Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Priyanka Gupta | -- | 4098 | 2022-06-21 15:24:37 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gupta, P.; Adhikari, A. Environmental Monitoring and Control of Listeria monocytogenes. Encyclopedia. Available online: https://encyclopedia.pub/entry/25021 (accessed on 07 February 2026).
Gupta P, Adhikari A. Environmental Monitoring and Control of Listeria monocytogenes. Encyclopedia. Available at: https://encyclopedia.pub/entry/25021. Accessed February 07, 2026.
Gupta, Priyanka, Achyut Adhikari. "Environmental Monitoring and Control of Listeria monocytogenes" Encyclopedia, https://encyclopedia.pub/entry/25021 (accessed February 07, 2026).
Gupta, P., & Adhikari, A. (2022, July 11). Environmental Monitoring and Control of Listeria monocytogenes. In Encyclopedia. https://encyclopedia.pub/entry/25021
Gupta, Priyanka and Achyut Adhikari. "Environmental Monitoring and Control of Listeria monocytogenes." Encyclopedia. Web. 11 July, 2022.
Copy Citation
Listeria monocytogenes is a serious public health hazard responsible for the foodborne illness listeriosis. L. monocytogenes is ubiquitous in nature and can become established in food production facilities, resulting in the contamination of a variety of food products, especially ready-to-eat foods. Effective and risk-based environmental monitoring programs and control strategies are essential to eliminate L. monocytogenes in food production environments.
Listeria monocytogenes
environmental monitoring programs
control methods
1. Introduction
Listeria monocytogenes continues to be a significant cause of foodborne illnesses. L. monocytogenes is a motile, facultative anaerobic, gram-positive, non-spore forming, rod-shaped bacteria which thrives between −0.4 °C to 50 °C [1]. L. monocytogenes was first described in 1926 by Murray et al. during investigating infected laboratory guinea pigs and rabbits [2], it was not until the 1980s that it was considered a serious public health hazard and a foodborne pathogen [3]. The bacteria occur ubiquitously in nature and has been found to be widely present in surface water, soil, plants, silage, sewage, slaughterhouse waste, and cow milk [4]. Its ability to thrive in various environmental stresses, such as low pH, high salt concentration, and low temperature, favors it as a foodborne pathogen [4].
Listeriosis is a serious foodborne illness caused by this pathogen, especially in susceptible populations, including children, pregnant women, the elderly, and individuals with compromised immune systems [5]. The symptoms of listeriosis include mild flu-like infection to severe cases of invasive infection, in which the bacteria spread from intestines to the blood, causing bloodstream infection, or central nervous system infection, causing meningitis and encephalitis [5]. In pregnant women, the infection may get transmitted from mother to neonate, causing spontaneous abortion or the birth of a premature infant with meningitis. The Centers for Disease Control and Prevention (CDC) estimates that listeriosis is the third leading cause of death from foodborne illnesses, with a fatality rate of 20 to 30% [6]. Epidemiological studies reveal that out of 13 identified serotypes (1/2a, 1/2b, 1/2c, 3a, 3b, 3c, 4a, 4ab, 4b, 4c, 4d, 4e and 7) of L. monocytogenes, three serotypes 1/2a, 1/2b, and 4b account for 90% of human listeriosis cases [7].
2. L. monocytogenes in Food Production Environments
The prevalence of L. monocytogenes in food production environments has been identified as a cause of many listeriosis outbreaks. For example, a listeriosis outbreak in 2011 that was linked to cantaloupe was originated from the food production environment [8]. Similarly, in 2015, the listeriosis outbreak linked to ice cream was also found to have originated in the food production environment [9]. L. monocytogenes may enter the food production environments through different routes, such as incoming raw material, equipment, employee activity, air flow, traffic flow, soil, water, and vegetation. The prevalence of L. monocytogenes in a food production environment depends on several factors, including the type of food, processing method, incoming raw material, the effectiveness of cleaning and sanitation protocols, the sanitary design of equipment and facilities, and employee training [10]. Many studies have demonstrated that some strains of L. monocytogenes, once entered into the food production environment, are not completely inactivated by cleaning and sanitation processes and persist for months or years in that environment [11][12]. Studies have used different molecular subtyping methods such as amplified fragment length polymorphism (AFLP), pulsed-field gel electrophoresis (PFGE), and whole genome sequence (WGS) to find persistent strains that are repeatedly isolated from a food production environment over a period of time [13][14][15][16][17][18][19][20], and some of these studies are summarized in Table 1.
Table 1. Examples of studies demonstrating that some L. monocytogenes strains persist in food processing environments over an extended period of time.
| Food Product | Time of Persistence | Serotypes | Country | Linked to Outbreak? | References |
|---|---|---|---|---|---|
| Bulk milk | 7 months | 1/2a | United States | No | [14] |
| Cold-smoked salmon | 9 months | 1/2a | France | No | [15] |
| Goat cheese | 11 months | 4b | United Kingdom | Yes | [17] |
| Pork | 1 year | Several | France | No | [18] |
| Salmon, seatrout, and their products | 1 year | 4 | Poland | No | [19] |
| Soft cheese | 5 years | ND | United States | Yes | [21] |
| Ice cream | 7 years | 1/2b | Finland | No | [20] |
| ND, not defined | |||||
Persistent L. monocytogenes is difficult to eliminate because it is present in a “niche” within the facility or equipment that can be difficult to clean and sanitize [12]. Such niches include cracks and crevices in different parts of the facility and equipment, such as floors, walls, drains, pipes, conveyors, mixers, slicers, freezers, condensers, gaskets, trollies, packaging machines, and so forth. These are the locations which are difficult to reach and where food particles and microorganisms tend to harbor. The persistence of L. monocytogenes in harborage sites depends on the efficacy of cleaning and sanitation process and the number of cells prior to and after cleaning and sanitation [16]. For example, if the reduction in the number of bacterial cells in harborage site after cleaning and sanitation is less than the increase in the number of cells due to growth, the bacterial strain persists in the harborage site. Conversely, transient strains of L. monocytogenes are removed with normal cleaning and sanitation and do not persist in a food production environment. Even with good cleaning and sanitation practices, transient strains may appear from time to time in an establishment and may be detected occasionally through testing.
Several scientists have questioned the relationship between the persistence of L. monocytogenes and its ability to form biofilms [22][23]. However, both concepts are not fully understood and require more in-depth discussion. Biofilms have been defined as the population of microbial cells adherent to each other and/or to the surfaces by producing a three-dimensional extracellular matrix [24][25]. The formation of biofilms occurs in sequential steps [26][27]: (1) attachment of planktonic bacteria to solid surface by electrostatic forces, van der Waals forces, and hydrophobic interactions, (2) proliferation of cells and formation of extracellular polymeric substances, (3) construction of multilayer cellular clusters with channels for the flow of nutrients and waste, and (4) biofilm formation and cell dispersion for subsequent colonization on other surfaces. L. monocytogenes is able to adhere to a variety of surfaces, including stainless steel, glass, propylene, rubber, quartz, marble, granite, and food surfaces such as chicken skin and beef surfaces [28][29][30]. L. monocytogenes in biofilms is protected from a variety of environmental stresses, such as UV light, desiccation, acids, and toxic metals, and may survive antimicrobial and sanitizing agents such as iodine, chlorine, and quaternary ammonium compounds [31]. For example, Russo et al. (2018) found that sodium hypochlorite (200 ppm, v/v), hydrogen peroxide (2%, v/v), and benzalkonium chloride (200 ppm, w/v) were not able to completely eradicate established biofilms in experimental conditions [31]. The study also suggested that subminimal concentrations of antimicrobial and sanitizing compounds may encourage the growth of the resistant population of L. monocytogenes. Some studies have shown that persistent strains show biofilm formation [23], while other studies have found no relationship between persistence and biofilm formation [22].
L. monocytogenes can be separated into lineages using different genotypic and phenotypic approaches. Initially, L. monocytogenes isolates were distinguished into two lineages using multi locus enzyme electrophoresis (MLEE) and PFGE [32][33][34][35], and later three genetic lineages were defined based on the sequences of virulence genes, ribotyping, and genomic microarrays [36][37][38][39][40][41][42][43]. As per Nadon et al., 2001, lineage I includes serotypes 1/2b, 3b, 3c, and 4b, lineage II includes serotypes 1/2a, 3a, and 1/2c, and lineage III includes 4a and 4c [38]. Lineage I and lineage II isolates have been frequently isolated from food production environments, although some studies have reported a higher frequency of lineage II isolates [44][45][46]. One of the reasons for a higher prevalence of lineage II can be that lineage II can outcompete lineage I isolates if they are present in the same environment [47][48][49]. Lineage III isolates are rarely found in food production environments. Some studies have reported the relationship between certain lineages of L. monocytogenes and the ability to form biofilms [22], while some studies have reported no relationship between lineages and the ability to form biofilms [50][51].
3. Novel Approaches to Control of L. monocytogenes
Different listericidal and listeriostatic approaches can be applied to control L. monocytogenes in foods and food production environments. Several conventional approaches, such as pasteurization, sterilization, freezing, chilling, acidification, fermentation, drying, filtration, antimicrobial agents, and additives have been used to control L. monocytogenes growth in foods. However, some of these approaches are harsh and adversely impact foods’ nutritional and sensory attributes [52]. In recent years, consumers have become more interested in buying food products that are minimally processed, free of additives, shelf-stable, and have a better nutritional and sensory value [53]. In order to fulfill these requirements, several novel control strategies have emerged recently. Figure 1 shows some novel approaches to control L. monocytogenes in foods and food production environments, based on thermal, non-thermal, biocontrol, natural, and chemical methods. Generally, a single approach is not effective in controlling L. monocytogenes, and a combination of approaches is required, also known as hurdle technology. For example, irradiation can be an effective approach to controlling L. monocytogenes in foods. However, some cells may survive and grow even after irradiation; hence, using an antimicrobial agent after irradiation may further suppress the growth of L. monocytogenes [54]. This deliberate and judicious combination of control strategies to create a series of hurdles that a microorganism is not able to overcome is called hurdle technology. Several studies have evaluated different hurdle strategies to control L. monocytogenes in food products. For example, Upadhyay et al., 2014 evaluated a combination of four plant-derived antimicrobial compounds (carvacrol, thymol, b-resorcylic acid, and caprylic acid), along with H2O2 and high-temperature treatment to control L. monocytogenes in cantaloupes [55]. In another study, Espina et al., 2014 applied a combination of pulsed electric field (PEF), mild heat, and natural essential oils to inactivate L. monocytogenes in liquid whole egg [56]. The study found that a combination of these techniques was as effective as ultra-pasteurization for killing L. monocytogenes but with a less detrimental impact on the product’s sensory attributes [56]. Combining two or more approaches can produce a synergistic effect by hitting different targets that disturb the homeostasis of microorganisms [57]. Several studies have successfully applied different combinations of conventional and innovative strategies to control L. monocytogenes in foods and food production environments [58][59][60][61][62].
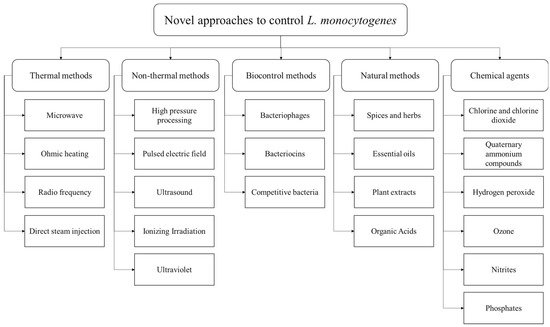
Figure 1. Novel approaches to control L. monocytogenes in food products and food production environments, categorized as thermal methods, non-thermal methods, biocontrol methods, natural methods, and chemical agents.
3.1. Thermal Methods
Conventionally, thermal processing methods such as pasteurization and sterilization are applied to control L. monocytogenes in foods. Direct hot air, steam, heat exchangers, and hot water baths are commonly used for the thermal processing of foods at different temperature-time combinations [63]. The temperature for pasteurization ranges from 60 to 80 °C to kill microorganisms and inactivate enzymes, whereas the temperature for sterilization is >100 °C to kill spores and spore-forming bacteria [64]. D-value is the heating time required to kill 90% of microorganisms or to reduce the microbial concentration by one log. The thermal resistance of microorganisms increases with an increase in the D value [53]. For example, the D-value of L. monocytogenes present in milk samples ranged from 1683.7 s to 0.7 s when milk samples were heated from 52.2 to 74.4 °C [65]. Post-package pasteurization of ready-to-eat foods is gaining recognition as a useful technique to reduce the risk of post-processing contamination of L. monocytogenes in food products [66][67]. Post-package pasteurization or sterilization is done by packing food in the container and heating the container using steam or hot water in a retort, such as a pressure cooker or autoclave [53]. However, not all food products are suitable for high-temperature treatments as they may reduce the organoleptic quality of the product.
Several novel interventions have emerged for precise and rapid heating of foods while maintaining their sensory and nutritional characteristics, such as microwave, radio frequency, ohmic heating, and direct steam injection. In the electromagnetic spectrum, both microwave and radio frequencies belong to non-ionizing radiation, with radio frequencies ranging from 30 to 300 MHz and microwaves ranging from 300 MHz to 300 GHz [68]. The primary mechanism involved in heating with microwave and radiofrequency is dielectric heating, which is based on the interaction of molecules with dipolar nature (e.g., water) and ionic charges in foods with electromagnetic radiation oscillating at a very high frequency [68]. The dielectric system provides non-contact, uniform, and volumetric heating of the product and has been extensively evaluated for its thermal and non-thermal antimicrobial activity. Sung and Kang, 2014 assessed the effectiveness of microwave heating for the inactivation of L. monocytogenes and other pathogenic microorganisms in salsa products [69]. The study found that microwave heating at 915 MHz could be an alternative to pasteurization, as it can kill microorganisms while maintaining the overall quality of the product [69]. Microbial destruction by microwave occurs by denaturation of cellular protein structure, causing rupturing of the cell membrane [53]. Awuah et al., 2005 evaluated the application of radiofrequency in inactivating Listeria in milk and found up to 5-log reduction at 1200 W, 65 °C, and 55.5 s [70]. Radiofrequency and microwave have been applied to control microorganisms in products such as fruit juices, meat products, ready-to-eat products, coconut water, catfish, eggs, and pasta products.
3.2. Non-Thermal Methods
High-pressure processing (HPP) is a novel non-thermal method where a high pressure above 100 MPa is applied to the product using pressurized liquid such as water. HPP causes the inactivation of microorganisms through the mechanism of denaturing cell membrane, unfolding protein structure, changing cell membrane fluidity, ribosome dissociation, leakage of intracellular components, and eventually cell disruption [71]. The effectiveness of HPP in the inactivation of L. monocytogenes depends on parameters such as temperature, applied pressure, holding time, and properties and composition of the food matrix. For example, HPP is a more effective technique in the inactivation of L. monocytogenes in liquid foods than solid foods [72]. HPP may not always cause microbial inactivation and may sub-lethally damage the microbial cells, which may recover later. Therefore, combining HPP with other hurdles can effectively kill L. monocytogenes, for example, Nassau et al., 2017 evaluated the effectiveness of a combination of endolysin with HPP to inactivate L. monocytogenes in a buffer and found that a combination of two techniques could result in a 5-log reduction of L. monocytogenes cells [59]. The study indicated that HPP, when applied individually at 300 MPa for 1 min at 30 °C, could reduce the cell count by only 0.3 log CFU, but when applied in combination with endolysin, it could cause an effective inactivation of L. monocytogenes even at lower pressure levels.
Another alternative non-thermal method for controlling L. monocytogenes is the pulsed electric field (PEF), wherein the inactivation of microorganisms takes place by using high electric field pulses (>18 kV/cm) for a short time [53]. A high voltage pulsed electric field disrupts the bacterial cell membrane, leading to releasing intracellular components and eventually killing the microorganism. Several factors influence the inactivation kinetics of PEF, including electric field strength, pulse length, pulse number, temperature, pH, and conductivity [73]. Gómez et al., 2005 assessed the effectiveness of PEF on the inactivation of L. monocytogenes in media of different pH (3.5–7.0) and found that PEF was more effective at lower pH and higher electric field strengths [74]. At pH 3.5, a treatment of 28 kV/cm for 400 μs was able to reduce 6.0 Log10 cycles of L. monocytogenes cells [74]. In recent years, PEF technology has been explored for controlling L. monocytogenes in various food products such as milk and dairy products, juices, and soups.
Ultrasound is another emerging non-thermal technology for processing food products. High powered ultrasound with a frequency of 20 to 100 kHz is used in food production environments to kill microorganisms through a mechanism called cavitation [75]. In this mechanism, gas bubbles are formed in the liquid medium as a result of sonication, the bubbles expand until a critical point is reached where ultrasonic energy is insufficient to retain the vapor phase of the bubbles, hence the bubbles become unstable and collapse, creating shock waves that damage the bacterial cell wall. Baumann et al., 2009 evaluated the efficacy of ultrasound for the removal of L. monocytogenes biofilms from stainless steel chips and found that power ultrasound (20 kHz, 100% amplitude, 120 W, 60 s) was effective in reducing recoverable cells (3.8 log CFU/mL reduction) [76]. When ultrasonication was combined with ozonation (ozone concentration 0.5 ppm), there were no recoverable cells after the treatment (reduction of 7.31-log CFU/mL) [76]. Ultrasound is an effective technology in food processing and inactivating microorganisms, but it may impact the quality of food products by creating free radicals, off-flavors, and changing the composition of the food matrix.
Ionizing irradiation involves exposing food to radiation which causes ionization on interaction, such as gamma rays (60Co and 137Cs), high energy electron beam, or X-rays. Irradiation can be used for the decontamination of products such as meat, poultry, egg products, fish products, and spices [77]. A study by Bari et al., 2005 indicated that a low dose of ionizing irradiation can be effective in reducing L. monocytogenes on fresh vegetables without significantly changing the color, texture, taste, and appearance of the product [78]. Irradiation in frozen foods allows a higher dose level before developing off-flavor, for example, in frozen poultry, the dose level can be at least two times higher as compared to chilled poultry [79]. In a study by Velasco et al., 2015, electron beam was applied to eliminate L. monocytogenes from soft cheeses [80]. The study found that irradiation was able to reduce the bacterial load, but injured cells were recovered during storage. Combining irradiation with other hurdles can be more effective compared to irradiation alone. For example, Mohamed et al., 2011 found that a combination of gamma radiation and nisin could be effective in eliminating L. monocytogenes in meat products [81]. One advantage of irradiation is that it can be used to treat food in packages to reduce the risk of post-process contamination. An irradiation dose of 30–50 kGy is used to reduce microbial contamination of foods called as radappertization [82]. A high irradiation dose may cause discoloration of the product, and release of radiation-induced off-odors and off-flavors during storage. Therefore, it is important to carefully determine the dose level and apply the minimum possible doses to achieve desired level of control.
Ultraviolet radiation has germicidal effect and is used to eliminate microbial load on surfaces, air, water, and is now approved for microbial reduction in foods and juices [83]. UV-C light (254 nm) is absorbed by most microorganisms which leads to alteration in microbial DNA by dimer formation, limiting the ability of microorganism to multiply and grow. Adhikari et al., 2015 evaluated the effectiveness of UV light for the inactivation of L. monocytogenes on fruit surfaces and found a higher inactivation on fruits with a smoother surface, such as apples (1.6 log CFU/g reduction at 3.75 kJ/m2), as compared to fruits with a rough surface such as cantaloupe (1.0 log CFU/g reduction at 11.9 kJ/m2) [83]. Kim et al., 2002 observed a >5 log reduction of L. monocytogenes on stainless steel after treating with UV-C (500 μW/cm2) for 3 min [84]. Similar observations were made by Sommers et al., 2010, indicating the efficacy of UV-C light for routine decontamination of food-contact surfaces [85].
3.3. Biocontrol Methods
Biocontrol methods against L. monocytogenes include bacteriophages, bacteriocins, and competitive bacteria. Bacteriophages are viruses that infect and kill bacteria for propagation. Bacteriophages are highly specific toward their target bacteria and have no detrimental effect on non-target microbes, which is considered a vital advantage for biocontrol specificity and sensitivity [86]. Bacteriophages can undergo two types of life cycle: lytic and lysogenic. In the lytic cycle, bacteriophage attaches to the bacterial cell, introduces phage DNA into the bacterial cell, utilizes bacterial machinery to encode and assemble new phage particles, and at the end, releases progeny phage into the environment by lysing the bacterial cell. In the lysogenic cycle, the phage genome is integrated into the host DNA and replicates with bacterial DNA. The lysogenic phages (also known as temperate phages) continue to replicate with the host cell until an unfavorable condition occurs, which initiates the lytic cycle. Temperate phages are not suitable for biocontrol as they may not result in host cell death, whereas lytic phages are considered suitable for biocontrol due to their virulence. Bacteriophages are not considered a risk to humans upon consumption due to their high specificity towards host bacterial cells, and consequently, some commercially produced bacteriophages have been recommended as GRAS (generally recognized as safe) by FDA, such as ListShieldTM and ListexTM P100 [86]. Gutiérrez et al., 2017 evaluated the effectiveness of ListShieldTM and ListexTM P100 against L. monocytogenes in biofilms and Spanish dry-cured ham [86]. The study found that both products effectively removed 72-h old biofilm from stainless steel surface after four-hour treatment at 12 °C. Application of ListShieldTM on Spanish dry-cured ham was effective in lysing 100% of strains examined, whereas ListexTM P100 was effective in lysing 64% of strains. The study suggested that these phage-based products can be useful for biocontrol of L. monocytogenes in food production environments. Phages can be applied to food products using different methods, such as spraying and dipping, or by using novel approaches such as immobilization on inert surfaces [87]. An alternative to using whole bacteriophages to control L. monocytogenes can be the application of endolysins. Endolysins are hydrolytic enzymes encoded by phage genome towards the end of lytic cycle to break the bacterial cell wall and release the progeny phages. Endolysins can be recombinantly produced and applied externally to bacterial cells without requiring bacteriophages. Ibarra-Sánchez evaluated the effectiveness of endolysin PlyP100 to control L. monocytogenes in Queso Fresco and compared it with nisin [88]. According to the study, PlyP100 showed bacterial reduction at varying L. monocytogenes inoculum levels, and showed no recovery at inoculum level of 1 log CFU/g. The endolysin was stable for 28 days and showed consistent antilisterial action. Nisin was not as effective as PlyP100 to control L. monocytogenes, however, a combination of the two showed a strong effect with no countable L. monocytogenes cells after 4 weeks of refrigeration [88].
3.4. Natural Methods
The application of natural and plant-derived antimicrobials, such as spices, herbs, essential oil, plant extract, and organic acids, is gaining attention for food preservation as an alternative to chemical preservatives. Natural antimicrobials have several benefits in addition to inhibiting microorganisms, for example, they increase flavor in the food, improve fragrance, improve medicinal value, and improve the nutritional quality of food. Spices and herbs are derived from different parts of the plants, such as clove from flower bud which contains antimicrobial compound eugenol, cinnamon from bark which contains cinnamic aldehyde, turmeric from rhizome which contains curcumin, mustard from seeds which contains allyl isothiocyanate, and thyme and oregano from leaves which contain thymol and carvacrol [89][90]. Numerous studies have evaluated the antimicrobial activities of spices and herbs against L. monocytogenes. Ting and Deibel, 1991 examined 13 species against L. monocytogenes and found that cloves had bactericidal effect and oregano had bacteriostatic effect at 0.5% or 1% concentration at 4 °C and 24 °C [89]. When tested against meat slurry, a 1% concentration of clove or oregano did not have much inhibitory impact on L. monocytogenes [89]. Essential oils are aromatic, volatile oils obtained from different parts of the plants, including flowers, leaves, seeds, buds, roots, bark, woods, fruits, and peels. They are extracted by pressing and distillation or supercritical fluid extraction. Antimicrobial activity of essential oils is due to the presence of different compounds such as terpenes, phenolic compounds, aldehydes, and esters, most of which are classified as GRAS. Studies have indicated that phenolic compounds in essential oils cause a change in the permeability of bacterial cell membrane, intervene in ATP (Adenosine 5′-triphosphate) formation, and disrupt proton motive force [90]. Several different essential oils have been evaluated for their effectiveness against L. monocytogenes. Sandasi et al., 2007 examined the effectiveness of five common essential oils (α-pinene, 1,8-cineole, (+)-limonene, linalool, and geranyl acetate) against biofilms [91]. Morshdy et al., 2021 examined essential oils (cinnamon bark oil, thyme oil, coriander oil, lavender oil, rosemary oil) against L. monocytogenes isolated from fresh retail chicken meat and found that cinnamon bark oil showed the highest antilisterial activity [92]. Studies have indicated that gram-positive bacteria are more sensitive to essential oils [90]. Essential oils due to their hydrophobic nature can easily pass through the cell wall of gram-positive bacteria, whereas the outer membrane of gram-negative bacteria possesses hydrophilic nature and limits the diffusion of essential oils [90]. One main disadvantage of using essential oils as antimicrobial agents is the production of strong aromas and off-flavors that can be undesirable in some food products.
3.5. Chemical Agents
In food production facilities, cleaning and sanitation are important steps to eliminate microorganisms and dirt from food-contact surfaces, equipment, floors, and walls. Various chemical agents, such as chlorine, chlorine dioxide, hydrogen peroxide, quaternary ammonium compounds, ozone, nitrites, and phosphates are used for cleaning and sanitation. Aqueous chlorine is an effective agent to control microbial growth; however, its antimicrobial activity decreases in alkaline conditions and leads to the formation of toxic reaction products such as chloramines and trihalomethanes (THMs) [93]. Chlorine dioxide (ClO2) is used as an alternative to chlorine, as it is more potent (~2 times oxidation capacity) in killing bacteria and is not affected by alkaline conditions and organic compounds. Researchers have investigated the application of ClO2 gas to disinfect several food products and food-contact surfaces [94][95][96]. ClO2 has more penetrability than aqueous ClO2 and has a better reach to microorganisms hidden in surface irregularities and biofilms [94]. Trinetta et al., 2013 evaluated the effectiveness of high-concentration short-time ClO2 for treating fresh produce and suggested it can be a useful technique for sanitizing produce in large-scale operations [97]. Luu et al., 2021 indicated that treatment with ClO2 gas (<5 mg/L) in gas permeable sachets could effectively reduce L. monocytogenes on strawberries and blueberries [98]. Results suggest that ClO2 gas has potential as a sanitizer for food processing; however, there can be economic and operational constraints in using this method on a large scale.
References
- Lilliana Radoshevich; Pascale Cossart; Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nature Reviews Genetics 2017, 16, 32-46, 10.1038/nrmicro.2017.126.
- E. G. D. Murray; R. A. Webb; M. B. R. Swann; A disease of rabbits characterised by a large mononuclear leucocytosis, caused by a hitherto undescribed bacillusBacterium monocytogenes (n.sp.). The Journal of Pathology and Bacteriology 1925, 29, 407-439, 10.1002/path.1700290409.
- Walter F. Schlech; Pierre M. Lavigne; Robert A. Bortolussi; Alexander C. Allen; E. Vanora Haldane; A. John Wort; Allen W. Hightower; Scott E. Johnson; Stanley H. King; Eric S. Nicholls; et al.Claire V. Broome Epidemic Listeriosis — Evidence for Transmission by Food. New England Journal of Medicine 1983, 308, 203-206, 10.1056/nejm198301273080407.
- Vengadesh Letchumanan; Peh-Chee Wong; Bey-Hing Goh; Long Chiau Ming; Priyia Pusparajah; Sunny Hei Wong; Nurul-Syakima Ab Mutalib; Learn-Han Lee; A review on the characteristics, taxanomy and prevalence of Listeria monocytogenes. Progress In Microbes & Molecular Biology 2018, 1, -, 10.36877/pmmb.a0000007.
- Caroline Charlier; Olivier Disson; Marc Lecuit; Maternal-neonatal listeriosis. Virulence 2020, 11, 391-397, 10.1080/21505594.2020.1759287.
- CDC. Listeria (Listeriosis). . CDC. Listeria (Listeriosis). . Retrieved 2022-7-11
- R. B. Tompkin; Control of Listeria monocytogenes in the Food-Processing Environment. Journal of Food Protection 2002, 65, 709-725, 10.4315/0362-028x-65.4.709.
- Outbreak of Listeriosis Linked to Whole Cantaloupes from Jensen Farms, Colorado (Final Update) . CDC. Retrieved 2022-7-11
- Multistate Outbreak of Listeriosis Linked to Blue Bell Creameries Products (Final Update) . CDC. Retrieved 2022-7-11
- Tobin Simonetti; Kari Peter; Yi Chen; Qing Jin; Guodong Zhang; Luke F. LaBorde; Dumitru Macarisin; Prevalence and Distribution of Listeria monocytogenes in Three Commercial Tree Fruit Packinghouses. Frontiers in Microbiology 2021, 12, -, 10.3389/fmicb.2021.652708.
- Carlo Spanu; Kieran Jordan; Listeria monocytogenes environmental sampling program in ready‐to‐eat processing facilities: A practical approach. Comprehensive Reviews in Food Science and Food Safety 2020, 19, 2843-2861, 10.1111/1541-4337.12619.
- Alexandra Belias; Genevieve Sullivan; Martin Wiedmann; Renata Ivanek; Factors that contribute to persistent Listeria in food processing facilities and relevant interventions: A rapid review. Food Control 2021, 133, 108579, 10.1016/j.foodcont.2021.108579.
- Tiina Autio; Riikka Keto-Timonen; Janne Lundén; Johanna Bjorkroth; Hannu Korkeala; Characterisation of Persistent and Sporadic Listeria monocytogenes Strains by Pulsed-Field Gel Electrophoresis (PFGE) and Amplified Fragment Length Polymorphism (AFLP). Systematic and Applied Microbiology 2003, 26, 539-545, 10.1078/072320203770865846.
- Wayne Muraoka; Clive Gay; Donald Knowles; Monica Borucki; Prevalence of Listeria monocytogenes Subtypes in Bulk Milk of the Pacific Northwest. Journal of Food Protection 2003, 66, 1413-1419, 10.4315/0362-028x-66.8.1413.
- G. Dauphin; C. Ragimbeau; P. Malle; Use of PFGE typing for tracing contamination with Listeria monocytogenes in three cold-smoked salmon processing plants. International Journal of Food Microbiology 2001, 64, 51-61, 10.1016/s0168-1605(00)00442-6.
- Brigitte Carpentier; Olivier Cerf; Review — Persistence of Listeria monocytogenes in food industry equipment and premises. International Journal of Food Microbiology 2011, 145, 1-8, 10.1016/j.ijfoodmicro.2011.01.005.
- J. McLauchlin; Melody H. Greenwood; Pia N. Pini; The occurrence of Listeria monocytogenes in cheese from a manufacturer associated with a case of listeriosis. International Journal of Food Microbiology 1990, 10, 255-262, 10.1016/0168-1605(90)90073-e.
- I Giovannacci; C Ragimbeau; S Queguiner; G Salvat; J.-L Vendeuvre; V Carlier; G Ermel; Listeria monocytogenes in pork slaughtering and cutting plants: use of RAPD, PFGE and PCR–REA for tracing and molecular epidemiology. International Journal of Food Microbiology 1999, 53, 127-140, 10.1016/s0168-1605(99)00141-5.
- Dagmara Mędrala; Waldemar Dąbrowski; Urszula Czekajło-Kołodziej; Elżbieta Daczkowska-Kozon; Anna Koronkiewicz; Ewa Augustynowicz; Marisa Manzano; Persistence of Listeria monocytogenes strains isolated from products in a Polish fish-processing plant over a 1-year period. Food Microbiology 2003, 20, 715-724, 10.1016/s0740-0020(02)00173-9.
- M K Miettinen; Characterization of Listeria monocytogenes from an ice cream plant by serotyping and pulsed-field gel electrophoresis. International Journal of Food Microbiology 1999, 46, 187-192, 10.1016/s0168-1605(98)00185-8.
- Listeria (Listeriosis)—Multistate Outbreak of Listeriosis Linked to Soft Cheeses Distributed by Karoun Dairies, Inc. (Final Update). . CDC. Retrieved 2022-7-11
- D. Djordjevic; M. Wiedmann; L. A. McLandsborough; Microtiter Plate Assay for Assessment of Listeria monocytogenes Biofilm Formation. Applied and Environmental Microbiology 2002, 68, 2950-2958, 10.1128/aem.68.6.2950-2958.2002.
- Janne M. Lundén; Maria K. Miettinen; Tiina J. Autio; Hannu Korkeala; Persistent Listeria monocytogenes Strains Show Enhanced Adherence to Food Contact Surface after Short Contact Times. Journal of Food Protection 2000, 63, 1204-1207, 10.4315/0362-028x-63.9.1204.
- Rodney M. Donlan; Biofilms: Microbial Life on Surfaces. Emerging Infectious Diseases 2002, 8, 881-890, 10.3201/eid0809.020063.
- Andrew G. Moltz; Scott E. Martin; Formation of Biofilms by Listeria monocytogenes under Various Growth Conditions. Journal of Food Protection 2004, 68, 92-97, 10.4315/0362-028x-68.1.92.
- Iñigo Lasa; Towards the identification of the common features of bacterial biofilm development.. International Microbiology 2006, 9, 21-8.
- Eliane Pereira da Silva; Elaine Cristina Pereira De Martinis; Current knowledge and perspectives on biofilm formation: the case of Listeria monocytogenes. Applied Microbiology and Biotechnology 2012, 97, 957-968, 10.1007/s00253-012-4611-1.
- Tingting Gu; Apisak Meesrisom; Yaguang Luo; Quynh N. Dinh; Sophia Lin; ManYun Yang; Arnav Sharma; Ruogu Tang; Jinde Zhang; Zhen Jia; et al.Patricia D. MillnerArne J. PearlsteinBoce Zhang Listeria monocytogenes biofilm formation as affected by stainless steel surface topography and coating composition. Food Control 2021, 130, 108275, 10.1016/j.foodcont.2021.108275.
- Xianming Shi; Xinna Zhu; Biofilm formation and food safety in food industries. Trends in Food Science & Technology 2009, 20, 407-413, 10.1016/j.tifs.2009.01.054.
- Sónia Silva; Pilar Teixeira; Rosário Oliveira; Joana Azeredo; Adhesion to and Viability of Listeria monocytogenes on Food Contact Surfaces. Journal of Food Protection 2008, 71, 1379-1385, 10.4315/0362-028x-71.7.1379.
- Pasquale Russo; Agni Hadjilouka; Luciano Beneduce; Vittorio Capozzi; Spiros Paramithiotis; Eleftherios H. Drosinos; Giuseppe Spano; Effect of different conditions on Listeria monocytogenes biofilm formation and removal. Czech Journal of Food Sciences 2018, 36, 208-214, 10.17221/199/2017-cjfs.
- J C Piffaretti; H Kressebuch; M Aeschbacher; J Bille; E Bannerman; J M Musser; R K Selander; J Rocourt; Genetic characterization of clones of the bacterium Listeria monocytogenes causing epidemic disease.. Proceedings of the National Academy of Sciences 1989, 86, 3818-3822, 10.1073/pnas.86.10.3818.
- William F. Bibb; Benjamin Schwartz; Bruce G. Gellin; Brian D. Plikaytis; Robert E. Weaver; Analysis of Listeria monocytogenes by multilocus enzyme electrophoresis and application of the method to epidemiologic investigations. International Journal of Food Microbiology 1989, 8, 233-239, 10.1016/0168-1605(89)90018-4.
- O F Rasmussen; T Beck; J E Olsen; L Dons; L Rossen; Listeria monocytogenes isolates can be classified into two major types according to the sequence of the listeriolysin gene.. Infection and Immunity 1991, 59, 3945-51.
- R Brosch; J Chen; J B Luchansky; Pulsed-field fingerprinting of listeriae: identification of genomic divisions for Listeria monocytogenes and their correlation with serovar.. Applied and Environmental Microbiology 1994, 60, 2584-92.
- Ole F. Rasmussen; Pernille Skouboe; Lone Dons; Lone Rossen; John E. Olsen; Listeria monocytogenes exists in at least three evolutionary lines: evidence from flagellin, invasive associated protein and listeriolysin O genes. Microbiology 1995, 141, 2053-2061, 10.1099/13500872-141-9-2053.
- M Wiedmann; J L Bruce; C Keating; A E Johnson; P L McDonough; C A Batt; Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential.. Infection and Immunity 1997, 65, 2707-16.
- C. A. Nadon; D. L. Woodward; C. Young; F. G. Rodgers; M. Wiedmann; Correlations between Molecular Subtyping and Serotyping of Listeria monocytogenes. Journal of Clinical Microbiology 2001, 39, 2704-2707, 10.1128/jcm.39.7.2704-2707.2001.
- Monica K. Borucki; So Hyun Kim; Douglas R. Call; Sandra C. Smole; Franco Pagotto; Selective Discrimination of Listeria monocytogenes Epidemic Strains by a Mixed-Genome DNA Microarray Compared to Discrimination by Pulsed-Field Gel Electrophoresis, Ribotyping, and Multilocus Sequence Typing. Journal of Clinical Microbiology 2004, 42, 5270-5276, 10.1128/jcm.42.11.5270-5276.2004.
- Douglas R. Call; Monica K. Borucki; Thomas E. Besser; Mixed-Genome Microarrays Reveal Multiple Serotype and Lineage-Specific Differences among Strains of Listeria monocytogenes. Journal of Clinical Microbiology 2003, 41, 632-639, 10.1128/jcm.41.2.632-639.2003.
- Michel Doumith; Christel Cazalet; Natalie Simoes; Lionel Frangeul; Christine Jacquet; Frank Kunst; Paul Martin; Pascale Cossart; Philippe Glaser; Carmen Buchrieser; et al. New Aspects Regarding Evolution and Virulence of Listeria monocytogenes Revealed by Comparative Genomics and DNA Arrays. Infection and Immunity 2004, 72, 1072-1083, 10.1128/iai.72.2.1072-1083.2004.
- Chaomei Zhang; Min Zhang; Jingliang Ju; Joseph Nietfeldt; John Wise; Philip M. Terry; Michael Olson; Stephen D. Kachman; Martin Wiedmann; Mansour Samadpour; et al.Andrew K. Benson Genome Diversification in Phylogenetic Lineages I and II of Listeria monocytogenes : Identification of Segments Unique to Lineage II Populations. Journal of Bacteriology 2003, 185, 5573-5584, 10.1128/jb.185.18.5573-5584.2003.
- Kendra Nightingale; Liselle Bovell; Ashley Grajczyk; Martin Wiedmann; Combined sigB allelic typing and multiplex PCR provide improved discriminatory power and reliability for Listeria monocytogenes molecular serotyping. Journal of Microbiological Methods 2007, 68, 52-59, 10.1016/j.mimet.2006.06.005.
- Domenico Meloni; Pietro Galluzzo; Anna Mureddu; Francesca Piras; Mansel Griffiths; Rina Mazzette; Listeria monocytogenes in RTE foods marketed in Italy: Prevalence and automated EcoRI ribotyping of the isolates. International Journal of Food Microbiology 2009, 129, 166-173, 10.1016/j.ijfoodmicro.2008.11.014.
- Dawn M. Norton; Janet M. Scarlett; Kelly Horton; David Sue; Joanne Thimothe; Kathryn J. Boor; Martin Wiedmann; Characterization and Pathogenic Potential of Listeria monocytogenes Isolates from the Smoked Fish Industry. Applied and Environmental Microbiology 2001, 67, 646-653, 10.1128/aem.67.2.646-653.2001.
- Deborah Corcoran; David Clancy; Micheál O’Mahony; Kathie Grant; Evelyn Hyland; Noel Shanaghy; Paul Whyte; James McLauchlin; Anne Moloney; Séamus Fanning; et al. Comparison of Listeria monocytogenes strain types in Irish smoked salmon and other foods. International Journal of Hygiene and Environmental Health 2006, 209, 527-534, 10.1016/j.ijheh.2006.06.001.
- Jesper Bartholin Bruhn; Birte Fonnesbech Vogel; Lone Gram; Merete Lunde; Are Halvor Aastveit; Janet Martha Blatny; Ingolf F. Nes; Bias in the Listeria monocytogenes Enrichment Procedure: Lineage 2 Strains Outcompete Lineage 1 Strains in University of Vermont Selective Enrichments. Applied and Environmental Microbiology 2005, 71, 721-727, 10.1128/aem.71.2.961-967.2005.
- Anna C. S. Porto; Laura Wonderling; Jeffrey E. Call; John B. Luchansky; Use of Pulsed-Field Gel Electrophoresis To Monitor a Five-Strain Mixture of Listeria monocytogenes in Frankfurter Packages†. Journal of Food Protection 2003, 66, 1465-1468, 10.4315/0362-028x-66.8.1465.
- Sava Buncic; Sheryl M Avery; Jocelyne Rocourt; Mirjana Dimitrijevic; Can food-related environmental factors induce different behaviour in two key serovars, 4b and 1/2a, of Listeria monocytogenes?. International Journal of Food Microbiology 2001, 65, 201-212, 10.1016/s0168-1605(00)00524-9.
- Min Seok Chae; Heidi Schraft; Lisbeth Truelstrup Hansen; Robert Mackereth; Effects of physicochemical surface characteristics of Listeria monocytogenes strains on attachment to glass. Food Microbiology 2006, 23, 250-259, 10.1016/j.fm.2005.04.004.
- M.L. Kalmokoff; John Austin; X.-D. Wan; G. Sanders; S. Banerjee; J.M. Farber; Adsorption, attachment and biofilm formation among isolates of Listeria monocytogenes using model conditions. Journal of Applied Microbiology 2001, 91, 725-734, 10.1046/j.1365-2672.2001.01419.x.
- Basheer Aaliya; Kappat Valiyapeediyekkal Sunooj; Muhammed Navaf; Plachikkattu Parambil Akhila; Cherakkathodi Sudheesh; Shabir Ahmed Mir; Sarasan Sabu; Abhilash Sasidharan; Moe Theingi Hlaing; Johnsy George; et al. Recent trends in bacterial decontamination of food products by hurdle technology: A synergistic approach using thermal and non-thermal processing techniques. Food Research International 2021, 147, 110514, 10.1016/j.foodres.2021.110514.
- Imran Khan; Charles Nkufi Tango; Sumaira Miskeen; Byong H. Lee; Deog-Hwan Oh; Hurdle technology: A novel approach for enhanced food quality and safety – A review. Food Control 2017, 73, 1426-1444, 10.1016/j.foodcont.2016.11.010.
- Aude Ndoti-Nembe; Khanh Dang Vu; Nicolas Doucet; Monique Lacroix; Effect of combination of essential oils and bacteriocins on the efficacy of gamma radiation againstSalmonellaTyphimurium and Listeria monocytogenes. International Journal of Radiation Biology 2013, 89, 794-800, 10.3109/09553002.2013.797621.
- Abhinav Upadhyay; Indu Upadhyaya; Shankumar Mooyottu; Anup Kollanoor Johny; Kumar Venkitanarayanan; Efficacy of plant-derived compounds combined with hydrogen peroxide as antimicrobial wash and coating treatment for reducing Listeria monocytogenes on cantaloupes. Food Microbiology 2014, 44, 47-53, 10.1016/j.fm.2014.05.005.
- Laura Espina; Silvia Monfort; Ignacio Álvarez; Diego García-Gonzalo; Rafael Pagán; Combination of pulsed electric fields, mild heat and essential oils as an alternative to the ultrapasteurization of liquid whole egg. International Journal of Food Microbiology 2014, 189, 119-125, 10.1016/j.ijfoodmicro.2014.08.002.
- Lothar Leistner; Basic aspects of food preservation by hurdle technology. International Journal of Food Microbiology 2000, 55, 181-186, 10.1016/s0168-1605(00)00161-6.
- F. Noci; Markus Walkling-Ribeiro; D.A. Cronin; D.J. Morgan; James Lyng; Effect of thermosonication, pulsed electric field and their combination on inactivation of Listeria innocua in milk. International Dairy Journal 2009, 19, 30-35, 10.1016/j.idairyj.2008.07.002.
- Tomas J. Van Nassau; Christian Lenz; Anna S. Scherzinger; Rudi F. Vogel; Combination of endolysins and high pressure to inactivate Listeria monocytogenes. Food Microbiology 2017, 68, 81-88, 10.1016/j.fm.2017.06.005.
- Alejandra Orihuel; Julieta Bonacina; María José Vildoza; Elena Bru; Graciela Vignolo; Lucila Saavedra; Silvina Fadda; Biocontrol of Listeria monocytogenes in a meat model using a combination of a bacteriocinogenic strain with curing additives. Food Research International 2018, 107, 289-296, 10.1016/j.foodres.2018.02.043.
- Andrew Chibeu; Louise Agius; Anli Gao; Parviz M. Sabour; Andrew M. Kropinski; S. Balamurugan; Efficacy of bacteriophage LISTEX™P100 combined with chemical antimicrobials in reducing Listeria monocytogenes in cooked turkey and roast beef. International Journal of Food Microbiology 2013, 167, 208-214, 10.1016/j.ijfoodmicro.2013.08.018.
- Abdollah Pirbalouti; Ebrahim Rahimi; Sayed Moosavi; Antimicrobial activity of essential oils of three herbs against Listeria monocytogenes on chicken frankfurters. Acta agriculturae Slovenica 2009, 95, 219-223, 10.2478/v10014-010-0013-1.
- Michael H. Taylor; Hsieh-Chin Tsai; Barbara Rasco; Juming Tang; Mei-Jun Zhu; Stability of Listeria monocytogenes in wheat flour during extended storage and isothermal treatment. Food Control 2018, 91, 434-439, 10.1016/j.foodcont.2018.04.008.
- Filipa V.M. Silva; Paul A. Gibbs; Thermal pasteurization requirements for the inactivation of Salmonella in foods. Food Research International 2012, 45, 695-699, 10.1016/j.foodres.2011.06.018.
- J. G. Bradshaw; J. T. Peeler; J. J. Corwin; J. M. Hunt; J. T. Tierney; E. P. Larkin; R. M. Twedt; Thermal Resistance of Listeria monocytogenes in Milk. Journal of Food Protection 1985, 48, 743-745, 10.4315/0362-028x-48.9.743.
- Ann M. Roering; Rachel K. Wierzba; Anne M. Ihnot; John B. Luchansky; PASTEURIZATION OF VACUUM-SEALED PACKAGES OF SUMMER SAUSAGE INOCULATED WITH LISTERIA MONOCYTOGENES. Journal of Food Safety 1998, 18, 49-56, 10.1111/j.1745-4565.1998.tb00201.x.
- P. M. Muriana; W. Quimby; C. A. Davidson; J. Grooms; Postpackage Pasteurization of Ready-to-Eat Deli Meats by Submersion Heating for Reduction of Listeria monocytogenes†. Journal of Food Protection 2002, 65, 963-969, 10.4315/0362-028x-65.6.963.
- Ashim K. Datta; P. Michael Davidson; Microwave and Radio Frequency Processing. Journal of Food Safety 2000, 65, 32-41, 10.1111/j.1745-4565.2000.tb00616.x.
- Hye-Jung Sung; Dong-Hyun Kang; Effect of a 915 MHz microwave system on inactivation of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in salsa. LWT - Food Science and Technology 2014, 59, 754-759, 10.1016/j.lwt.2014.05.058.
- G.B. Awuah; H.S. Ramaswamy; A. Economides; K. Mallikarjunan; Inactivation of Escherichia coli K-12 and Listeria innocua in milk using radio frequency (RF) heating. Innovative Food Science & Emerging Technologies 2005, 6, 396-402, 10.1016/j.ifset.2005.06.002.
- Akbar Bahrami; Zahra Moaddabdoost Baboli; Keith Schimmel; Seid Mahdi Jafari; Leonard Williams; Efficiency of novel processing technologies for the control of Listeria monocytogenes in food products. Trends in Food Science & Technology 2019, 96, 61-78, 10.1016/j.tifs.2019.12.009.
- Kiera M. Considine; Alan Kelly; Gerald F. Fitzgerald; Colin Hill; Roy D. Sleator; High-pressure processing – effects on microbial food safety and food quality. FEMS Microbiology Letters 2008, 281, 1-9, 10.1111/j.1574-6968.2008.01084.x.
- P C Wouters; N Dutreux; J P Smelt; H L Lelieveld; Effects of pulsed electric fields on inactivation kinetics of Listeria innocua.. Applied and Environmental Microbiology 1999, 65, 5364-71.
- N. Gómez; D. García; I. Álvarez; S. Condón; J. Raso; Modelling inactivation of Listeria monocytogenes by pulsed electric fields in media of different pH. International Journal of Food Microbiology 2005, 103, 199-206, 10.1016/j.ijfoodmicro.2004.11.033.
- P Piyasena; E Mohareb; R.C McKellar; Inactivation of microbes using ultrasound: a review. International Journal of Food Microbiology 2003, 87, 207-216, 10.1016/s0168-1605(03)00075-8.
- Adam R. Baumann; Scott E. Martin; Hao Feng; Removal of Listeria monocytogenes Biofilms from Stainless Steel by Use of Ultrasound and Ozone. Journal of Food Protection 2009, 72, 1306-1309, 10.4315/0362-028x-72.6.1306.
- Muhammad Tanveer Munir; Michel Federighi; Control of Foodborne Biological Hazards by Ionizing Radiations. Foods 2020, 9, 878, 10.3390/foods9070878.
- M. L. Bari; M. Nakauma; S. Todoriki; Vijay K. Juneja; K. Isshiki; S. Kawamoto; Effectiveness of Irradiation Treatments in Inactivating Listeria monocytogenes on Fresh Vegetables at Refrigeration Temperature†. Journal of Food Protection 2005, 68, 318-323, 10.4315/0362-028x-68.2.318.
- J Farkas; Irradiation as a method for decontaminating food: A review. International Journal of Food Microbiology 1998, 44, 189-204, 10.1016/s0168-1605(98)00132-9.
- Raquel Velasco; Juan A. Ordóñez; Use of E-beam radiation to eliminate Listeria monocytogenes from surface mould cheese. International Microbiology 2014, 18, 33-40, 10.2436/20.1501.01.232.
- Hussein Mohamed; Fathi A. Elnawawi; Ahmed E. Yousef; Nisin Treatment To Enhance the Efficacy of Gamma Radiation against Listeria monocytogenes on Meat. Journal of Food Protection 2011, 74, 193-199, 10.4315/0362-028x.jfp-10-288.
- Rahman Alfarobbi; Nadya Anggraini; Preservation of Foodstuffs with Gamma Ray Irradiation Technology for Decreasing Pathogen Bacteria on Food and Maintain Sustainable Food Security: A Review. SSRN Electronic Journal 2018, ., ., 10.2139/ssrn.3201078.
- Achyut Adhikari; Roopesh M. Syamaladevi; Karen Killinger; Shyam S. Sablani; Ultraviolet-C light inactivation of Escherichia coli O157:H7 and Listeria monocytogenes on organic fruit surfaces. International Journal of Food Microbiology 2015, 210, 136-142, 10.1016/j.ijfoodmicro.2015.06.018.
- T. Kim; J. L. Silva; T. C. Chen; Effects of UV Irradiation on Selected Pathogens in Peptone Water and on Stainless Steel and Chicken Meat. Journal of Food Protection 2002, 65, 1142-1145, 10.4315/0362-028x-65.7.1142.
- Christopher H. Sommers; Joseph E. Sites; Michael Musgrove; ULTRAVIOLET LIGHT (254 NM) INACTIVATION OF PATHOGENS ON FOODS AND STAINLESS STEEL SURFACES. Journal of Food Safety 2010, 30, 470-479, 10.1111/j.1745-4565.2010.00220.x.
- Diana Gutiérrez; Lorena Rodriguez-Rubio; Lucía Fernández; Beatriz Martínez; Ana Rodríguez; Pilar García; Applicability of commercial phage-based products against Listeria monocytogenes for improvement of food safety in Spanish dry-cured ham and food contact surfaces. Food Control 2017, 73, 1474-1482, 10.1016/j.foodcont.2016.11.007.
- H. Anany; W. Chen; R. Pelton; M. W. Griffiths; Biocontrol of Listeria monocytogenes and Escherichia coli O157:H7 in Meat by Using Phages Immobilized on Modified Cellulose Membranes. Applied and Environmental Microbiology 2011, 77, 6379-6387, 10.1128/aem.05493-11.
- Luis A. Ibarra-Sánchez; Maxwell L. Van Tassell; Michael J. Miller; Antimicrobial behavior of phage endolysin PlyP100 and its synergy with nisin to control Listeria monocytogenes in Queso Fresco. Food Microbiology 2018, 72, 128-134, 10.1016/j.fm.2017.11.013.
- W.T. Evert Ting; Kurt E. Deibel; SENSITIVITY OF LISTERIA MONOCYTOGENES TO SPICES AT TWO TEMPERATURES. Journal of Food Safety 1991, 12, 129-137, 10.1111/j.1745-4565.1991.tb00071.x.
- Mojtaba Yousefi; Nasim Khorshidian; Hedayat Hosseini; Potential Application of Essential Oils for Mitigation of Listeria monocytogenes in Meat and Poultry Products. Frontiers in Nutrition 2020, 7, ., 10.3389/fnut.2020.577287.
- M. Sandasi; C.M. Leonard; Alvaro Viljoen; The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control 2008, 19, 1070-1075, 10.1016/j.foodcont.2007.11.006.
- Alaa Eldin M. A. Morshdy; Mohammed S. Al-Mogbel; Mohamed E. M. Mohamed; Mohamed Tharwat Elabbasy; Azza K. Elshafee; Mohamed A. Hussein; Bioactivity of Essential Oils for Mitigation of Listeria monocytogenes Isolated from Fresh Retail Chicken Meat. Foods 2021, 10, 3006, 10.3390/foods10123006.
- Alicia Marín; Juan A. Tudela; Yolanda Garrido; Sofía Albolafio; Natalia Hernández; Silvia Andújar; Ana Allende; Maria I. Gil; Chlorinated wash water and pH regulators affect chlorine gas emission and disinfection by-products. Innovative Food Science & Emerging Technologies 2020, 66, 102533, 10.1016/j.ifset.2020.102533.
- Kaye V. Sy; Melinda B. Murray; M. David Harrison; Larry R. Beuchat; Evaluation of Gaseous Chlorine Dioxide as a Sanitizer for Killing Salmonella, Escherichia coli O157:H7, Listeria monocytogenes, and Yeasts and Molds on Fresh and Fresh-Cut Produce. Journal of Food Protection 2005, 68, 1176-1187, 10.4315/0362-028x-68.6.1176.
- Valentina Trinetta; Richa Vaid; Qin Xu; Richard Linton; Mark Morgan; Inactivation of Listeria monocytogenes on ready-to-eat food processing equipment by chlorine dioxide gas. Food Control 2012, 26, 357-362, 10.1016/j.foodcont.2012.02.008.
- Xiuxiu Sun; Jinhe Bai; Christopher Ference; Zhe Wang; Yifan Zhang; Jan Narciso; KeQuan Zhou; Antimicrobial Activity of Controlled-Release Chlorine Dioxide Gas on Fresh Blueberries†. Journal of Food Protection 2014, 77, 1127-1132, 10.4315/0362-028x.jfp-13-554.
- V. Trinetta; R.H. Linton; M.T. Morgan; The application of high-concentration short-time chlorine dioxide treatment for selected specialty crops including Roma tomatoes (Lycopersicon esculentum), cantaloupes (Cucumis melo ssp. melo var. cantaloupensis) and strawberries (Fragaria×ananassa). Food Microbiology 2013, 34, 296-302, 10.1016/j.fm.2012.12.010.
- Phillip Luu; Vijay Singh Chhetri; Marlene E. Janes; Joan M. King; Achyut Adhikari; Efficacy of gaseous chlorine dioxide in reducing Salmonella enterica, E. coli O157:H7, and Listeria monocytogenes on strawberries and blueberries. LWT 2021, 141, 110906, 10.1016/j.lwt.2021.110906.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
909
Revision:
1 time
(View History)
Update Date:
12 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




