: An insight on plant growth-promoting properties and microbial safety aspects. Sci. Total Environ. 2019, 682, 779–799.
- Xia, X.; Zheng, D.; Zhong, H.; Qin, B.; Gurr, G.M.; Vasseur, L.; Lin, H.; Bai, J.; He, W.; You, M. DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS ONE 2013, 8, e68852.
- Mahar, A.; Al-Siyabi, A.; Elawad, S.; Hague, N.; Gowen, S. Application of toxins from the entomopathogenic bacterium, Xenorhabdus nematophila, for the control of insects on foliage. Commun. Agric. Appl. Biol. Sci. 2006, 71, 233–238.
- Shi, Y.-M.; Bode, H.B. Chemical language and warfare of bacterial natural products in bacteria–nematode–insect interactions. Nat. Prod. Rep. 2018, 35, 309–335.
- Zug, R.; Hammerstein, P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. 2015, 90, 89–111.
- Nikolouli, K.; Colinet, H.; Renault, D.; Enriquez, T.; Mouton, L.; Gibert, P.; Sassu, F.; Cáceres, C.; Stauffer, C.; Pereira, R. Sterile insect technique and Wolbachia symbiosis as potential tools for the control of the invasive species Drosophila suzukii. J. Pest. Sci. 2018, 91, 489–503.
- Kajtoch, Ł.; Kolasa, M.; Kubisz, D.; Gutowski, J.M.; Ścibior, R.; Mazur, M.A.; Holecová, M. Using host species traits to understand the Wolbachia infection distribution across terrestrial beetles. Sci. Rep. 2019, 9, 847.
- Liu, Q.; Zhou, J.; Zhang, C.; Dong, Q.; Ning, S.; Hui, D. Absence of complementary sex determination in Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae). Peer J. Prepr. 2019, 7, e27871v27871.
- Gaunet, A.; Dincă, V.; Dapporto, L.; Montagud, S.; Vodă, R.; Schär, S.; Badiane, A.; Font, E.; Vila, R. Two consecutive Wolbachia-mediated mitochondrial introgressions obscure taxonomy in Palearctic swallowtail butterflies (Lepidoptera, Papilionidae). Zool. Scr. 2019, 48, 507–519.
- Arai, H.; Hirano, T.; Akizuki, N.; Abe, A.; Nakai, M.; Kunimi, Y.; Inoue, M.N. Multiple infection and reproductive manipulations of Wolbachia in Homona magnanima (Lepidoptera: Tortricidae). Microb. Ecol. 2019, 77, 257–266.
- Fukui, T.; Kawamoto, M.; Shoji, K.; Kiuchi, T.; Sugano, S.; Shimada, T.; Suzuki, Y.; Katsuma, S. The endosymbiotic bacterium Wolbachia selectively kills male hosts by targeting the masculinizing gene. PLoS Pathog. 2015, 11, e1005048.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
779
Revisions:
2 times
(View History)
Update Date:
14 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




