Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Srimanta Manna | -- | 1285 | 2022-07-11 13:15:42 | | | |
| 2 | Conner Chen | Meta information modification | 1285 | 2022-07-12 02:46:15 | | | | |
| 3 | Conner Chen | Meta information modification | 1285 | 2022-07-14 08:37:35 | | | | |
| 4 | Srimanta Manna | + 2 word(s) | 1287 | 2022-07-17 17:50:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Manna, S. Copper-Catalyzed Intramolecular Borylative Coupling with Imines. Encyclopedia. Available online: https://encyclopedia.pub/entry/25007 (accessed on 07 February 2026).
Manna S. Copper-Catalyzed Intramolecular Borylative Coupling with Imines. Encyclopedia. Available at: https://encyclopedia.pub/entry/25007. Accessed February 07, 2026.
Manna, Srimanta. "Copper-Catalyzed Intramolecular Borylative Coupling with Imines" Encyclopedia, https://encyclopedia.pub/entry/25007 (accessed February 07, 2026).
Manna, S. (2022, July 11). Copper-Catalyzed Intramolecular Borylative Coupling with Imines. In Encyclopedia. https://encyclopedia.pub/entry/25007
Manna, Srimanta. "Copper-Catalyzed Intramolecular Borylative Coupling with Imines." Encyclopedia. Web. 11 July, 2022.
Copy Citation
Copper-catalyzed enantioselective borylative cyclization with various electrophiles via difunctionalization of unsaturated hydrocarbons is a powerful tool for the generation of interesting boron-containing carbocycles and heterocycles processes involving a chiral organocopper intermediate. Alkenes, allenes, and alkynes are versatile and easily accessible substrates that can be subjected to a wide range of reactions to produce densely functionalized, enantioenriched products.
copper catalysis
enantioselective
borylation
1. Introduction
Copper-catalyzed diastereo- and enantioselective borylative coupling represent a powerful tool to build complex, functionalized molecular architecture rapidly and efficiently from simple precursors in modern organic chemistry [1][2][3][4][5]. These methods have found a sustainable future in catalytic reaction for exploring new regions of chemical space including step- and atom-economical processes [6]. In the past few decades, numerous transition metal catalysts have been utilized for the functionalization of alkenes [7][8], alkynes [9], allenes, [10] and many other hydrocarbons [11] for dense functionalization of the desired chemical reaction delivering high-value, multifunctionalized, enantioenriched, stereodefined products [12]. In recent years, copper catalysts have been shown to achieve this goal in this field because they are more abundant and less toxic than other noble transition metal catalysts [13][14]. Copper-catalyzed functionalization of unsaturated hydrocarbons has proven to be an effective method [15]. Indeed, a wide range of unsaturated hydrocarbons is suitable for access to functionalized organocopper reagents, resulting in products with widely disubstituted functional molecules [16]. Alkenes, alkynes, and enynes, in particular, are extremely versatile and robust feedstocks. These chemicals have been used in a variety of effective catalytic applications to produce a wide range of useful densely bifunctionalized products. In particular, an enantioselective intramolecular borylative cyclization of a diverse range of hydrocarbons with various electrophiles such as carbonyl, imine, and electron-withdrawing alkene for the construction of boron-containing carbocycles and heterocycles using copper catalysis is emerging as a key theme in modern synthesis [10][15][17]. Since the first report of copper-catalyzed borylative intramolecular cyclization of allylic carbonates by Ito and Sawamura [18], the cyclization reaction has grown in popularity and been extensively studied [19].
2. Copper-Catalyzed Intramolecular Borylative Coupling with Imines
2.1. Copper-Catalyzed Intramolecular Borylative Cyclization with Alkenes and Imines
The copper-catalyzed enantioselective borylative cyclization of aldimine derivative of styrenes was disclosed by You and co-workers in 2018, affording 2,3-disubstituted indolines [20]. Following this report, Xiong groups simultaneously developed a similar enantioselective intramolecular borylative coupling of aldimine of 2-aminostyrene derivatives 1 to access boron-containing indolines [21]. A broad range of styrene derivatives reacted smoothly. A general mechanism for borylative reactions was shown in Scheme 1C. Initially, the active species (L1Cu-Bpin) is generated after reaction with B2pin2, ligand and base. Then, the active species (L1Cu-Bpin) react with styrene to furnish an organocopper intermediate 1a, which equilibrium to 1a’ followed by intramolecular cyclization gives boron-containing 2,3-disubstituted indolines. The active catalyst L1Cu-Bpin is regenerated in presence of base and B2pin2. An enantioselective coupling reaction was reported by both groups using (S,S)-Ph-BPE as a chiral ligand. A variety of aryl substituted imines was well tolerated and gave high enantioselectivities (Scheme 1B). This methodology offers a simple way to potentially lead regio-, diastereo-, and enantioselective chiral 2,3-disubstituted indolines with the versatile boron functional group. At nearly the same time, Shen, Xu, and co-workers unveiled another borylative diastereoselective coupling of aldimine of 2-aminostyrene derivatives using dppf L2 as a ligand (Scheme 2) [22]. The ligand dppf was found to be the best for diastereoselective reaction. Borylative indoles were used for further application to access indoline-derived scaffolds. Interestingly, cis-tetrahydroindenoindole was synthesized through a palladium-catalyzed intramolecular Suzuki coupling of 2-bromoaryl part of cis-2,3 disubstituted indolines.
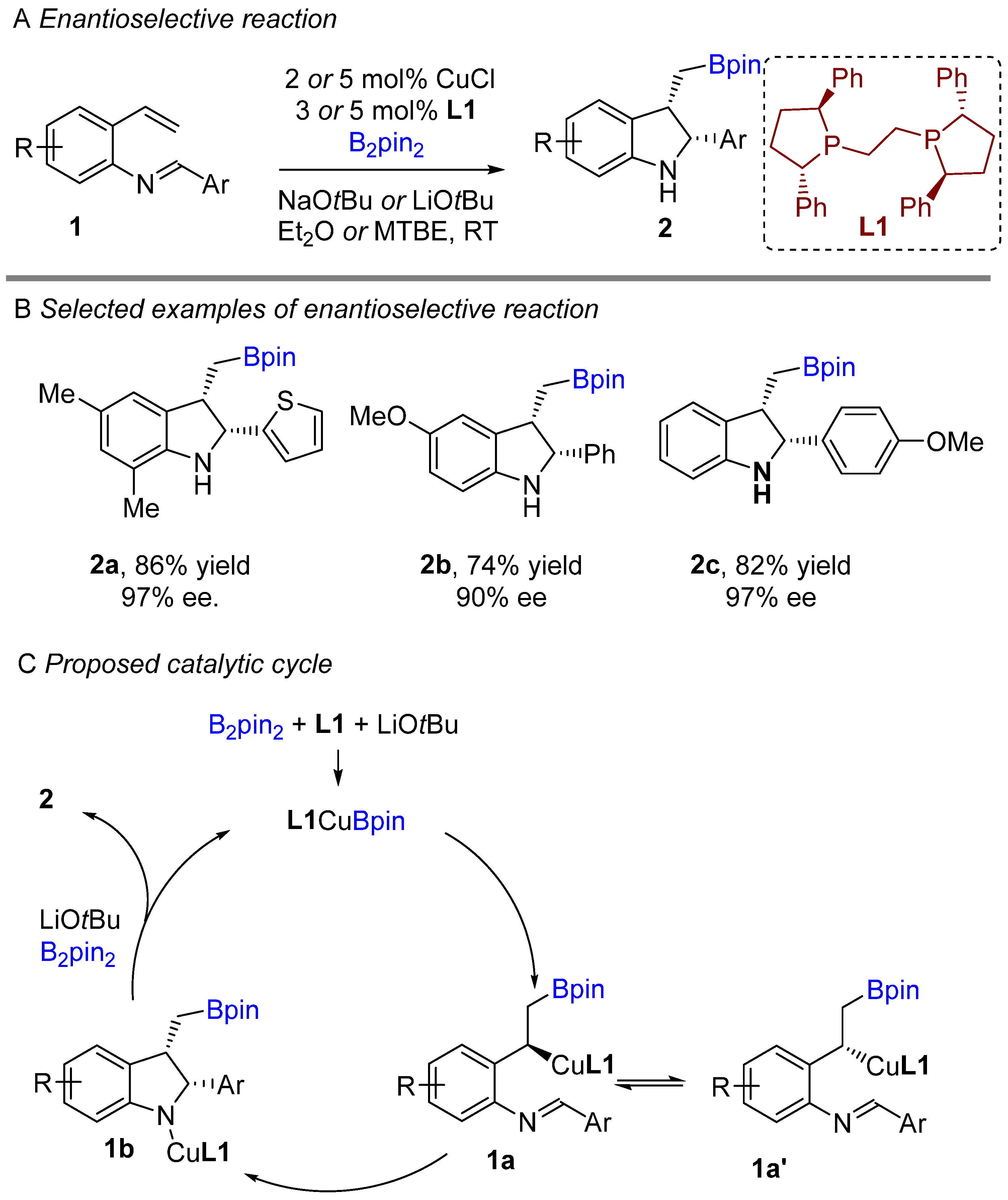
Scheme 1. Copper-catalyzed intramolecular borylative cyclization for the synthesis of 2,3-disubstituted indolines.
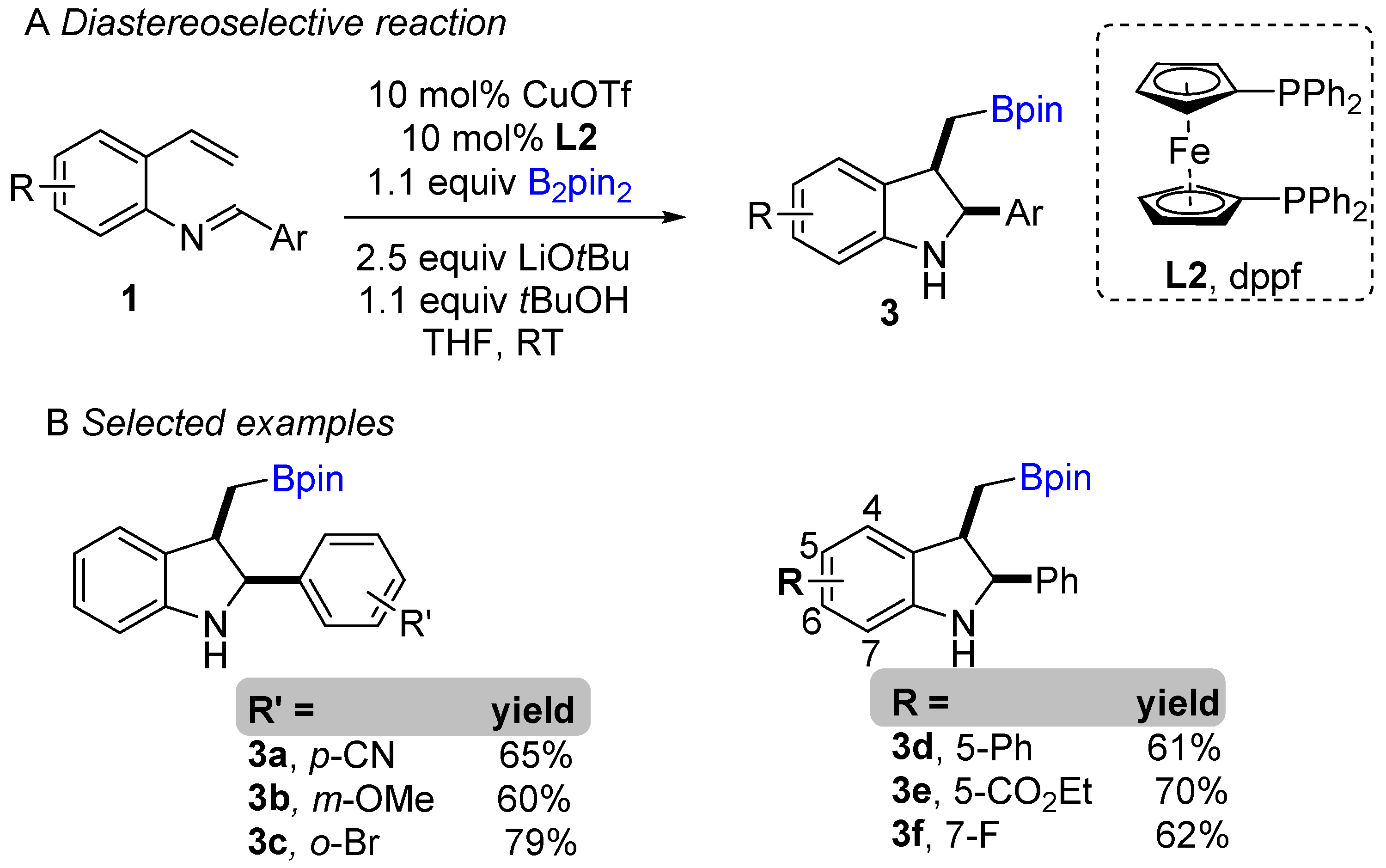
Scheme 2. Copper-catalyzed intramolecular borylative cyclization for the synthesis of 2,3-disubstituted indolines.
2.2. Copper-Catalyzed Intramolecular Borylative Cyclization with 1,3-Dienes and Imines
Following previous works on copper-catalyzed intramolecular cyclization alkenes, the Yun group reported a graceful intramolecular copper-catalyzed borylative coupling of 1-dienylarenes 4 with tethered imines in 2018 (Scheme 3A) [23]. A range of aryl and heterocyclic aldimines as well as various ketimines were well tolerated, providing rapid access to 2,3-disubstituted cis-1-benzo[b]azepines with high diastereoselectivity. Interestingly, a mixture of (E/Z)-dienyl arenes, instead of pure dienyl arene could be used to obtain high diastereoselectivity. Following the racemic reaction, asymmetric reactions were developed using the N-heterocyclic carbene (NHC) ligand (L3). Chiral NHC ligands are well known for copper-catalyzed borylative coupling (Scheme 3C). However, a conceivable drawback with this method is that lower enantioselectivity (41% ee) was achieved after using pure (E)-dienyl arene bearing aldimine ((E)-4). The suggested mechanism proposed by the authors involves the initial formation of the active LCuBpin species by reaction with B2pin2, base and copper salt. Then, the active species undergoes 1,4-borylcupration with the mixture of (E/Z)-1,3-dienes to furnish (Z)-σ-allylcopper intermediate 4a (Scheme 4). The allylcopper 4a attacks the imine intramolecularly, generating an intermediate 4b via a favoured 6-membered ring transition state TS-A with a large group (R3) in the less hindered equatorial position.
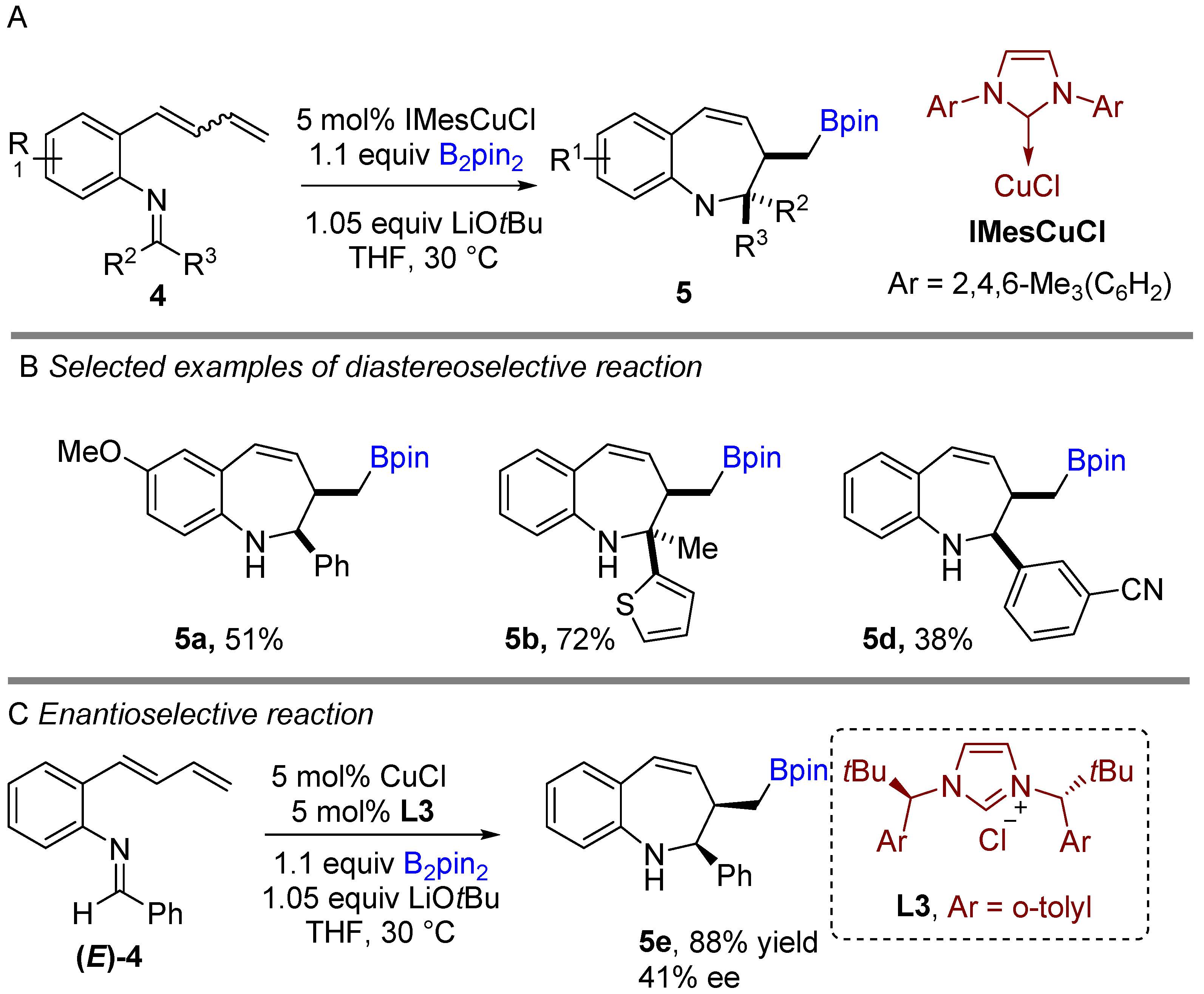
Scheme 3. Copper-catalyzed intramolecular borylative cyclization for the synthesis of 2,3-disubstituted cis-1-benzo[b]azepines.
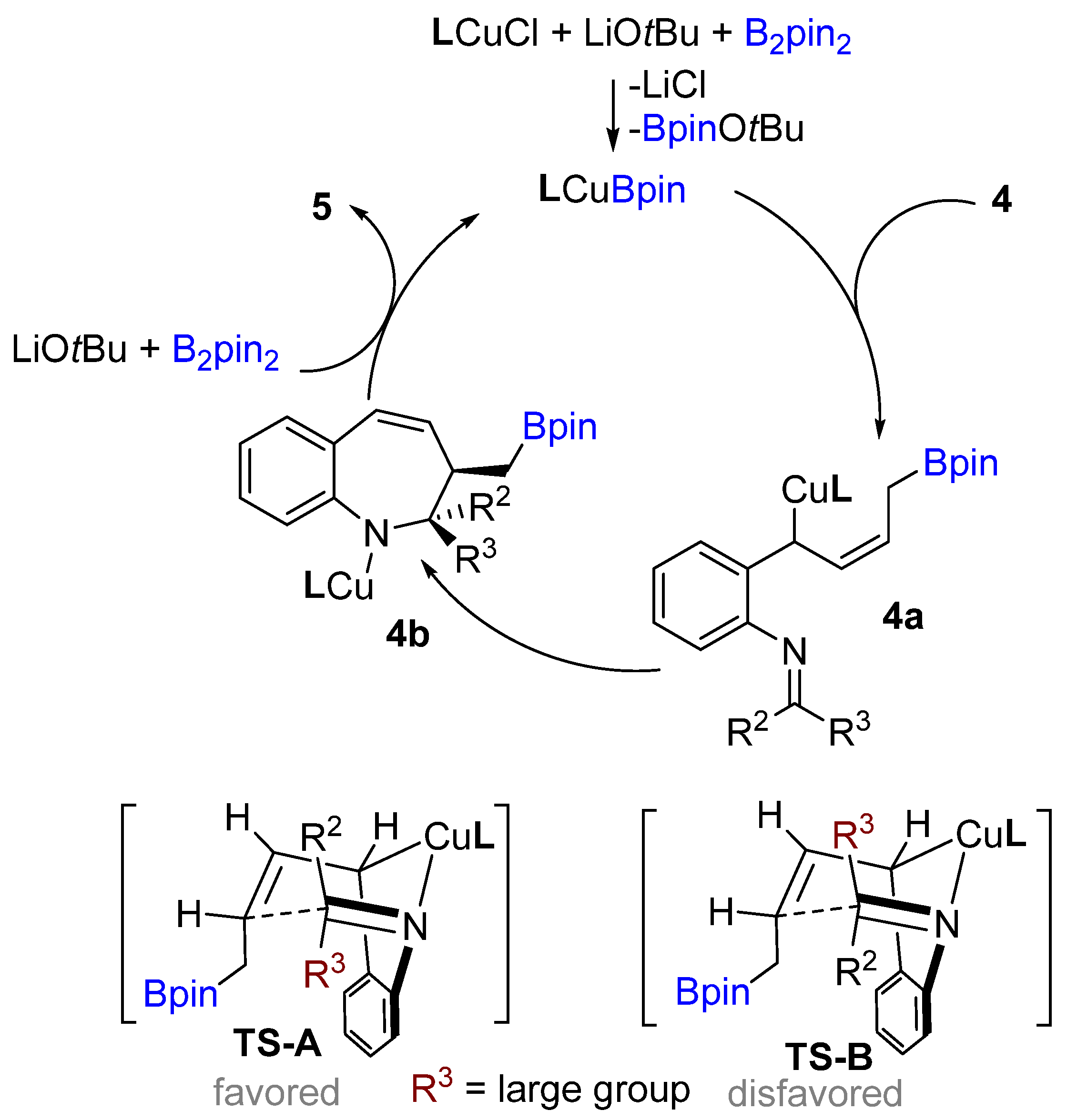
Scheme 4. A. Proposed catalytic cycle.
2.3. Copper-Catalyzed Intramolecular Borylative Cyclization with Conjugated Alkenes and Imines
An enantioselective copper-catalyzed conjugate intramolecular borylation/cyclization of electron-deficient alkenes with aldimines was developed by Lautens and co-workers using bis (pinacolato)diboronreagent (Bi2pin2) as boron sources to produce enantioenriched tetrahydroquinoline scaffolds (Scheme 5A) [24]. Initially, the active species (L4Cu-Bpin) generate in situ after transmetallation of L4Cu-OtBu with B2pin2 followed by 1,4-addition into the alkene to produce organocopper intermediate 5a. The organocopper species then cyclizes with imine to afford borylated tetrahydroquinoline, followed by routine oxidation with NaBO3•4H2O furnish alcohol-containing products. The authors screened several bisphospine ligands. Josiphos proved to be the best ligand in terms of enantio- and diastereoselectivity. This method worked well with a wide range of electron-deficient alkenes and delivered excellent enantioselectivities. Substrates bearing various aryl imines were well tolerated with representative procedures for the preparation of enantioenrich tetrahydroquinoline scaffolds. The established method was further applied to composing a variety of valuable building blocks.
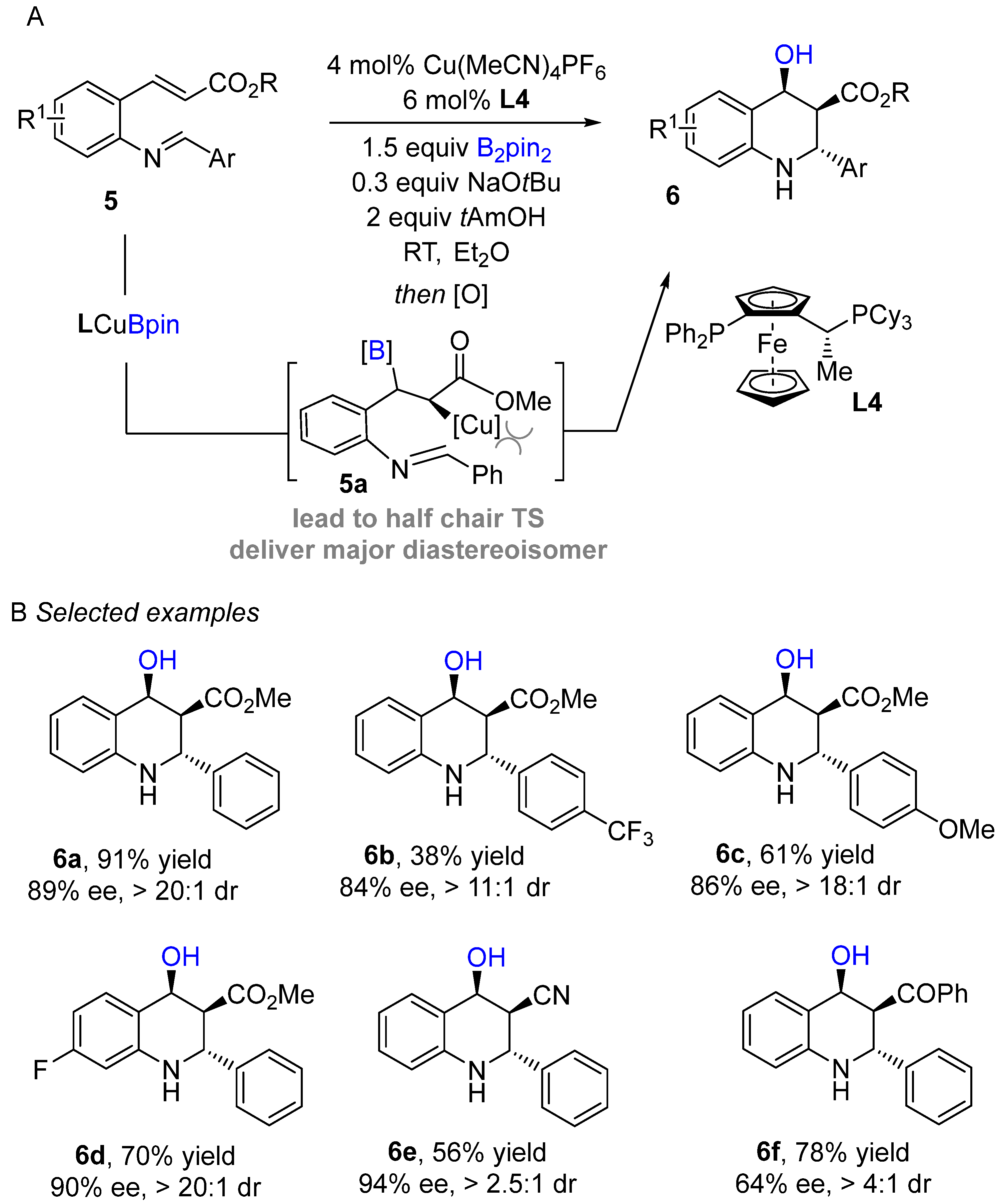
Scheme 5. Copper-catalyzed intramolecular borylation cyclization for the synthesis of tetrahydroquinoline.
2.4. Copper-Catalyzed Intramolecular Borylative Cyclization with Alkenes and Amidines
In 2021, Procter and co-workers demonstrated a copper-catalyzed borylative intramolecular coupling of quinazolinone-bearing alkenes to prepare boron-containing heterocycles (Scheme 6A) [25]. By far, this was the introductory report on enantioselective borylative coupling of aliphatic alkenes as well as substituted styrenes with amidines to obtain boron-bearing quinazoline scaffolds. This method proceeds very smoothly with high enantiocontrol and diastereocontrol. Interestingly, ligand L6 was found to be finest when unsubstituted alkene (R1 H) was used for the coupling reaction. The use of the ligand L5 for intramolecular borylative coupling with aryl-substituted alkenes (R1 aryl) provided excellent diastereo- and enantioselectivities. Various substitutions on the aryl ring of the amidine component were well tolerated, and delivered pyrroloquinazolines featuring quaternary stereocenters with high enantio- and diastereocontrol. Various aryl-substituted alkenes were successfully used in the borylative cyclization and formation of two adjacent stereocentres to yield pyrroloquinazolinones with very good to excellent enantio- and diastereocontrol. This method demonstrates the use of amidine derivatives for the borylative coupling in following transformations to access more riveting complex structures.
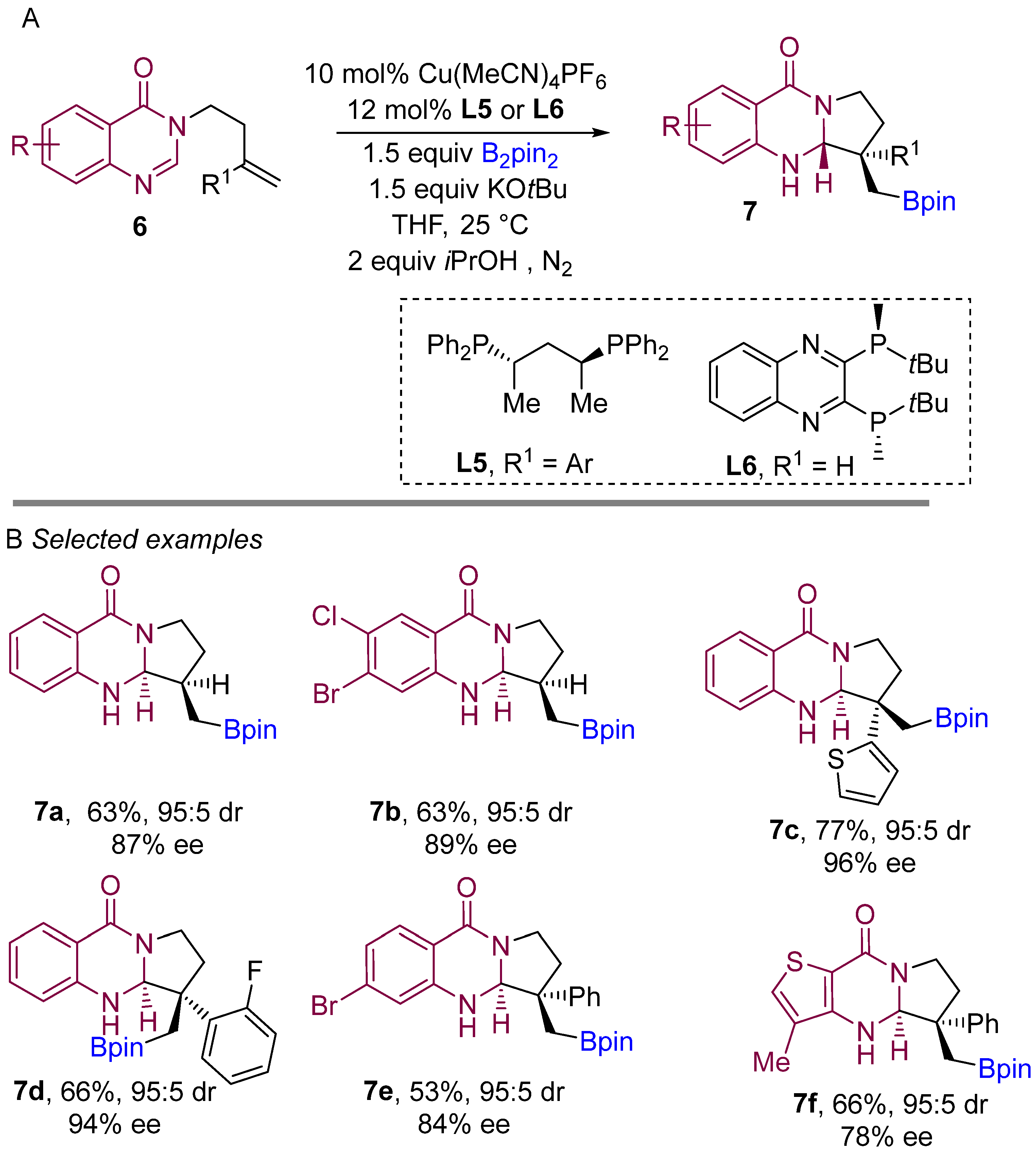
Scheme 6. Copper-catalyzed borylative cyclization for the synthesis of quinazolinones.
According to the proposed mechanism by the authors, an active LCu-Bpin intermediate is formed initially by reacting with B2pin2, base, and copper salt. Then, the active species (LCu-Bpin) undergo enantioselective borocupration across the double bond of the alkene to generate intermediate 6a (Scheme 7). The authors proposed stereochemical models for forming the most favourable enantiomers. Then, the LCu-Bpin intermediate interacts with alkenes in which the phenyl group of the substrate is oriented toward the ligand’s less hindered position, resulting in a major enantiomer A. The intermediate 6b is then formed by diastereoselective cyclization of the amidine carbon-nitrogen double bond via TS-1b. Finally, the active catalyst (LCu-Bpin) is regenerated in the presence of base, alcohol, and B2pin2.
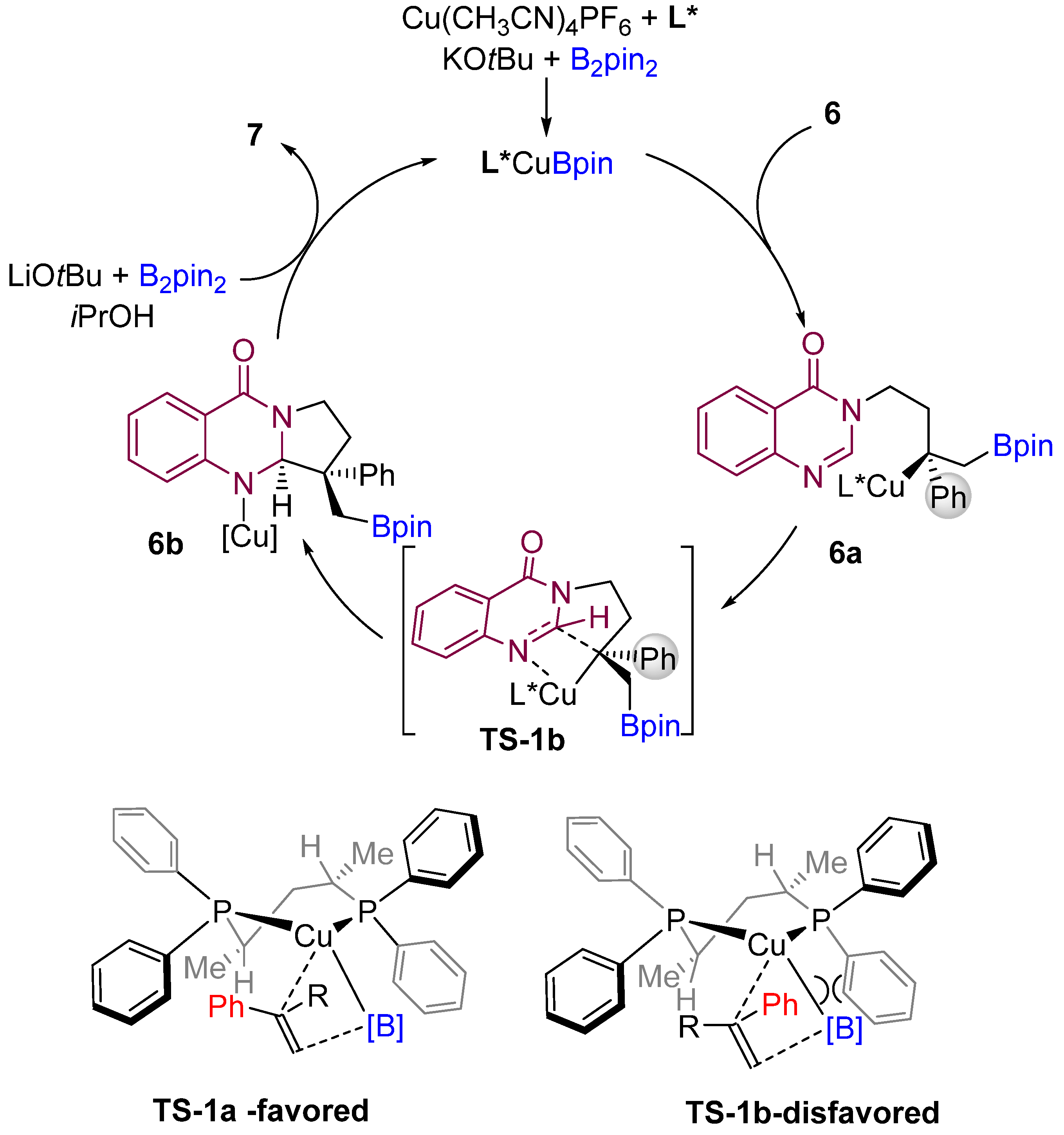
Scheme 7. Proposed mechanism and model for the origin of stereocontrol. (L* = chiral ligand).
References
- Manna, S.; Dherbassy, Q.; Perry, G.J.P.; Procter, D.J. Enantio- and Diastereoselective Synthesis of Homopropargyl Amines by Copper-Catalyzed Coupling of Imines, 1,3-Enynes, and Diborons. Angew. Chem. Int. Ed. 2020, 59, 4879–4882.
- Sun, Y.; Zhou, Y.; Shi, Y.; del Pozo, J.; Torker, S.; Hoveyda, A.H. Copper–Hydride-Catalyzed Enantioselective Processes with Allenyl Boronates. Mechanistic Nuances, Scope, and Utility in Target-Oriented Synthesis. J. Am. Chem. Soc. 2019, 141, 12087–12099.
- Meng, F.; Haeffner, F.; Hoveyda, A.H. Diastereo- and Enantioselective Reactions of Bis(pinacolato)diboron, 1,3-Enynes, and Aldehydes Catalyzed by an Easily Accessible Bisphosphine–Cu Complex. J. Am. Chem. Soc. 2014, 136, 11304–11307.
- Huang, Y.; Torker, S.; Li, X.; del Pozo, J.; Hoveyda, A.H. Racemic Vinylallenes in Catalytic Enantioselective Multicomponent Processes: Rapid Generation of Complexity through 1,6-Conjugate Additions. Angew. Chem. Int. Ed. 2019, 58, 2685–2691.
- Lee, Y.; Jang, H.; Hoveyda, A.H. Vicinal Diboronates in High Enantiomeric Purity through Tandem Site-Selective NHC−Cu-Catalyzed Boron−Copper Additions to Terminal Alkynes. J. Am. Chem. Soc. 2009, 131, 18234–18235.
- Trost, B.M. Atom Economy—A Challenge for Organic Synthesis: Homogeneous Catalysis Leads the Way. Angew. Chem. Int. Ed. 1995, 34, 259–281.
- Tan, Y.-X.; Zhang, F.; Xie, P.-P.; Zhang, S.-Q.; Wang, Y.-F.; Li, Q.-H.; Tian, P.; Hong, X.; Lin, G.-Q. Rhodium(III)-Catalyzed Asymmetric Borylative Cyclization of Cyclohexadienone-Containing 1,6-Dienes: An Experimental and DFT Study. J. Am. Chem. Soc. 2019, 141, 12770–12779.
- Pardo-Rodríguez, V.; Buñuel, E.; Collado-Sanz, D.; Cárdenas, D.J. Pd-catalyzed borylative cyclisation of 1,7-enynes. Chem. Commun. 2012, 48, 10517–10519.
- Hsieh, J.-C.; Hong, Y.-C.; Yang, C.-M.; Mannathan, S.; Cheng, C.-H. Nickel-catalyzed highly chemo- and stereoselective borylative cyclization of 1,6-enynes with bis(pinacolato)diboron. Org. Chem. Front. 2017, 4, 1615–1619.
- Zhao, Y.-S.; Tang, X.-Q.; Tao, J.-C.; Tian, P.; Lin, G.-Q. Efficient access to cis-decalinol frameworks: Copper(i)-catalyzed borylative cyclization of allene cyclohexanediones. Org. Biomol. Chem. 2016, 14, 4400–4404.
- Wu, X.; Lin, H.-C.; Li, M.-L.; Li, L.-L.; Han, Z.-Y.; Gong, L.-Z. Enantioselective 1,2-Difunctionalization of Dienes Enabled by Chiral Palladium Complex-Catalyzed Cascade Arylation/Allylic Alkylation Reaction. J. Am. Chem. Soc. 2015, 137, 13476–13479.
- Kanti Das, K.; Manna, S.; Panda, S. Transition metal catalyzed asymmetric multicomponent reactions of unsaturated compounds using organoboron reagents. Chem. Commun. 2021, 57, 441–459.
- Talbot, F.J.T.; Dherbassy, Q.; Manna, S.; Shi, C.; Zhang, S.; Howell, G.P.; Perry, G.J.P.; Procter, D.J. Copper-Catalyzed Borylative Couplings with C−N Electrophiles. Angew. Chem. Int. Ed. 2020, 59, 20278–20289.
- Dherbassy, Q.; Manna, S.; Talbot, F.J.T.; Prasitwatcharakorn, W.; Perry, G.J.P.; Procter, D.J. Copper-catalyzed functionalization of enynes. Chem. Sci. 2020, 11, 11380–11393.
- Whyte, A.; Torelli, A.; Mirabi, B.; Zhang, A.; Lautens, M. Copper-Catalyzed Borylative Difunctionalization of π-Systems. ACS Catal. 2020, 10, 11578–11622.
- He, C.-Y.; Li, Q.-H.; Wang, X.; Wang, F.; Tian, P.; Lin, G.-Q. Copper-Catalyzed Asymmetric Borylative Cyclization of Cyclohexadienone-Containing 1,6-Dienes. Adv. Synth. Catal. 2020, 362, 765–770.
- Yoon, W.S.; Han, J.T.; Yun, J. Divergent Access to Benzocycles through Copper-Catalyzed Borylative Cyclizations. Adv. Synth. Catal. 2021, 363, 4953–4959.
- Ito, H.; Kosaka, Y.; Nonoyama, K.; Sasaki, Y.; Sawamura, M. Synthesis of Optically Active Boron–Silicon Bifunctional Cyclopropane Derivatives through Enantioselective Copper(I)-Catalyzed Reaction of Allylic Carbonates with a Diboron Derivative. Angew. Chem. Int. Ed. 2008, 47, 7424–7427.
- Iwamoto, H.; Ozawa, Y.; Hayashi, Y.; Imamoto, T.; Ito, H. Conformationally Fixed Chiral Bisphosphine Ligands by Steric Modulators on the Ligand Backbone: Selective Synthesis of Strained 1,2-Disubstituted Chiral cis-Cyclopropanes. J. Am. Chem. Soc. 2022, 144, 10483–10494.
- Li, D.; Kim, J.; Yang, J.W.; Yun, J. Copper-Catalyzed Asymmetric Synthesis of Borylated cis-Disubstituted Indolines. Chem. Asian J. 2018, 13, 2365–2368.
- Zhang, G.; Cang, A.; Wang, Y.; Li, Y.; Xu, G.; Zhang, Q.; Xiong, T.; Zhang, Q. Copper-Catalyzed Diastereo- and Enantioselective Borylative Cyclization: Synthesis of Enantioenriched 2,3-Disubstituted Indolines. Org. Lett. 2018, 20, 1798–1801.
- Wang, H.-M.; Zhou, H.; Xu, Q.-S.; Liu, T.-S.; Zhuang, C.-L.; Shen, M.-H.; Xu, H.-D. Copper-Catalyzed Borylative Cyclization of Substituted N-(2-Vinylaryl)benzaldimines. Org. Lett. 2018, 20, 1777–1780.
- Li, D.; Park, Y.; Yun, J. Copper-Catalyzed Regioselective and Diastereoselective Synthesis of Borylated 1-Benzoazepines. Org. Lett. 2018, 20, 7526–7529.
- Larin, E.M.; Loup, J.; Polishchuk, I.; Ross, R.J.; Whyte, A.; Lautens, M. Enantio- and diastereoselective conjugate borylation/Mannich cyclization. Chem. Sci. 2020, 11, 5716–5723.
- Dherbassy, Q.; Manna, S.; Shi, C.; Prasitwatcharakorn, W.; Crisenza, G.E.M.; Perry, G.J.P.; Procter, D.J. Enantioselective Copper-Catalyzed Borylative Cyclization for the Synthesis of Quinazolinones. Angew. Chem. Int. Ed. 2021, 60, 14355–14359.
More
Information
Subjects:
Chemistry, Organic
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Entry Collection:
Organic Synthesis
Revisions:
4 times
(View History)
Update Date:
17 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




