Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shih-Min Hsia | -- | 1563 | 2022-07-12 10:45:48 | | | |
| 2 | Jessie Wu | -2 word(s) | 1561 | 2022-07-13 04:04:17 | | | | |
| 3 | Jessie Wu | Meta information modification | 1561 | 2022-07-14 08:17:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, K.; Yu, Y.; Chen, H.; Chiang, Y.; Ali, M.; Shieh, T.; Hsia, S. Glycyrrhiza glabra (Licorice)-Containing Herbs in Cancer Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/25045 (accessed on 08 February 2026).
Wang K, Yu Y, Chen H, Chiang Y, Ali M, Shieh T, et al. Glycyrrhiza glabra (Licorice)-Containing Herbs in Cancer Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/25045. Accessed February 08, 2026.
Wang, Kai-Lee, Ying-Chun Yu, Hsin-Yuan Chen, Yi-Fen Chiang, Mohamed Ali, Tzong-Ming Shieh, Shih-Min Hsia. "Glycyrrhiza glabra (Licorice)-Containing Herbs in Cancer Treatment" Encyclopedia, https://encyclopedia.pub/entry/25045 (accessed February 08, 2026).
Wang, K., Yu, Y., Chen, H., Chiang, Y., Ali, M., Shieh, T., & Hsia, S. (2022, July 12). Glycyrrhiza glabra (Licorice)-Containing Herbs in Cancer Treatment. In Encyclopedia. https://encyclopedia.pub/entry/25045
Wang, Kai-Lee, et al. "Glycyrrhiza glabra (Licorice)-Containing Herbs in Cancer Treatment." Encyclopedia. Web. 12 July, 2022.
Copy Citation
Cancer is one of the leading causes of premature death and a significant barrier to increasing life expectancy in almost every country in the world. Licorice belongs to the genus Glycyrrhiza, and radix glycyrrhizae (RG) is the dried roots and rhizomes of licorice. Licorice, as well as licorice-purified compounds, has the potential to abrogate the onset and progression of different malignancy cancers, both in vitro and in vivo. Moreover, previous studies also suggest that licorice is a beneficial medicine plant used as a cure for nausea and vomiting.
licorice
cancers
chemotherapy
1. Utility of Licorice-Containing Herbs in Cancer
1.1. Licorice Introduction
Licorice belongs to the genus Glycyrrhiza, and radix glycyrrhizae (RG) is the dried roots and rhizomes of licorice. Licorice is commonly used as a natural sweetener and in herbal medicine. It mainly acts as a supplement in Western countries, for products such as herbal teas, soft drinks, and tobacco products. However, it is regarded as a medicine in Asia. Licorice is utilized to relieve pain, phlegm, spasms, cough, and dyspnea. The abundant active ingredients in licorice demonstrate efficacy in many different biological and physiological functions. To date, more than 300 bioactive compounds have been identified in licorice, including ~100 types of triterpenoid saponins and sapogenins, and ~300 kinds of phenolic compounds [1]. However, it has been found that the cultivated geographical area, the state of plant maturity, environmental conditions (including the pH of the soil, temperature, and weather), harvesting, and processing all affect the content of the bioactive compounds in licorice [2]. For example, the triterpenoid saponins in licorice, especially glycyrrhizic acid (GL; approximately 1.84% to 9.82% of licorice, depending on the sources and methods of extraction), are the major constituents and bioactive ingredients of licorice [3][4][5]. Flavonoids (approximately 1.78% to 4.82% of licorice, depending on the sources and methods of extraction) are the other main bioactive compound found in licorice, including isoliquiritigenin (ISL), isoliquiritin, and liquiritigenin, etc. [3][6]. In the 2010 edition of the Chinese Pharmacopoeia, GL and isoliquiritigenin were selected as the biomarkers for licorice, and it is stated that their content should exceed 2% and 0.5%, respectively [7]. Interestingly, researchers found that these two major compounds (GL and ISL) appear in many studies related to chemotherapy, which will be discussed further in a later section.
Licorice can be simply categorized into three Glycyrrhiza species: Glycyrrhiza uralensis Fisch., Glycyrrhiza glabra L., and Glycyrrhiza inflata Bat. [8]. In China, G. uralensis, G. glabra, and G. inflata are considered equivalent, and are combined and utilized as licorice without discrimination in the 2015 edition of the Chinese Pharmacopoeia. However, the morphological characteristics of the three Glycyrrhiza species show differences in the root, rhizome, seed, fruit, and inflorescence, as well as in the leaf and stem height. It is difficult to identify these licorice species accurately based only on their root or rhizome morphology [9].
There are significant differences between the species, which were established by the analytical methods of numerous studies aimed at separating and quantifying the active ingredients in licorice samples. These studies report that different licorice species have species-specific markers (Table 1); for example, the content of major flavonoids (liquiritin, liquiritigenin, and isoliquiritin) in G. uralensis is higher than that in G. glabra, and glycycoumarin only exists in G. uralensis [10][11][12][13][14]. Glycyrrhizin, 50 times sweeter than sugar and especially suitable for children, is evenly distributed in the three species [13]. The amount of isoliquiritigenin (2′,4′,4-trihydroxychalcone, ISL), one of the major bioactive compounds in licorice, is higher in G. uralensis than in G. glabra and G. inflata. [15][16].
Table 1. Most common Glycyrrhiza species used as medicine.
| Glycyrrhiza Species | Region | Specific Content | Ref |
|---|---|---|---|
| Glycyrrhiza uralensis (Glycyrrhiza radix) | China Northeastern Far east Russia |
Owning the highest content of flavonoids (liquiritin, liquiritigenin, and isoliquiritin). Glycycoumarin only represented in G. uralensis. |
[10][11][12][13][14][17] |
| Isotrifloliol, licoricone, neoglycyrol, glycyrin, and licorisoflavan A in G. uralensis are higher. | [10] | ||
| Glyinflanin D/G and licoflavone B are absent. | [18] | ||
| Glycyrrhiza glabra | Italy Spain China Russia Iran Central Asia |
Owning the highest content of 18α-glycyrrhizic acid and 18β-glycyrrhizic acid. | [19] |
| Higher content of saponins–licorice saponin K2/H2, licorice saponin B2, and licorice saponin G2/yunganoside K2. Quercetin absent in G. glabra. |
[18][20][21] | ||
| The highest content of apiosides (liquiritin apioside, isoliquiritin apioside, licuraside). | [14] | ||
| Abundant 8-cyclized isoprenyl isoflavanes (e.g., glabridin and 4′-O-methylglabridin). | [13] | ||
| Polysaccharide content in G. glabra is the highest. | [22][23] | ||
| Glycyrrhiza | China, Asia | Highest content of triterpene saponins. | [9][13] |
| inflata | Chalcone derivatives such as licochalcone (A, B, C, E), kanzonol C, and echinatin in G. inflata are higher. | [13][18][24] | |
| The content of quercetin is higher than that in G. uralensis. | [18][20][21] | ||
| Highest content of prenylated chalcones. | [18] |
1.2. Chemopreventive Activities of Licorice
Licorice and its derivatives exert anti-inflammatory and antioxidant effects, suggesting their potential as chemopreventive or therapeutic agents. For example, licorice can be used for reducing inflammation and allergic responses, as well as preventing liver damage [8][25][26]. Licorice extract also acts as a moderate hypocholesterolemic nutrient, and a potent antioxidant agent, to prevent cardiovascular disease [27]. Clinical data suggest that licorice, or its bioactive components, prevent dyspepsia and hyperlipidemia [28][29]. When taken concurrently with a glycyrrhizin-containing product, licorice is shown to afford hepatoprotection during alcohol consumption [30]. Three randomized clinical trials claim that Glycyrrhiza acts as a mucoadhesive film, improving oral mucositis during radiotherapy [31][32][33]. Moreover, preoperative gargling with a licorice solution reduces postoperative sore throat, thus, revealing its analgesic properties [34]. The clinical trials of licorice are summarized in Table 2.
Table 2. Licorice and its components applied in chemopreventive clinical trials.
| Name | Disease/Disorder | Dose/Duration | Trial | Location/ Identifier No. |
Ref | |||
|---|---|---|---|---|---|---|---|---|
| Patient (n) | Experiment Group |
Control Group |
Outcome | |||||
| Extract of G. glabra | Radiotherapy Head or neck |
Oral 100 c.c/Bid 2 weeks |
n = 37 | Extract of G. glabra | Placebo (radiotherapy) |
Prevent oral mucositis | IRCT201203012464N4 Iran Tehran University of medical science |
[31] |
| G. glabra (yashtimadhu) |
Radiotherapy Head or neck |
Oral 5 g/Bid 6 weeks |
n = 127 | G. glabra | Placebo (radiotherapy) |
Prevent oral mucositis | Himalayan Institute of Medical Sciences, Dehradun, India | [32] |
| Licorice | Radiotherapy Head or neck |
Mouth wash |
n = 60 | Licorice mucoadhesive film | Placebo mucoadhesive film | Prevent oral mucositis | Isfahan University of Medical Sciences, Isfahan, Iran | [33] |
| Licorice extract |
Randomized Double-blind |
Oral 1 g/Tid |
n = 236 | +licorice extract |
Sugar water | Pain relieving | NCT02968823 | [34] |
| Licorice | Dyspepsia | 380 mg/Bid 4 weeks |
n = 120 | +licorice | N.A. | Improved H. pylori eradication | IRCT2014061718124N | [28][29] |
| Glycyrrhizin | Alcohol consuming | Oral 0.1–0.3% 12 days |
n = 24 | +Licorice | Placebo (alcohol) |
Hepato-protection | N.A. | [30] |
2. Bioactive Components of Licorice
Licorice root contains a variety of bioactive components, including alkaloids, polysaccharides, polyamines, triterpenes, phenolic acids, flavones, flavans, chalcones, flavonoids, and isoflavonoids. Among them, only a few can be characterized and isolated from licorice. Only the components studied for chemoprevention are discussed (See Table 3), such as glycyrrhetic acid (GA) and chalcone-type derivative isoliquiritigenin (ISL) (Figure 1).
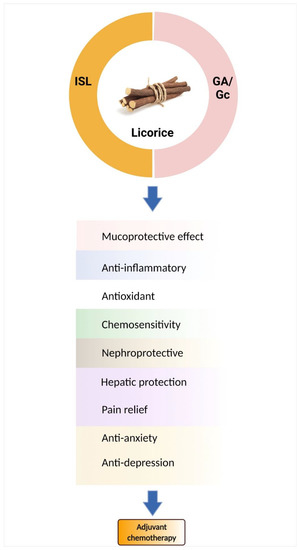
Figure 1. Licorice and its active components are candidates for chemo-combinations. Glycyrrhizin (Gc)/glycyrrhetic acid (GA) and isoliquiritigenin (ISL) mediate many mechanisms to improve chemotherapy-induced adverse effects.
Glycyrrhizin demonstrates immunomodulatory actions in vitro, stimulating T lymphocytes for IL-2 production [35]. An anti-inflammatory effect is associated with glycyrrhizinic and glycyrrhetic acid, via an inhibition of corticosteroid metabolism and production [36]. To extend the corticosteroid effects, it is broadly classified into immunological and metabolic effects [37]. From a metabolic perspective, the active form of glycyrrhizin, glycyrrhetic acid, influences energy metabolism and fat distribution by mediating fatty acid oxidation–related genes [38][39]. To emphasize the role of antioxidants, pretreatment with glycyrrhizinic acid could decrease free radicals and increase the level of reduced glutathione (GSH) [40][41]. Licorice extract and glycyrrhizic acid could reduce ROS-mediating p53 activation, and promote p21 expression against cisplatin-induced nephrotoxicity in vitro [42]. In an animal model, glycyrrhizic acid (GA) and 18β-glycyrrhetinic acid (18βGA) are represented as chemoprotectants, through the modulation of the NF-κB and Nrf2 pathways to reduce cisplatin-induced nephrotoxicity [43]. Overall, glycyrrhizin has been widely studied for combination chemotherapies involving cisplatin, 5-Fluorouracil, radiation, doxorubicin, paclitaxel, etc. [43][44][45][46][47][48][49][50][51][52][53].
Isoliquiritigenin, one of the major bioactive compounds found in licorice, shares the same basic pharmacologic effects as Glycyrrhiza and exerts more biological activity, especially in its anti-tumor effects [54]. In a CT-26 murine colon animal model, ISL suppresses cisplatin-induced kidney/liver damage by mediating nitric oxide, lipid peroxidation, and GSH levels [55]. Based on the antioxidant properties of ISL, it shows a protective effect on cisplatin-induced toxicity, through regulating the oxidative ER stress hormesis. [56]. In addition, to target the anti-inflammatory effects, ISL also inhibits IL-6, IL-12, and TNF-α production [57]. Many studies suggest that licorice extract or licorice-derived active components benefit chemotherapy (Figure 1). Table 3 summarizes the licorice components associated with chemopreventive activities, mainly focusing on glycyrrhizin and ISL. However, some components of licorice present unwanted side effects; therefore, Kampo medicine is another option to improve chemotherapy-induced adverse effects.
Table 3. Licorice compounds, mechanisms of action and potential chemopreventions.
| Compounds | Pharmacological Group | Chemotherapy | Therapeutic Actions/Mechanism | Ref |
|---|---|---|---|---|
| Glycyrrhizinic acid | Triterpenoid saponin | 5-Fluorouracil |
|
[44][45] |
| Cisplatin |
|
[43] | ||
| Cisplatin/radiation |
|
[46][47][48] | ||
| Erlotinib/cisplatin |
|
[49] | ||
| Doxorubicin |
|
[51][52][58] | ||
| Paclitaxel |
|
[50][59] | ||
|
[53] | |||
| N.A. |
|
[60][61] | ||
| Glycyrrhizin | Cyclosporine (CsA) |
|
[62] | |
| Isoliquiritigenin | Trans-chalcone (flavonoid) | Cisplatin |
|
[56][63][64][65] |
|
[55][66] | |||
| 5-Fluorouracil |
|
[67] | ||
|
[68] | |||
| Doxorubicin |
|
[69] | ||
|
[70][71] |
References
- Deutch, M.R.; Grimm, D.; Wehland, M.; Infanger, M.; Krüger, M. Bioactive Candy: Effects of Licorice on the Cardiovascular System. Foods 2019, 8, 495.
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339.
- Zhang, Q.; Ye, M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. A 2009, 1216, 1954–1969.
- Ong, E.S.; Len, S.M. Pressurized hot water extraction of berberine, baicalein and glycyrrhizin in medicinal plants. Anal. Chim. Acta 2003, 482, 81–89.
- Charpe, T.; Rathod, V. Extraction of glycyrrhizic acid from licorice root using ultrasound: Process intensification studies. Chem. Eng. Processing Process Intensif. 2012, 54, 37–41.
- Cui, Y.M.; Yu, L.J.; Ao, M.Z.; Yang, Y.; Hu, J. . Zhong Yao Cai 2006, 29, 838–841.
- The People’s Republic of China. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2010.
- Yang, R.; Yuan, B.C.; Ma, Y.S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017, 55, 5–18.
- Tao, W.; Duan, J.; Zhao, R.; Li, X.; Yan, H.; Li, J.; Guo, S.; Yang, N.; Tang, Y. Comparison of three officinal Chinese pharmacopoeia species of Glycyrrhiza based on separation and quantification of triterpene saponins and chemometrics analysis. Food Chem. 2013, 141, 1681–1689.
- Zhu, Z.; Tao, W.; Li, J.; Guo, S.; Qian, D.; Shang, E.; Su, S.; Duan, J.A. Rapid determination of flavonoids in licorice and comparison of three licorice species. J. Sep. Sci. 2016, 39, 473–482.
- Xie, J.; Zhang, Y.; Wang, W.; Hou, J. Identification and Simultaneous Determination of Glycyrrhizin, Formononetin, Glycyrrhetinic Acid, Liquiritin, Isoliquiritigenin, and Licochalcone A in Licorice by LC-MS/MS. Acta Chromatogr. 2014, 26, 507–516.
- Kondo, K.; Shiba, M.; Nakamura, R.; Morota, T.; Shoyama, Y. Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information. Biol. Pharm. Bull. 2007, 30, 1271–1277.
- Song, W.; Qiao, X.; Chen, K.; Wang, Y.; Ji, S.; Feng, J.; Li, K.; Lin, Y.; Ye, M. Biosynthesis-Based Quantitative Analysis of 151 Secondary Metabolites of Licorice to Differentiate Medicinal Glycyrrhiza Species and Their Hybrids. Anal. Chem. 2017, 89, 3146–3153.
- Li, G.; Nikolic, D.; van Breemen, R.B. Identification and Chemical Standardization of Licorice Raw Materials and Dietary Supplements Using UHPLC-MS/MS. J. Agric. Food Chem. 2016, 64, 8062–8070.
- Nomura, T.; Fukai, T. Phenolic constituents of licorice (Glycyrrhiza species). Fortschr. Chem. Org. Nat. 1998, 73, 1–140.
- Yang, R.; Li, W.; Yuan, B.; Ren, G.; Wang, L.; Cheng, T.; Liu, Y. The genetic and chemical diversity in three original plants of licorice, Glycyrriza uralensis Fisch., Glycyrrhiza inflata Bat. and Glycyrrhiza glabra L. Pak. J. Pharm. Sci. 2018, 31, 525–535.
- Qiao, X.; Liu, C.F.; Ji, S.; Lin, X.H.; Guo, D.A.; Ye, M. Simultaneous determination of five minor coumarins and flavonoids in Glycyrrhiza uralensis by solid-phase extraction and high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Planta Med. 2014, 80, 237–242.
- Rizzato, G.; Scalabrin, E.; Radaelli, M.; Capodaglio, G.; Piccolo, O. A new exploration of licorice metabolome. Food Chem. 2017, 221, 959–968.
- Yang, R.; Li, W.D.; Yuan, B.C.; Ma, Y.; Zhou, S.; Liu, C.S.; Liu, Y. Simultaneous determination of 18 α-glycyrrhizic acid and 18 β-glycyrrhizic acid in three licorice samples from different origin by HPLC. Pharm. Anal. 2016, 36, 1065–1071.
- Liao, W.C.; Lin, Y.H.; Chang, T.M.; Huang, W.Y. Identification of two licorice species, Glycyrrhiza uralensis and Glycyrrhiza glabra, based on separation and identification of their bioactive components. Food Chem. 2012, 132, 2188–2193.
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC-MS, LC-MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72.
- Wei, L.; Song, X.B.; Sun, C.R.; Xia, Q. Content determination of polysaccharides in Radix Glycyrrhizae from three different species. Tianjin J. Tradit. Chin. Med. 2013, 30, 47–49.
- Zhao, L.; Cheng, Z.M.; Shu-Yong, M.U.; Zhu, J.W.; Pan, H.X. Content of Glycyrrhizic Acid and Polysaccharide of Cultivated Glycyrrhiza Root. Arid Land Geogr. 2005, 28, 843–848.
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071.
- Li, J.Y.; Cao, H.Y.; Liu, P.; Cheng, G.H.; Sun, M.Y. Glycyrrhizic acid in the treatment of liver diseases: Literature review. Biomed. Res. Int. 2014, 2014, 872139.
- Shin, Y.W.; Bae, E.A.; Lee, B.; Lee, S.H.; Kim, J.A.; Kim, Y.S.; Kim, D.H. In vitro and in vivo antiallergic effects of Glycyrrhiza glabra and its components. Planta. Med. 2007, 73, 257–261.
- Fuhrman, B.; Volkova, N.; Kaplan, M.; Presser, D.; Attias, J.; Hayek, T.; Aviram, M. Antiatherosclerotic effects of licorice extract supplementation on hypercholesterolemic patients: Increased resistance of LDL to atherogenic modifications, reduced plasma lipid levels, and decreased systolic blood pressure. Nutrition 2002, 18, 268–273.
- Hajiaghamohammadi, A.A.; Zargar, A.; Oveisi, S.; Samimi, R.; Reisian, S. To evaluate of the effect of adding licorice to the standard treatment regimen of Helicobacter pylori. Braz. J. Infect. Dis. 2016, 20, 534–538.
- Madisch, A.; Holtmann, G.; Mayr, G.; Vinson, B.; Hotz, J. Treatment of functional dyspepsia with a herbal preparation. A double-blind, randomized, placebo-controlled, multicenter trial. Digestion 2004, 69, 45–52.
- Chigurupati, H.; Auddy, B.; Biyani, M.; Stohs, S.J. Hepatoprotective Effects of a Proprietary Glycyrrhizin Product during Alcohol Consumption: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Phytother. Res. 2016, 30, 1943–1953.
- Najafi, S.; Koujan, S.E.; Manifar, S.; Kharazifard, M.J.; Kidi, S.; Hajheidary, S. Preventive Effect of Glycyrrhiza Glabra Extract on Oral Mucositis in Patients Under Head and Neck Radiotherapy: A Randomized Clinical Trial. J. Dent. 2017, 14, 267–274.
- Mamgain, R.K.; Gupta, M.; Mamgain, P.; Verma, S.K.; Pruthi, D.S.; Kandwal, A.; Saini, S. The efficacy of an ayurvedic preparation of yashtimadhu (Glycyrrhiza glabra) on radiation-induced mucositis in head-and-neck cancer patients: A pilot study. J. Cancer Res. Ther. 2020, 16, 458–462.
- Pakravan, F.; Salehabad, N.H.; Karimi, F.; Isfahani, M.N. Comparative Study of the Effect of Licorice Muco-adhesive Film on Radiotherapy Induced Oral Mucositis, A Randomized Controlled Clinical Trial. Gulf J. Oncol. 2021, 1, 42–47.
- Ruetzler, K.; Fleck, M.; Nabecker, S.; Pinter, K.; Landskron, G.; Lassnigg, A.; You, J.; Sessler, D.I. A randomized, double-blind comparison of licorice versus sugar-water gargle for prevention of postoperative sore throat and postextubation coughing. Anesth. Analg. 2013, 117, 614–621.
- Zhang, Y.H.; Isobe, K.; Nagase, F.; Lwin, T.; Kato, M.; Hamaguchi, M.; Yokochi, T.; Nakashima, I. Glycyrrhizin as a promoter of the late signal transduction for interleukin-2 production by splenic lymphocytes. Immunology 1993, 79, 528–534.
- Ojima, M.; Satoh, K.; Gomibuchi, T.; Itoh, N.; Kin, S.; Fukuchi, S.; Miyachi, Y. The inhibitory effects of glycyrrhizin and glycyrrhetinic acid on the metabolism of cortisol and prednisolone--in vivo and in vitro studies. Nihon Naibunpi Gakkai Zasshi 1990, 66, 584–596.
- Yoh, T.; Nakashima, T.; Sumida, Y.; Kakisaka, Y.; Nakajima, Y.; Ishikawa, H.; Sakamoto, Y.; Okanoue, T.; Mitsuyoshi, H. Effects of glycyrrhizin on glucocorticoid signaling pathway in hepatocytes. Dig. Dis. Sci. 2002, 47, 1775–1781.
- James, B. The Use of Liquorice in Weight Reduction. Lancet 1956, 268, 996.
- Armanini, D.; Nacamulli, D.; Francini-Pesenti, F.; Battagin, G.; Ragazzi, E.; Fiore, C. Glycyrrhetinic acid, the active principle of licorice, can reduce the thickness of subcutaneous thigh fat through topical application. Steroids 2005, 70, 538–542.
- Beskina, O.A.; Abramov, A.; Gabdulkhakova, A.G.; Miller, A.V.; Safronova, V.G.; Zamaraeva, M.V. Possible mechanisms of antioxidant activity of glycyrrhizic acid. Biomed. Khim. 2006, 52, 60–68.
- Armanini, D.; De Palo, C.B.; Mattarello, M.J.; Spinella, P.; Zaccaria, M.; Ermolao, A.; Palermo, M.; Fiore, C.; Sartorato, P.; Francini-Pesenti, F.; et al. Effect of licorice on the reduction of body fat mass in healthy subjects. J. Endocrinol. Investig. 2003, 26, 646–650.
- Ju, S.M.; Kim, M.S.; Jo, Y.S.; Jeon, Y.M.; Bae, J.S.; Pae, H.O.; Jeon, B.H. Licorice and its active compound glycyrrhizic acid ameliorates cisplatin-induced nephrotoxicity through inactivation of p53 by scavenging ROS and overexpression of p21 in human renal proximal tubular epithelial cells. Eur. Rev. Med. Pharm. Sci. 2017, 21, 890–899.
- Wu, C.H.; Chen, A.Z.; Yen, G.C. Protective Effects of Glycyrrhizic Acid and 18β-Glycyrrhetinic Acid against Cisplatin-Induced Nephrotoxicity in BALB/c Mice. J. Agric. Food Chem. 2015, 63, 1200–1209.
- Kim, M.; Park, S.C.; Lee, D.Y. Glycyrrhizin as a Nitric Oxide Regulator in Cancer Chemotherapy. Cancers 2021, 13, 5762.
- Zeeshan, M.; Atiq, A.; Ain, Q.U.; Ali, J.; Khan, S.; Ali, H. Evaluating the mucoprotective effects of glycyrrhizic acid-loaded polymeric nanoparticles in a murine model of 5-fluorouracil-induced intestinal mucositis via suppression of inflammatory mediators and oxidative stress. Inflammopharmacology 2021, 29, 1539–1553.
- Deng, Q.P.; Wang, M.J.; Zeng, X.; Chen, G.G.; Huang, R.Y. Effects of Glycyrrhizin in a Mouse Model of Lung Adenocarcinoma. Cell Physiol. Biochem. 2017, 41, 1383–1392.
- Wakamatsu, T.; Nakahashi, Y.; Hachimine, D.; Seki, T.; Okazaki, K. The combination of glycyrrhizin and lamivudine can reverse the cisplatin resistance in hepatocellular carcinoma cells through inhibition of multidrug resistance-associated proteins. Int. J. Oncol. 2007, 31, 1465–1472.
- Zhu, X.; Cong, J.; Lin, Z.; Sun, J.; Yang, B.; Li, A. Inhibition of HMGB1 Overcomes Resistance to Radiation and Chemotherapy in Nasopharyngeal Carcinoma. Onco Targets Ther. 2020, 13, 4189–4199.
- Kabe, Y.; Koike, I.; Yamamoto, T.; Hirai, M.; Kanai, A.; Furuhata, R.; Tsugawa, H.; Harada, E.; Sugase, K.; Hanadate, K.; et al. Glycyrrhizin Derivatives Suppress Cancer Chemoresistance by Inhibiting Progesterone Receptor Membrane Component 1. Cancers 2021, 13, 3265.
- Shi, L.; Tang, C.; Yin, C. Glycyrrhizin-modified O-carboxymethyl chitosan nanoparticles as drug vehicles targeting hepatocellular carcinoma. Biomaterials 2012, 33, 7594–7604.
- Tian, G.; Pan, R.; Zhang, B.; Qu, M.; Lian, B.; Jiang, H.; Gao, Z.; Wu, J. Liver-Targeted Combination Therapy Basing on Glycyrrhizic Acid-Modified DSPE-PEG-PEI Nanoparticles for Co-delivery of Doxorubicin and Bcl-2 siRNA. Front. Pharmacol. 2019, 10, 4.
- Wang, Q.S.; Gao, L.N.; Zhu, X.N.; Zhang, Y.; Zhang, C.N.; Xu, D.; Cui, Y.L. Co-delivery of glycyrrhizin and doxorubicin by alginate nanogel particles attenuates the activation of macrophage and enhances the therapeutic efficacy for hepatocellular carcinoma. Theranostics 2019, 9, 6239–6255.
- Honda, H.; Nagai, Y.; Matsunaga, T.; Saitoh, S.; Akashi-Takamura, S.; Hayashi, H.; Fujii, I.; Miyake, K.; Muraguchi, A.; Takatsu, K. Glycyrrhizin and isoliquiritigenin suppress the LPS sensor toll-like receptor 4/MD-2 complex signaling in a different manner. J. Leukoc. Biol. 2012, 91, 967–976.
- Wang, K.L.; Yu, Y.C.; Hsia, S.M. Perspectives on the Role of Isoliquiritigenin in Cancer. Cancers 2021, 13, 115.
- Lee, C.K.; Son, S.H.; Park, K.K.; Park, J.H.; Lim, S.S.; Chung, W.Y. Isoliquiritigenin inhibits tumor growth and protects the kidney and liver against chemotherapy-induced toxicity in a mouse xenograft model of colon carcinoma. J. Pharm. Sci. 2008, 106, 444–451.
- Gómez-Sierra, T.; Medina-Campos, O.N.; Solano, J.D.; Ibarra-Rubio, M.E.; Pedraza-Chaverri, J. Isoliquiritigenin Pretreatment Induces Endoplasmic Reticulum Stress-Mediated Hormesis and Attenuates Cisplatin-Induced Oxidative Stress and Damage in LLC-PK1 Cells. Molecules 2020, 25, 4442.
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Kim, S.; Lee, Y.M.; Koh, Y.S.; Kim, Y.H. Flavonoids from Astragalus membranaceus and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch. Pharm. Res. 2014, 37, 186–192.
- Lv, X.; Zhu, Y.; Deng, Y.; Zhang, S.; Zhang, Q.; Zhao, B.; Li, G. Glycyrrhizin improved autophagy flux via HMGB1-dependent Akt/mTOR signaling pathway to prevent Doxorubicin-induced cardiotoxicity. Toxicology 2020, 441, 152508.
- Lei, X.; Hu, X.; Zhang, T.; Zhang, J.; Wu, C.; Hong, W.; Jiang, Y.; Wang, Q.; Xie, Y.; Zhao, Y.; et al. HMGB1 release promotes paclitaxel resistance in castration-resistant prostate cancer cells via activating c-Myc expression. Cell. Signal. 2020, 72, 109631.
- Hisaoka-Nakashima, K.; Tomimura, Y.; Yoshii, T.; Ohata, K.; Takada, N.; Zhang, F.F.; Nakamura, Y.; Liu, K.; Wake, H.; Nishibori, M.; et al. High-mobility group box 1-mediated microglial activation induces anxiodepressive-like behaviors in mice with neuropathic pain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 347–362.
- Cao, Z.Y.; Liu, Y.Z.; Li, J.M.; Ruan, Y.M.; Yan, W.J.; Zhong, S.Y.; Zhang, T.; Liu, L.L.; Wu, R.; Wang, B.; et al. Glycyrrhizic acid as an adjunctive treatment for depression through anti-inflammation: A randomized placebo-controlled clinical trial. J. Affect. Disord. 2020, 265, 247–254.
- Ren, C.A.; Li, Y.X.; Cui, J.Y.; Sheng, Z.X.; Ran, X.H.; Wang, B.H.; Zhang, M.H. Efficacy of glycyrrhizin combined with cyclosporine in the treatment of non-severe aplastic anemia. Chin. Med. J. 2013, 126, 2083–2086.
- Patricia Moreno-Londoño, A.; Bello-Alvarez, C.; Pedraza-Chaverri, J. Isoliquiritigenin pretreatment attenuates cisplatin induced proximal tubular cells (LLC-PK1) death and enhances the toxicity induced by this drug in bladder cancer T24 cell line. Food Chem. Toxicol. 2017, 109, 143–154.
- Hu, F.W.; Yu, C.C.; Hsieh, P.L.; Liao, Y.W.; Lu, M.Y.; Chu, P.M. Targeting oral cancer stemness and chemoresistance by isoliquiritigenin-mediated GRP78 regulation. Oncotarget 2017, 8, 93912–93923.
- Alshangiti, A.M.; Togher, K.L.; Hegarty, S.V.; Sullivan, A.M.; O’Keeffe, G.W. The dietary flavonoid isoliquiritigenin is a potent cytotoxin for human neuroblastoma cells. Neuronal Signal. 2019, 3, NS20180201.
- Rui-Zhi, T.; Ke-Huan, X.; Yuan, L.; Xiao, L.; Bing-Wen, Z.; Tong-Tong, L.; Li, W. Renoprotective effect of isoliquiritigenin on cisplatin-induced acute kidney injury through inhibition of FPR2 in macrophage. J. Pharm. Sci. 2022, 148, 56–64.
- Jin, H.; Seo, G.S.; Lee, S.H. Isoliquiritigenin-mediated p62/SQSTM1 induction regulates apoptotic potential through attenuation of caspase-8 activation in colorectal cancer cells. Eur. J. Pharm. 2018, 841, 90–97.
- Yamazaki, S.; Morita, T.; Endo, H.; Hamamoto, T.; Baba, M.; Joichi, Y.; Kaneko, S.; Okada, Y.; Okuyama, T.; Nishino, H.; et al. Isoliquiritigenin suppresses pulmonary metastasis of mouse renal cell carcinoma. Cancer Lett. 2002, 183, 23–30.
- Al-Qahtani, W.H.; Alshammari, G.M.; Ajarem, J.S.; Al-Zahrani, A.Y.; Alzuwaydi, A.; Eid, R.; Yahya, M.A. Isoliquiritigenin prevents Doxorubicin-induced hepatic damage in rats by upregulating and activating SIRT1. Biomed. Pharm. 2022, 146, 112594.
- Zhou, J.X.; Wink, M. Reversal of Multidrug Resistance in Human Colon Cancer and Human Leukemia Cells by Three Plant Extracts and Their Major Secondary Metabolites. Medicines 2018, 5, 123.
- Youns, M.; Fu, Y.J.; Zu, Y.G.; Kramer, A.; Konkimalla, V.B.; Radlwimmer, B.; Sültmann, H.; Efferth, T. Sensitivity and resistance towards isoliquiritigenin, doxorubicin and methotrexate in T cell acute lymphoblastic leukaemia cell lines by pharmacogenomics. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010, 382, 221–234.
More
Information
Subjects:
Integrative & Complementary Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
941
Revisions:
3 times
(View History)
Update Date:
14 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




