Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guilong Zhang | -- | 2781 | 2022-07-10 15:14:18 | | | |
| 2 | Jason Zhu | Meta information modification | 2781 | 2022-07-11 03:12:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Feng, C.; Xu, Z.; Tang, X.; Cao, H.; Tang, J.; Zhang, G. Estrogen-Related Receptor α. Encyclopedia. Available online: https://encyclopedia.pub/entry/24978 (accessed on 07 February 2026).
Feng C, Xu Z, Tang X, Cao H, Tang J, Zhang G. Estrogen-Related Receptor α. Encyclopedia. Available at: https://encyclopedia.pub/entry/24978. Accessed February 07, 2026.
Feng, Chun, Zhaowei Xu, Xiaojie Tang, Haifei Cao, Jiangwei Tang, Guilong Zhang. "Estrogen-Related Receptor α" Encyclopedia, https://encyclopedia.pub/entry/24978 (accessed February 07, 2026).
Feng, C., Xu, Z., Tang, X., Cao, H., Tang, J., & Zhang, G. (2022, July 10). Estrogen-Related Receptor α. In Encyclopedia. https://encyclopedia.pub/entry/24978
Feng, Chun, et al. "Estrogen-Related Receptor α." Encyclopedia. Web. 10 July, 2022.
Copy Citation
Bone homeostasis is maintained with the balance between bone formation and bone resorption, which is involved in the functional performance of osteoblast and osteoclast. Disruption of this equilibrium usually causes bone disorders including osteoporosis, osteoarthritis, and osteosclerosis. In addition, aberrant activity of bone also contributes to the bone metastasis that frequently occurs in the late stage of aggressive cancers. Orphan nuclear receptor estrogen-related receptor (ERRα) has been demonstrated to control the bone cell fate and the progression of tumor cells in bone through crosstalk with various molecules and signaling pathways.
ERRα
bone
osteoclast
osteoblast
1. Structure and Function of Estrogen-Related Receptor α (ERRα)
Estrogen-related receptor α (ERRα) belong to a subfamily of nuclear receptors and consist of three members: ERRα, ERRβ, and ERRγ. Owing to lack of endogenous ligands in vivo, they are also called orphan nuclear receptors [1]. ERRα was firstly identified from a cDNA library by using the DNA-binding domain (DBD) sequence of estrogen receptor α (ERα) as a probe [1]. Similar to other steroid hormone receptors, ERRα has an N-terminal located ligand-independent transcription activation domain, a centrally located DBD encompassing two zinc fingers that mediate the interaction between the specific DNA motif and the receptor, and a C-terminal located domain that is responsible for ligand dependent transaction (also called ligand binding domain (LBD)). Between the DBD and LBD, a poorly conserved hinge domain mainly mediates the homodimer formation [2][3], the schematic diagram of structure domains between human ERRs and ERα is illustrated in Figure 1. Despite the high structural conservation among ERRα and ERα, the ligand of ERα, such as 17β-estradiol, cannot activate its transcriptional activity. Subsequent research revealed that ERRα recognized and binded on the estrogen-related response element (ERRE) but not the estrogen r element (ERE) on the promoter region of target genes to activate the transcription expression [4]. However, many target genes of ERα (like pS2 and LTF) were simultaneously regulated by the two receptors in breast cancer cells [5][6]. In addition, the transcriptional activity of ERRα is largely dependent on its co-regulators such as peroxisome proliferator-activated receptor γ coactivator-1 (PGC-1) α and PGC-1β, which is also distinguished from ERα that prefers to cooperate with SRC/p160 family [7].
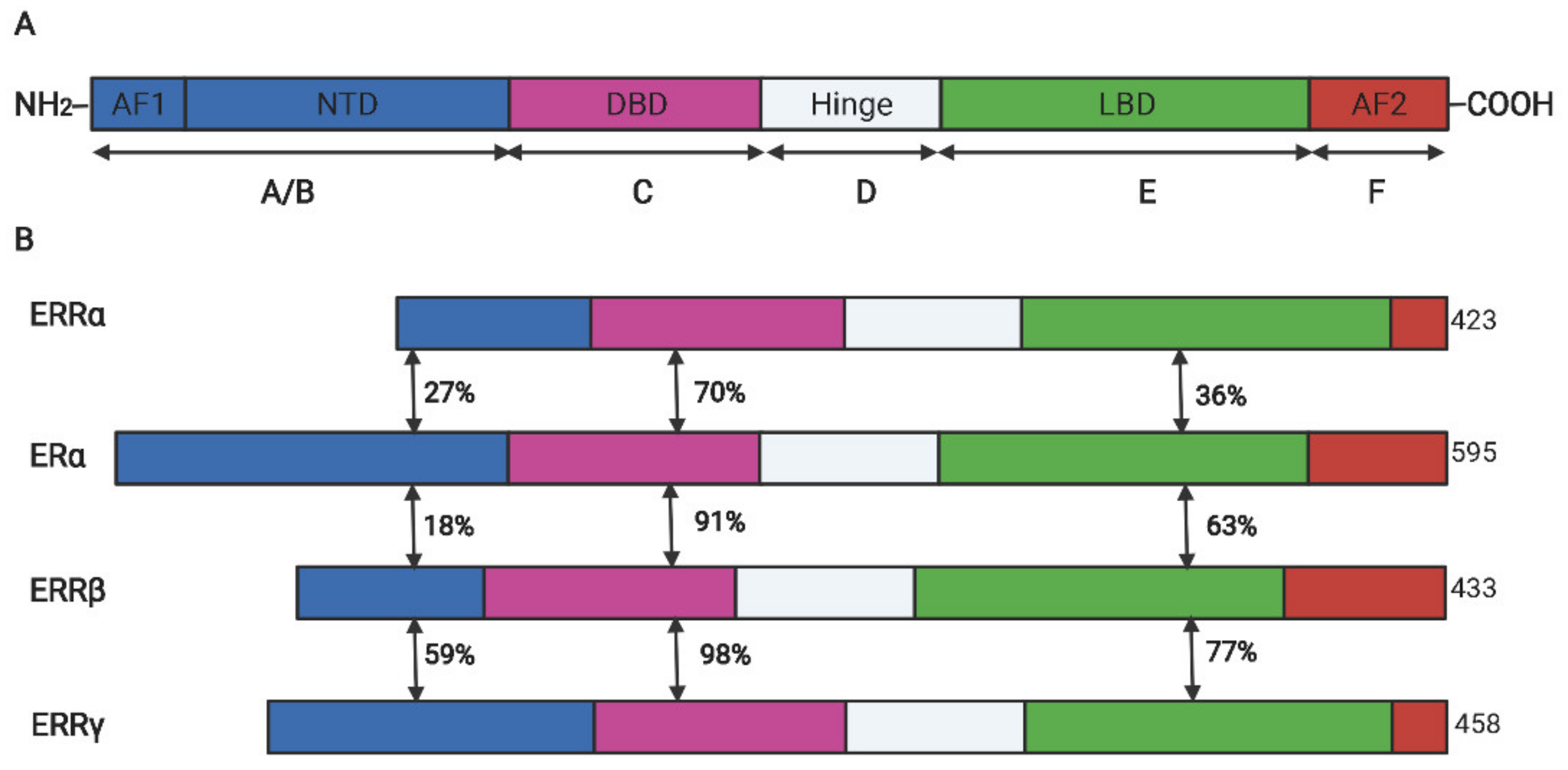
Figure 1. The schematic diagram for the protein domains of ERα and ERR isoforms. (A). The conserved structure of nuclear receptors. (B). The domain similarity between ERRs and ERα. DBD, DNA binding domain; LBD, ligand binding domain; AF, activation functional domain; NTD, N-terminal domain; The double-headed arrows indicated the sequence similarity between two receptors.
ERRα has also been clarified to exhibit various regulatory functions in physiological and pathological processes, including energy metabolism [8], organ development, immune response [9][10], and tumorigenesis [11][12]. ERRα is strongly expressed in high energy consumption tissues (such as heart, liver, muscle, and adipose tissues), and it can regulate lipid metabolism and thermogenesis in both white adipose tissues (WAT) and brown adipose tissues (BAT) [13]. Mice with ERRα knockout (KO) rapidly exhibited hypothermia under low-temperature conditions, and the expression and activity of uncoupling protein 1 (UCP1) induced by adrenalin in BAT was largely dependent on the presence and activity of ERRα [14][15]. In addition, ERRα was proved to modulate glycolytic metabolism via regulating the expression of essential enzymes including phosphofructokinase, hexokinase 2, and enolase 1 [16]. ERRα cooperating with PGC-1α controlled the mitochondria metabolism and oxidative response to sensor the cellular ROS levels [17]. Beyond the function in metabolism, ERRα also acted in the innate immune response via regulating mitochondria ROS production and autophagy [9][10]. Furthermore, the high abundance of ERRα was frequently detected in many types of solid tumors (including colon, endometrium, bladder, ovary, breast, and prostate cancers), and its upregulation was associated with more aggressive phenotypes, drug resistance, and poor prognosis [11][18]. Mechanism exploration found that ERRα facilitated the proliferation and metastasis of tumor cells via upregulating oncogenes transcription and inducing metabolic reprogramming [12]. However, the role of ERRα in different cancers appears to be inconsistent. For example, ERRα almost functioned as an oncogenic factor in many types of carcinomas, but could serve as a favorable biomarker for the tamoxifen sensitivity in the patients with triple-negative breast cancer (TNBC) [19]. An alternative probability is that the functional performance of ERRα is tightly dependent on the specific cellular environment and post-translational modifications (PTMs) [18][20]. In addition, many specific inhibitors or natural products that function as agonists or antagonists of ERRα were identified, and these compounds have been implicated in the treatment of cancers or metabolism-disorder diseases [3][21]. Therefore, it is essential to explore the defined role of ERRα in a certain tumor model and achieve the precise therapy using the specific pharmacochemistry targeting on ERRα.
2. The Regulatory Role of ERRα in Osteoblasts
Epidemiological investigation showed that single nucleotide polymorphism (SNP) within ESRRA was closely associated with increased bone mineral density (BMD) of premenopausal women [22], and in situ hybridization assay found that ESRRA was consistently expressed in ossification zones during the mouse embryonic development stages, and it could directly bind to the promoter and activate the OPN transcription. These results indicated that ERRα may be involved in the process of the osteoblast progenitors differentiation and bone metabolism [23]. Indeed, ERRα was initially indicated to serve as a positive modulator for osteoblast differentiation and bone formation [24]; in vitro differentiation of osteoblast progenitors originating from rat calvaria or bone marrow was also accompanied with the continuous expression of ERRα, knockdown of ERRα by shRNA significantly impaired the proliferation and differentiation of osteoblast progenitors, while overexpression of this receptor increased the differentiation markers [25][26]. Moreover, estrogen could enhance the ESRRA transcription in a dose-dependent manner in the early differentiation stage of osteoblast progenitor cells [27][28], inhibition of ERRα in a culture medium induced a decreased colony number of mineralized cells [25], and the progenitor cells with ERRα deficiency were inclined to differentiate into adipocytes as evidenced through the increasing lipid droplet accumulation and expression of adipocyte markers (such as PPARγ or aP2). These studies suggested that ERRα served as a switch factor to favor the precursor cells differentiating into osteoblasts but not adipocytes [28][29]. Furthermore, other models from mice and humans also supported the promoted effect of ERRα on the osteoblast differentiation and functional exhibition, Rajalin et al., isolated the mouse mesenchymal stem cell from bone marrow and conducted ex vivo differentiation culture; they found that knockdown of ERRα induced the decreased cell proliferation, osteoblastic differentiation, and mineralization with reduced expression of bone sialoprotein (BSP) [30]. Cai et al., demonstrated that the mRNA and protein levels of ERRα were significantly increased during the late stage of osteogenic differentiation of human periodontal ligament cells (hPDLSCs), and the receptor maintained the osteogenic differentiation via regulating the ALP activity and osteogenesis-related genes expression [31], and another group established that ERRα interacted cooperatively with PGC-1α in the promoter region of osteocalcin and enhanced its transcription expression to facilitate the osteogenesis [32]. In the human MSCs model, Huang et al., demonstrated that ERRα could directly bind to ERRE on the Gls promoter and transactivate its transcription, then further regulated glutamine anaplerosis and mitochondrial function, and this effect was required for osteogenic differentiation and impaired by aging [33]. Overall, these data indicated that ERRα functioned as a driving factor for osteoblast differentiation and was resistant to bone loss.
However, an increasing number of studies proposed that ERRα preferred to repress osteoblast differentiation and caused bone loss in vitro and in vivo. Firstly, the association between ESRRA SNP and increased BMD was not replicable in another set of independent clinical data [34]. Two different groups consistently demonstrated that ERRα-KO mice exhibited increased BMD and enhanced osteoblast activity when compared with control, osteoblast progenitors derived from mice with ERRα deficiency had elevated expression of differential markers and were more liable to differentiate to matured phenotype but not adipocyte lineage [35][36]. One of groups further showed that the animals with ERRα-KO had resistance to the bone loss induced by aging or estrogen withdrawal mimicked with ovariectomy, indicating that targeting the receptor in menopause could prevent excessive bone loss and reduce the rate of fracture [36]. Moreover, Gallet et al., generated a conditional ESRRA-KO mouse in which ERRα was normally presented during the early differentiation stage of osteoblasts but lost its function in maturation; they found that ERRα could effectively respond to estrogen deficiency and inhibit the osteoblast differentiate and osteogenesis in the later maturation stage but not the early stage [37]. This phenomenon was consistent with the regulatory role of ERRα investigated in the mineralization of osteoblasts [35][38]. OPN had been verified to be the inhibitor of mineralization and one of the target genes of ERRα, ERRα deficiency was accompanied with a dramatic reduction of OPN and impairment of mineralization [39]. Consistently, OPN-/- mice were also resistant to the bone loss induced by estrogen withdrawal, this phenotype was similar to ERRα-KO animals [40]. Mechanism investigation revealed that ERRα might serve as a dual functional factor to modulate the activation of signaling pathways and osteogenesis-related genes expression (such as Runx2 and OPN), and this effect was mainly dependent on the presence of its cofactor PGC-1β [41][42]. Studies using different models, such as different cell lines or mouse strains, may obtain inconsistent and contrary results. Therefore, more comprehensive research deserves to be conducted to uncover the defined role of ERRα in osteoblast differentiation and bone homeostasis.
3. The Role of ERRα in Osteoclasts
Bone remodeling is not only associated with osteoblasts-mediated bone formation, but involves bone resorption regulated by the osteoclasts. Bonnelye et al., conducted the in vitro culture experiments and revealed that ERRα exhibited little effect on osteoclast differentiation but promoted adhesion and migration of osteoclasts via regulating the expression of OPN and integrin β3 chain and the alteration of cytoskeleton [43]. This conclusion was partially consistent with the role of ERRα in breast cancer [11]. However, in vivo studies using ERRα-KO animals challenged this conclusion. They found a specific phenotype with increased bone formation and reduced bone absorption in an ESRRA-/- mouse model owing to the decreased number and activity of osteoclasts [35][44]; mechanism investigation revealed that ERRα cooperated with PGC-1β and controlled the mitochondria biosynthesis and oxidative activity, which indirectly enhanced the osteoclast differentiation [44]. They further demonstrated that ERRα served as the key factor in modulating the RANKL- and rosiglitazone-mediated bone loss and functional performance of osteoclasts [44]. Consistently, PGC-1β was parallel with ERRα expression during the osteoclasts transition and the knockout of PGC-1β in mice exhibited osteopetrosis with abnormal morphology and the impaired bone-resorption activity of osteoclasts [45]. Subsequently, the significant role of ERRα in cholesterol metabolism and bone homeostasis was confirmed by Wei et al. [46] who identified that cholesterol acted as an endogenous ligand for ERRα to activate its transcriptional activity; this effect induced the inhibition of proinflammatory cytokinesis production by macrophage and facilitated the osteoclast differentiation [46]. Concomitantly, pharmacological inhibition on cholesterol synthesis and bone resorption by statin and bisphosphonate were also modulated by ERRα [46]. Considering the origin of osteoclast and macrophage from embryogenesis, ERRα may act as rheostat factor in the differentiation of macrophage and osteoclast. Congruously, MYC promoted the oxidative metabolism and drove osteoclastogenesis, the process of which was largely dependent on the ERRα action [47]. These fundamental studies suggested that the inhibition of ERRα by molecules or natural products may prevent or improve the bone loss induced by aging or hormone withdrawal. Indeed, Zheng et al., identified that carnosic acid, isolated from Rosmarinus officinalis L., could decrease the transcription activity of ERRα via reducing the interaction between ERRα and PGC-1β and triggering proteasomal degradation of ERRα, then suppress RANKL-mediated osteoclastogenesis and ovariectomy-induced bone loss [48]. Andrographolide, another natural compound targeted on ERRα from virtual docking screening, had been proven to suppress the formation of ERRα/PGC-1β complex and further reduce glutaminase metabolism during osteoclast differentiation. Inhibition on this regulatory axis attenuated osteoclasts-mediated bone resorption and bone loss in vivo [21]. Taken together, these data illustrated that ERRα functioned as a promoting effector to facilitate osteoclast differentiation and bone absorption involving various mechanism networks, targeting on the receptor would be a promising strategy for degenerative bone disorders.
4. The Role of ERRα in Cartilage and Osteoarthritis
Osteoarthritis (OA) is characteristically associated with chronic inflammatory pathological feature and closely associated with articular cartilage dysfunction (including abnormal proliferation and erasion of cartilage) [29][49][50]. During the development of osteoarthritis, the elevated iNOS, IFNs, and TNFs levels in the microenvironment were detected, and LPS or TNF-γ induced the aberrant activation of NF-κB signaling in the pro-inflammatory (M1) macrophages, which could generate pro-inflammatory cytokinesis (such as TNF-γ, IL-6 and IL-12) to form a positive feedback loop and result in the degradation of cartilage [51]. ERRα has been reported to be involved in the innate immune response and functional performance of macrophage via modulating autophagy or TLR4/TRAF6/NF-κB axis [10][52]. Consistently, ERRα also performed a dual role in cartilage formation and OA progression. The effect of ERRα on cartilage formation was initially involved in the regulation of SOX-9, Bonnely et al., reported that the receptor upregulated Sox-9 transcription and induced the differentiation and proliferation of chondrocytes [53]. The chondrocytes isolated from the OA patients treated with XCT790 (the specific reverse agonist of ERRα) showed the downregulation of SOX-9 expression in a dose dependent manner. These results were proven by Kim et al., in a zebrafish embryo model, where ERRα knockdown led to the malformation of pharyngeal arch cartilage [54]. In addition, ERRα also contributed to the cartilage degradation which is associated with IL-1β and MMP-13. IL-1β stimulation could increase the ERRα expression via the PGE2/cAMP/PKA signaling pathway, ERRα reversely activated the MMP-13 expression in OA progression [55]. This process may involve the macrophage action mediated by ERRα/NF-κB signaling, but this possibility needs to be further investigated. The comprehensive effect of ERRα on cartilage and OA had been reviewed by Bonnelye and Tang [29][56], but more systematic research is indispensable to reveal the crosstalk between inflammatory signaling pathways and ERRα action in cartilage.
5. The Role of ERRα in Bone Metastasis
According to the different mechanisms involved, bone metastasis is mainly classified into two types: osteoblastic (bone-forming) metastasis and osteolytic (bone-resorbing) metastasis, which are closely associated with abnormal activity of osteoblasts or osteoclasts, respectively. Considering the essential role of ERRα in cancer metastasis and osteocyte differentiation, the role of ERRα in bone metastasis was also investigated. Similar to the complicated action of ERRα in bone metabolism, its role in bone metastasis was also controversial. Although the studies regarding the regulation of ERRα in bone metastasis were mainly produced from the same research group, the conclusion appears to be evolutionary and inconsistent. Initially, ERRα was shown to enhance the growth in situ but repress the proliferation of breast cancer cells in bone via upregulating the osteoclastogenesis inhibitor OPG and angiogenic growth factor VEGF in a mouse xenograft model of metastatic human breast cancer. A primary breast tumor with overexpression of ERRα exhibited highly vascularized and increased growth ability, while the receptor inhibited the differentiation and activity of osteoclasts, and further limited the tumorigenesis in bone [57]. However, the study conducted in castration-resistant prostate cancer (CRPC), which had higher aggression and frequently occurring bone metastasis, obtained a distinctive conclusion: they found that the overexpression of ERRα significantly increased the progression of CRPC and metastasis in bone, and mechanism investigation revealed that the receptor enhanced the bone remodeling via increasing the expression of metastatic factors (such as VEGF-A, WNT5A, TGFβ1, and periostin) and generating a favoring-growth tumor environment [58]. In addition, Vargas et al., also found that ERRα promoted orthotopic breast cancer cell dissemination to bone but not lung metastasis in a mouse breast cancer model through increasing the expression of RANK, which acted as the receptor of RANKL and guided the cancer cells’ migration into the bone microenvironment. In line with this, RANKL stimulation induced a higher activity of mTOR signaling in 4T1 cells with ERRα overexpression, and pharmaceutical inhibition of ERRα by C29 complex inhibited the tumorigenesis and bone metastatic events [59]. Interestingly, Bouchet et al., subsequently revealed that ERRα inhibited the growth of breast cancer cells after tumor cell anchorage in the bone via activating the immune response in bone microenvironment. This phenomenon could be achieved via ERRα-mediated induction of chemokines (CCL17 and CCL20) and reduction of TGFβ3, which recruited the CD8 positive T cells to trigger immune cytotoxic action and metastasis repression [60]. The reason that ERRα appears to function inconsistently or reversely in different cancer types or stages of bone metastasis is a critical issue to be explored. One alternative probability is that prostate cancer predominantly prefers osteoblastic lesions, and the metastasis from breast cancer mostly exhibits osteolytic lesions [61], which may explain the diverse role of ERRα between prostate cancer and breast cancer. With regard to the different function of ERRα between dissemination of tumor cells to bone and the growth of metastases, the distinguished microenvironment and signaling stimulation may account for these results [62][63]. Therefore, an appropriate metastatic model and comprehensive analysis of regulatory network will illustrate the important effect of ERRα on the initiation and progression of bone metastasis.
References
- Giguère, V.; Yang, N.; Segui, P.; Evans, R.M. Identification of a new class of steroid hormone receptors. Nature 1988, 331, 91–94.
- Greschik, H.; Wurtz, J.M.; Sanglier, S.; Bourguet, W.; van Dorsselaer, A.; Moras, D.; Renaud, J.P. Structural and functional evidence for ligand-independent transcriptional activation by the estrogen-related receptor 3. Mol. Cell 2002, 9, 303–313.
- Tripathi, M.; Yen, P.M.; Singh, B.K. Estrogen-Related Receptor Alpha: An Under-Appreciated Potential Target for the Treatment of Metabolic Diseases. Int. J. Mol. Sci. 2020, 21, 1645.
- Giguère, V. To ERR in the estrogen pathway. Trends Endocrinol. Metab. TEM 2002, 13, 220–225.
- Zhang, Z.; Teng, C.T. Estrogen receptor-related receptor alpha 1 interacts with coactivator and constitutively activates the estrogen response elements of the human lactoferrin gene. J. Biol. Chem. 2000, 275, 20837–20846.
- Lu, D.; Kiriyama, Y.; Lee, K.Y.; Giguère, V. Transcriptional regulation of the estrogen-inducible pS2 breast cancer marker gene by the ERR family of orphan nuclear receptors. Cancer Res. 2001, 61, 6755–6761.
- Huss, J.M.; Kopp, R.P.; Kelly, D.P. Peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-α and -γ. Identification of novel leucine-rich interaction motif within PGC-1α. J. Biol. Chem. 2002, 277, 40265–40274.
- Villena, J.A.; Kralli, A. ERRα: A metabolic function for the oldest orphan. Trends Endocrinol. Metab. TEM 2008, 19, 269–276.
- Sonoda, J.; Laganière, J.; Mehl, I.R.; Barish, G.D.; Chong, L.W.; Li, X.; Scheffler, I.E.; Mock, D.C.; Bataille, A.R.; Robert, F.; et al. Nuclear receptor ERRα and coactivator PGC-1β are effectors of IFN-γ-induced host defense. Genes Dev. 2007, 21, 1909–1920.
- Kim, S.Y.; Yang, C.S.; Lee, H.M.; Kim, J.K.; Kim, Y.S.; Kim, Y.R.; Kim, J.S.; Kim, T.S.; Yuk, J.M.; Dufour, C.R.; et al. ESRRA (estrogen-related receptor α) is a key coordinator of transcriptional and post-translational activation of autophagy to promote innate host defense. Autophagy 2018, 14, 152–168.
- Deblois, G.; St-Pierre, J.; Giguère, V. The PGC-1/ERR signaling axis in cancer. Oncogene 2013, 32, 3483–3490.
- Deblois, G.; Giguère, V. Oestrogen-related receptors in breast cancer: Control of cellular metabolism and beyond. Nat. Rev. Cancer 2013, 13, 27–36.
- Villena, J.A.; Hock, M.B.; Chang, W.Y.; Barcas, J.E.; Giguère, V.; Kralli, A. Orphan nuclear receptor estrogen-related receptor α is essential for adaptive thermogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 1418–1423.
- Porter, C. Quantification of UCP1 function in human brown adipose tissue. Adipocyte 2017, 6, 167–174.
- Gantner, M.L.; Hazen, B.C.; Eury, E.; Brown, E.L.; Kralli, A. Complementary Roles of Estrogen-Related Receptors in Brown Adipocyte Thermogenic Function. Endocrinology 2016, 157, 4770–4781.
- Cai, Q.; Lin, T.; Kamarajugadda, S.; Lu, J. Regulation of glycolysis and the Warburg effect by estrogen-related receptors. Oncogene 2013, 32, 2079–2086.
- Vernier, M.; Dufour, C.R.; McGuirk, S.; Scholtes, C.; Li, X.; Bourmeau, G.; Kuasne, H.; Park, M.; St-Pierre, J.; Audet-Walsh, E.; et al. Estrogen-related receptors are targetable ROS sensors. Genes Dev. 2020, 34, 544–559.
- Yang, Y.; Li, S.; Li, B.; Li, Y.; Xia, K.; Aman, S.; Yang, Y.; Ahmad, B.; Zhao, B.; Wu, H. FBXL10 promotes ERRα protein stability and proliferation of breast cancer cells by enhancing the mono-ubiquitylation of ERRα. Cancer Lett. 2021, 502, 108–119.
- Manna, S.; Bostner, J.; Sun, Y.; Miller, L.D.; Alayev, A.; Schwartz, N.S.; Lager, E.; Fornander, T.; Nordenskjöld, B.; Yu, J.J.; et al. ERRα Is a Marker of Tamoxifen Response and Survival in Triple-Negative Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1421–1431.
- Crevet, L.; Vanacker, J.M. Regulation of the expression of the estrogen related receptors (ERRs). Cell. Mol. Life Sci. 2020, 77, 4573–4579.
- Huang, T.; Fu, X.; Wang, N.; Yang, M.; Zhang, M.; Wang, B.; Chen, T.; Majaz, S.; Wang, H.; Wong, C.W.; et al. Andrographolide prevents bone loss via targeting estrogen-related receptor-α-regulated metabolic adaption of osteoclastogenesis. Br. J. Pharmacol. 2021, 178, 4352–4367.
- Laflamme, N.; Giroux, S.; Loredo-Osti, J.C.; Elfassihi, L.; Dodin, S.; Blanchet, C.; Morgan, K.; Giguère, V.; Rousseau, F. A frequent regulatory variant of the estrogen-related receptor α gene associated with BMD in French-Canadian premenopausal women. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005, 20, 938–944.
- Bonnelye, E.; Vanacker, J.M.; Dittmar, T.; Begue, A.; Desbiens, X.; Denhardt, D.T.; Aubin, J.E.; Laudet, V.; Fournier, B. The ERR-1 orphan receptor is a transcriptional activator expressed during bone development. Mol. Endocrinol. 1997, 11, 905–916.
- Bonnelye, E.; Aubin, J.E. Estrogen receptor-related receptor α: A mediator of estrogen response in bone. J. Clin. Endocrinol. Metab. 2005, 90, 3115–3121.
- Bonnelye, E.; Merdad, L.; Kung, V.; Aubin, J.E. The orphan nuclear estrogen receptor-related receptor α (ERRα) is expressed throughout osteoblast differentiation and regulates bone formation in vitro. J. Cell Biol. 2001, 153, 971–984.
- Bonnelye, E.; Aubin, J.E. Differential expression of estrogen receptor-related receptor α and estrogen receptors α and β in osteoblasts in vivo and in vitro. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2002, 17, 1392–1400.
- Liu, D.; Zhang, Z.; Gladwell, W.; Teng, C.T. Estrogen stimulates estrogen-related receptor α gene expression through conserved hormone response elements. Endocrinology 2003, 144, 4894–4904.
- Bonnelye, E.; Kung, V.; Laplace, C.; Galson, D.L.; Aubin, J.E. Estrogen receptor-related receptor α impinges on the estrogen axis in bone: Potential function in osteoporosis. Endocrinology 2002, 143, 3658–3670.
- Bonnelye, E.; Aubin, J.E. An energetic orphan in an endocrine tissue: A revised perspective of the function of estrogen receptor-related receptor α in bone and cartilage. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 225–233.
- Rajalin, A.M.; Pollock, H.; Aarnisalo, P. ERRα regulates osteoblastic and adipogenic differentiation of mouse bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2010, 396, 477–482.
- Cai, C.; Yuan, G.J.; Huang, Y.; Yang, N.; Chen, X.; Wen, L.; Wang, X.; Zhang, L.; Ding, Y. Estrogen-related receptor α is involved in the osteogenic differentiation of mesenchymal stem cells isolated from human periodontal ligaments. Int. J. Mol. Med. 2013, 31, 1195–1201.
- Wang, H.; Wang, J. Estrogen-related receptor alpha interacts cooperatively with peroxisome proliferator-activated receptor-gamma coactivator-1alpha to regulate osteocalcin gene expression. Cell Biol. Int. 2013, 37, 1259–1265.
- Huang, T.; Liu, R.; Fu, X.; Yao, D.; Yang, M.; Liu, Q.; Lu, W.W.; Wu, C.; Guan, M. Aging Reduces an ERRalpha-Directed Mitochondrial Glutaminase Expression Suppressing Glutamine Anaplerosis and Osteogenic Differentiation of Mesenchymal Stem Cells. Stem Cells 2017, 35, 411–424.
- Giroux, S.; Elfassihi, L.; Cole, D.E.C.; Rousseau, F. Replication of associations between LRP5 and ESRRA variants and bone density in premenopausal women. Osteoporos. Int. 2008, 19, 1769–1775.
- Delhon, I.; Gutzwiller, S.; Morvan, F.; Rangwala, S.; Wyder, L.; Evans, G.; Studer, A.; Kneissel, M.; Fournier, B. Absence of Estrogen Receptor-Related-α Increases Osteoblastic Differentiation and Cancellous Bone Mineral Density. Endocrinology 2009, 150, 4463–4472.
- Teyssier, C.; Gallet, M.; Rabier, B.; Monfoulet, L.; Dine, J.; Macari, C.; Espallergues, J.; Horard, B.; Giguère, V.; Cohen-Solal, M.; et al. Absence of ERRα in female mice confers resistance to bone loss induced by age or estrogen-deficiency. PLoS ONE 2009, 4, e7942.
- Gallet, M.; Saïdi, S.; Haÿ, E.; Photsavang, J.; Marty, C.; Sailland, J.; Carnesecchi, J.; Tribollet, V.; Barenton, B.; Forcet, C.; et al. Repression of osteoblast maturation by ERRα accounts for bone loss induced by estrogen deficiency. PLoS ONE 2013, 8, e54837.
- Gallet, M.; Vanacker, J.M. ERR receptors as potential targets in osteoporosis. Trends Endocrinol. Metab. TEM 2010, 21, 637–641.
- Vanacker, J.M.; Delmarre, C.; Guo, X.; Laudet, V. Activation of the osteopontin promoter by the orphan nuclear receptor estrogen receptor related alpha. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 1998, 9, 1007–1014.
- Yoshitake, H.; Rittling, S.R.; Denhardt, D.T.; Noda, M. Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption. Proc. Natl. Acad. Sci. USA 1999, 96, 8156–8160.
- Auld, K.L.; Berasi, S.P.; Liu, Y.; Cain, M.; Zhang, Y.; Huard, C.; Fukayama, S.; Zhang, J.; Choe, S.; Zhong, W.; et al. Estrogen-related receptor α regulates osteoblast differentiation via Wnt/β-catenin signaling. J. Mol. Endocrinol. 2012, 48, 177–191.
- Kammerer, M.; Gutzwiller, S.; Stauffer, D.; Delhon, I.; Seltenmeyer, Y.; Fournier, B. Estrogen Receptor α (ERα) and Estrogen Related Receptor α (ERRα) are both transcriptional regulators of the Runx2-I isoform. Mol. Cell. Endocrinol. 2013, 369, 150–160.
- Bonnelye, E.; Saltel, F.; Chabadel, A.; Zirngibl, R.A.; Aubin, J.E.; Jurdic, P. Involvement of the orphan nuclear estrogen receptor-related receptor α in osteoclast adhesion and transmigration. J. Mol. Endocrinol. 2010, 45, 365–377.
- Wei, W.; Wang, X.; Yang, M.; Smith, L.C.; Dechow, P.C.; Sonoda, J.; Evans, R.M.; Wan, Y. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 2010, 11, 503–516.
- Ishii, K.A.; Fumoto, T.; Iwai, K.; Takeshita, S.; Ito, M.; Shimohata, N.; Aburatani, H.; Taketani, S.; Lelliott, C.J.; Vidal-Puig, A.; et al. Coordination of PGC-1β and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat. Med. 2009, 15, 259–266.
- Wei, W.; Schwaid, A.G.; Wang, X.; Wang, X.; Chen, S.; Chu, Q.; Saghatelian, A.; Wan, Y. Ligand Activation of ERRα by Cholesterol Mediates Statin and Bisphosphonate Effects. Cell Metab. 2016, 23, 479–491.
- Bae, S.; Lee, M.J.; Mun, S.H.; Giannopoulou, E.G.; Yong-Gonzalez, V.; Cross, J.R.; Murata, K.; Giguère, V.; van der Meulen, M.; Park-Min, K.H. MYC-dependent oxidative metabolism regulates osteoclastogenesis via nuclear receptor ERRα. J. Clin. Investig. 2017, 127, 2555–2568.
- Zheng, Z.G.; Cheng, H.M.; Zhou, Y.P.; Zhu, S.T.; Thu, P.M.; Li, H.J.; Li, P.; Xu, X. Dual targeting of SREBP2 and ERRα by carnosic acid suppresses RANKL-mediated osteoclastogenesis and prevents ovariectomy-induced bone loss. Cell Death Differ. 2020, 27, 2048–2065.
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11, 111.
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534.
- Lopa, S.; Leijs, M.J.; Moretti, M.; Lubberts, E.; van Osch, G.J.; Bastiaansen-Jenniskens, Y.M. Arthritic and non-arthritic synovial fluids modulate IL10 and IL1RA gene expression in differentially activated primary human monocytes. Osteoarthr. Cartil. 2015, 23, 1853–1857.
- Yuk, J.M.; Kim, T.S.; Kim, S.Y.; Lee, H.M.; Han, J.; Dufour, C.R.; Kim, J.K.; Jin, H.S.; Yang, C.S.; Park, K.S.; et al. Orphan Nuclear Receptor ERRα Controls Macrophage Metabolic Signaling and A20 Expression to Negatively Regulate TLR-Induced Inflammation. Immunity 2015, 43, 80–91.
- Bonnelye, E.; Zirngibl, R.A.; Jurdic, P.; Aubin, J.E. The orphan nuclear estrogen receptor-related receptor-α regulates cartilage formation in vitro: Implication of Sox9. Endocrinology 2007, 148, 1195–1205.
- Kim, Y.I.; No Lee, J.; Bhandari, S.; Nam, I.K.; Yoo, K.W.; Kim, S.J.; Oh, G.S.; Kim, H.J.; So, H.S.; Choe, S.K.; et al. Cartilage development requires the function of Estrogen-related receptor alpha that directly regulates sox9 expression in zebrafish. Sci. Rep. 2015, 5, 18011.
- Bonnelye, E.; Reboul, P.; Duval, N.; Cardelli, M.; Aubin, J.E. Estrogen receptor-related receptor α regulation by interleukin-1β in prostaglandin E(2)- and cAMP-dependent pathways in osteoarthritic chondrocytes. Arthritis Rheum. 2011, 63, 2374–2384.
- Tang, J.; Liu, T.; Wen, X.; Zhou, Z.; Yan, J.; Gao, J.; Zuo, J. Estrogen-related receptors: Novel potential regulators of osteoarthritis pathogenesis. Mol. Med. 2021, 27, 5.
- Fradet, A.; Sorel, H.; Bouazza, L.; Goehrig, D.; Dépalle, B.; Bellahcène, A.; Castronovo, V.; Follet, H.; Descotes, F.; Aubin, J.E.; et al. Dual function of ERRα in breast cancer and bone metastasis formation: Implication of VEGF and osteoprotegerin. Cancer Res. 2011, 71, 5728–5738.
- Fradet, A.; Bouchet, M.; Delliaux, C.; Gervais, M.; Kan, C.; Benetollo, C.; Pantano, F.; Vargas, G.; Bouazza, L.; Croset, M.; et al. Estrogen related receptor alpha in castration-resistant prostate cancer cells promotes tumor progression in bone. Oncotarget 2016, 7, 77071–77086.
- Vargas, G.; Bouchet, M.; Bouazza, L.; Reboul, P.; Boyault, C.; Gervais, M.; Kan, C.; Benetollo, C.; Brevet, M.; Croset, M.; et al. ERRα promotes breast cancer cell dissemination to bone by increasing RANK expression in primary breast tumors. Oncogene 2019, 38, 950–964.
- Bouchet, M.; Lainé, A.; Boyault, C.; Proponnet-Guerault, M.; Meugnier, E.; Bouazza, L.; Kan, C.W.S.; Geraci, S.; El-Moghrabi, S.; Hernandez-Vargas, H.; et al. ERRα Expression in Bone Metastases Leads to an Exacerbated Antitumor Immune Response. Cancer Res. 2020, 80, 2914–2926.
- Logothetis, C.J.; Lin, S.H. Osteoblasts in prostate cancer metastasis to bone. Nat. Rev. Cancer 2005, 5, 21–28.
- Clézardin, P.; Coleman, R.; Puppo, M.; Ottewell, P.; Bonnelye, E.; Paycha, F.; Confavreux, C.B.; Holen, I. Bone metastasis: Mechanisms, therapies, and biomarkers. Physiol. Rev. 2021, 101, 797–855.
- Zhang, W.; Bado, I.; Wang, H.; Lo, H.C.; Zhang, X.H. Bone Metastasis: Find Your Niche and Fit in. Trends Cancer 2019, 5, 95–110.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
806
Revisions:
2 times
(View History)
Update Date:
11 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




