Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sithara Radhakrishnan | -- | 7481 | 2022-07-09 09:52:56 | | | |
| 2 | Vivi Li | -34 word(s) | 7447 | 2022-07-11 04:13:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Radhakrishnan, S.; Lakshmy, S.; Santhosh, S.; Kalarikkal, N.; Chakraborty, B.; Rout, C.S. Electrochemical Glucose Sensors Based on 2D Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/24963 (accessed on 07 February 2026).
Radhakrishnan S, Lakshmy S, Santhosh S, Kalarikkal N, Chakraborty B, Rout CS. Electrochemical Glucose Sensors Based on 2D Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/24963. Accessed February 07, 2026.
Radhakrishnan, Sithara, Seetha Lakshmy, Shilpa Santhosh, Nandakumar Kalarikkal, Brahmananda Chakraborty, Chandra Sekhar Rout. "Electrochemical Glucose Sensors Based on 2D Materials" Encyclopedia, https://encyclopedia.pub/entry/24963 (accessed February 07, 2026).
Radhakrishnan, S., Lakshmy, S., Santhosh, S., Kalarikkal, N., Chakraborty, B., & Rout, C.S. (2022, July 09). Electrochemical Glucose Sensors Based on 2D Materials. In Encyclopedia. https://encyclopedia.pub/entry/24963
Radhakrishnan, Sithara, et al. "Electrochemical Glucose Sensors Based on 2D Materials." Encyclopedia. Web. 09 July, 2022.
Copy Citation
Diabetes is a health disorder that necessitates constant blood glucose monitoring. The industry is always interested in creating novel glucose sensor devices because of the great demand for low-cost, quick, and precise means of monitoring blood glucose levels. Electrochemical glucose sensors, among others, have been developed and are now frequently used in clinical research. Nonetheless, despite the substantial obstacles, these electrochemical glucose sensors face numerous challenges. Because of their excellent stability, vast surface area, and low cost, various types of 2D materials have been employed to produce enzymatic and nonenzymatic glucose sensing applications.
glucose sensor
2D materials
electrochemical sensor
wearable and flexible sensor
photoelectrochemical sensors
1. Introduction
Diabetes was the seventh leading cause of mortality worldwide in 2016, according to the World Health Organization (WHO). Diabetes-related early death has been continuously rising. There were around 1.6 million deaths globally in 2019. According to the WHO, diabetes is a major cause of various health effects, including blindness, lethargy, kidney failure, strokes, numbness, and heart attacks [1]. Our mental pictures of diabetes are of overweight people and the elderly. According to current ICMR studies, over 96,000 children suffer from diabetes, with nearly 16,000 new cases diagnosed each year in children under the age of 14. As a result, there is a risk that diagnoses may be overlooked and treatment will be delayed, resulting in a potentially fatal illness [2]. Thus, glucose monitoring is an important tool for diabetes diagnosis [3].
Several types of biosensors have been developed for this purpose. They include invasive, non-invasive glucose monitoring systems, and continuous glucose monitoring systems (CGM). In this sector, reliable and accurate glucose monitoring is always a major difficulty. Recently, a report by Nava et al. provided a systematic review of the comparative diagnostic accuracy of CGM devices in preterm infants. They used a bivariate model to summarize the receiver operating characteristic curve (ROC) curve and extract the area under the curve (AUC). According to their findings, CGM systems have low sensitivity for detecting hypoglycemia in preterm newborns, but good accuracy for identifying hyperglycemia [4]. As a result, new approaches are continually being developed, and minimally invasive glucose monitoring technologies have sparked a lot of interest in this field. A biosensor’s main components include:
- (1)
-
A biological element that distinguishes the analyte from other substances;
- (2)
-
A transducer, which converts the biorecognition event into a quantifiable signal;
- (3)
-
A processing system that converts a quantifiable signal into a readable signal [5].
Various types of transducers employed for the detection are calorimetric, thermometric, magnetic, optical, piezoelectric, electrochemical transducers, etc. Calorimetric transducers rely on changes in heat energy caused by chemical reactions between the bio-analyte and enzymes [6]. Thermistors are commonly used to detect temperature changes. Magnetic transducers detect changes in the magnetic properties of magnetic fields such as direction, intensity, and flux [7]. Optical transducers make use of the interaction of the optical field with bio components [8]. Piezoelectric transducers operate on the basis of a change in the frequency of oscillations that is proportional to the mass bound to the surface of the electrode [9]. Finally, electrochemical transducers rely on changes in electrical signals caused by electrochemical processes at the electrode surface, which are directly proportional to analyte concentration [5]. Among all these, electrochemical glucose sensors have a high potential due to several appealing features such as low cost, fast response time, high sensitivity, ease of operation, and excellent miniaturization and construction potential for portable glucose sensing devices for point-of-care (POC) applications [10]. The advantage and disadvantages are given below in Table 1.
Table 1. Advantages and disadvantages of electrochemical glucose sensors based on 2D materials.
| Pros | Cons |
|---|---|
| Low power requirements, linear output, and good resolution Excellent repeatability and accuracy Less expensive Fast response time with high sensitivity and low detection limit Multianalyte detection [11] |
Narrow or limited temperature range Short or limited shelf life |
The transduction techniques used in electrochemical glucose sensors are amperometry, conductometry; capacitive, impedance spectroscopy; voltammetry and potentiometry (ion-selective) field effect transistor. Its features are discussed below in Table 2.
Table 2. Different types of electrochemical sensors.
| Amperometry | Conductometry | Voltammetry | Potentiometry |
|---|---|---|---|
| Amperometry means the measurement of the current flow in a closed loop of cells using an excitation signal produced by the generator Advantages are
|
Response of the conductometric enzyme biosensors is mainly due to protons generated by a biocatalytic reaction inside the layer of immobilized enzyme Advantages are
Not very specific (less selective) [13] |
Here measurements are related to the recording of either the current–time or the current–voltage relationship by applying known potential varying between the WE and REF electrodes Advantages are Simplicity, sensitivity, speed, and low costs |
In this technique, negligible bias current flows as the potential between a working electrode and a reference electrode is measured across some interface Advantages are
Not very specific (less selective) [14] |
With the great advancements in nanotechnology over the last decade, there has been a surge in research interest in nanomaterial-based electrochemical glucose sensors. Combining glucose biosensors with the distinct properties of developing nanostructures based on two-dimensional (2D) advanced materials has resulted in significantly better sensor performance in terms of sensitivity and selectivity [15]. These recent advances have also improved the key sensor properties related to biofouling, long-term use, limit of detection, and wearability.
Reviews on electrochemical glucose sensors have already been published, encompassing their operational principles [16], history [17], important breakthroughs, and the issues these electrochemical glucose sensors confront. There are also papers discussing the utilization of nanostructures for electrochemical glucose sensors [10]. For example, graphene-based electrochemical sensors for the detection of glucose were well-reviewed by Zhang et al. [18]. After graphene, a large number of 2D materials were discovered and are also widely used in electrochemical glucose sensing. However, comprehensive reviews discussing the 2D materials for enzymatic and non-enzymatic electrochemical sensors are rare.
The first half of this entry presents the mechanisms of electrochemical glucose sensors, followed by a discussion of recent advancements based on the use of 2D materials to improve glucose sensor performance. The second section focuses on wearable glucose sensors and provides a comprehensive analysis of different innovative non-invasive detection concepts, as well as associated limitations, challenges, and prospects. The fast advancement of wearable and mobile technologies has sparked considerable interest among researchers in non-invasive glucose biosensing. The last decade has also witnessed the development of photoelectrochemical glucose sensors. Despite its late beginnings, PEC glucose sensing has grown rapidly as a significant branch of electrochemical glucose detection. There are some noteworthy reviews available that state advancements in PEC sensing and performance. However, no review concentrating on PEC glucose sensing based on 2D materials has been described thus far.
To further understand the achievements of the last decade in depth, the readers must go through recent topics such as nanomaterials-based electrochemical glucose sensors, graphene-based electrochemical glucose sensors, PEC sensors, advances in wearable sensors, and so on [1][11][19][20].
This entry covers the state of the art of electrochemical glucose sensors based on 2D materials developed primarily from 2010 onwards. Figure 1 shows the summary of the entry.
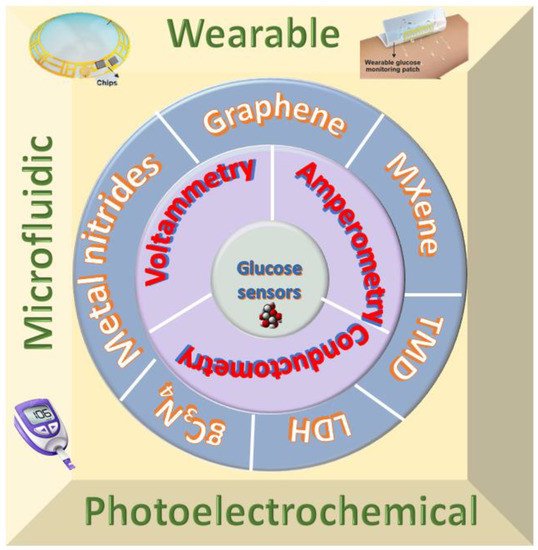
Figure 1. Summary of recent advancement of two-dimensional materials for electrochemical glucose sensors.
Commercialy Available Electrochemical Glucose Sensors
The Graphene Market and 2D Materials Assessment Report (2021–2031) predicted 18 primary application areas and the transition of graphene biosensors from the laboratory to the commercial market in the coming decades. According to this assessment analysis, the graphene market is expected to expand from $100 million in 2020 to $700 million by 2031 [21]. This success will take time and will be the result of ongoing global research and commercialization initiatives. There is a race to commercialize graphene-based glucose sensors, and their introduction will transform the way diseases are diagnosed and treated. Patients will obtain test results quickly, diagnoses will arrive on-site in minutes, and treatment can begin immediately, saving important and precious time. For example, in partnership with integrated graphene, researchers from the University of Bath recently developed an electrochemical sensor whose function is unaffected by changes in pH or temperature. Boronic acid is used in the new sensor, which is connected to a graphene foam surface. On top, an electroactive polymer layer bind to the boronic acid. When glucose is present, it binds to the boronic acid competitively, displacing the polymer. The sensor generates an electric current proportional to the amount of polymer displaced, allowing the concentration of glucose in the sample to be precisely detected [22]. Even though this 2D-material-based glucose sensor shows quite satisfactory properties for glucose sensing, it should be noted that the electrochemical glucose sensors in the current market are still based on noble metal catalysts. However, there was a trend for graphene-based electrochemical glucose sensors. The global market trends and opportunities show that glucose sensors based on 2D materials are on an upward trend.
2. 2D Materials for Electrochemical Glucose Sensing
2.1. Graphene
Since Novoselov and Geim discovered graphene in 2004, it has been a prominent carbon material. Graphene is a type of carbon material that features a honeycomb-like crystal lattice and an ultrathin one-atom-thick sheet structure. Graphene-based materials show potential application in electrochemical glucose sensing. Table 3 shows the properties of graphene that facilitate glucose sensing. The quantity of research publications and reviews on graphene and graphene-hybrids for electrochemical sensing has been steadily increasing in recent years. Different research groups have thoroughly examined graphene-based electrochemical glucose sensors. Zhang et al. [18] reviewed the current achievements in the preparation of graphene-based material for electrochemical glucose sensing. G. Gnana Kumar reviewed non-enzymatic electrochemical glucose sensors based on graphene [18]. According to these reviews, graphene is a promising material for electrochemical glucose detection. Comparing GO and rGO, pristine graphene has less interfacial contact; hence GO and rGO were widely utilized in high-performance glucose sensors. Since the application of graphene-based electrochemical glucose sensors was already reviewed in 2018 and 2017, researchers will focus here on the studies done after 2018.
Table 3. Properties of 2D materials required for sensing.
| 2D Materials | Properties Required for Sensing |
|---|---|
| Graphene |
|
| MXene |
|
| TMDs |
|
| LDH |
|
2.1.1. Enzymatic Glucose Sensors Based on Graphene
Enzyme immobilization on various nanostructured materials (organic or inorganic composites, nanoparticles and 2D materials) has recently received considerable attention in electrochemical sensors because it improves enzyme activity and selectivity towards specific target analytes. Considering the combination of an organic or inorganic composite with a 2D material, Baek et al. modified the gold chip with Cu nanoflower- gold NPs-decorated GO nanofibers. Here GO nanofibers were electrospun over a gold chip that was further decorated with Au nanoparticles following Cu nanoflowers; then, the gold chip was covered with 1% Nafion as a binding agent. With the addition of glucose, it interacts with O2 in the presence of Cu-nanoflower@AuNPs-GO NFs to form H2O2 using an enzymatic catalytic process, resulting in a significant increase in electric current. The doping of AuNPs over GO NFs helps to enhance the sensitivity of glucose. The growth mechanism for Cu-nanoflower follows the nucleation and growth phases. While the Cu2+ ion interacts with the phosphate anion in PBS solution to form a copper phosphate crystal, the amide backbone in proteins (HRP and GOx) coordinates with the crystal to form the Cu–protein complex. This complex acts as a seed for the nanoflower, and the nuclei expand over time to form the nanoflower’s petals. A multi-layered nanoflower structure might be formed by a sequence of these successive processes. The Cu-nanoflower reacts with the glucose itself, forming a synergetic action with the GO NFs and AuNPs. Its stability was bolstered by the GO NFs as a backer. Because the 3D-structured AuNPs increase the surface area of the AuNPs, an intrinsic peroxidase-like activity could help HRP improve the electrochemical activities [26]. The immobilization of the enzyme over the nanoflowers such as Cu helps to enhance the sensitivity, durability as well as stability. In addition to this, GO NFs as a supporter also enhance the stability. This sensor shows excellent current even after 20 days suggesting the potential for field use.
Another electrochemical sensor was fabricated by Mao et al. He used rGO to improve the sensitivity and selectivity of the ZnO nanorod biosensor. In this work, the ZnO nanorods were hydrothermally synthesized over a polyethylene terephthalate (PET) substrate. The ZnO/PET working electrode was then coated with electrodeposited rGO, and AuNPs were scattered on the surface, resulting in ZnO/rGO/Au/PET. Lastly, the GOx was physically adsorbed on the electrode’s surface, yielding a GOx/rGO/ZnO/Au/PET glucose sensor with a sensitivity of 56.32 µA mM −1 cm−2 and a linear range of 0.1 to 12 mM [27]. However, under 10 cycles of bending, this working electrode exhibits good current responsiveness. However, after 15 cycles, the device’s performance is poor because GOx and rGO peel away from the ZNO nanorod, resulting in microcrack development [27]. Hossain and Slaughter have suggested a hybrid glucose biosensor employing MWCNTs and graphene with great sensitivity and selectivity. A one-step solvothermal approach was used to make a solution containing both MWCNTs functionalized with carboxylic groups and chemically-derived graphene. PtNPs were electrochemically deposited onto a thin layer formed by drop-casting this suspension onto an Au electrode. Finally, GOx was immobilized and coated with Nf on the nanostructured electrode. The hybrid biosensor was built with a sensitivity of 26.5 µA mM−1cm−2 and a linear detection range of 0.5 to 13.5 mM [28].
2.1.2. Non-Enzymatic Glucose Sensors Based on Graphene
A large number of transition metal nanoparticles such as platinum (Pt), gold (Au) and palladium (Pd) were synthesized and employed in non-enzymatic sensors. These metal nanoparticles have an electronic structure having unpaired-d electrons, and unfilled d-orbitals help in the electrocatalytic activity of glucose. In order to enhance the electrocatalytic activity of glucose, several supports have been investigated, among which GO serves as a suitable supporting material. Because of its high catalytic activity and stability, platinum is commonly employed as an electrocatalytic electrode material. Sakar and colleagues describe a unique design of glucose sensor with graphene Schottky diodes consisting of a graphene (G)/platinum oxide (PtO)/n-silicon (Si) heterostructure, taking into mind the benefits of combining graphene with the platinum electrode. They discovered that the platinum oxide film thickness affects the sensor’s sensitivity and that raising the PtO film thickness can increase the sensor’s sensitivity by up to 150%. This was ascribed to an increase in the number of active sites for glucose oxidation and the thickness of the graphene layer, resulting in increased charge carrier mobility and concentration. The device works by glucose molecule oxidation over the surface of the suggested heterostructure electrode due to the catalytic activity of the PtO thin film. Aside from that, the glucose molecules are broken down into gluconolactone, which creates gluconic acid, H2, and electrons. The current was measured from the graphene surface after applying a forward bias to the Si terminal. The physisorption interaction between PtO (metal-semiconductor Schottky junction) and graphene caused a shift in graphene’s fermi-level location and p-doping [29]. Here graphene is employed as both a protective and sieving layer to protect the PtO film and further enhance the sensor stability and selectivity.
Compared to Pt-based electrodes, the main advantage of using Au-based electrodes for glucose sensing is the higher current response, which allows for higher sensitivity and the ability to detect glucose at a neutral pH. However, the main drawback of Au-based electrodes is the low glucose oxidation efficiency on the Au electrode surface, which can be mitigated by using arrays of nanoelectrodes separated by non-electroactive materials. Furthermore, because these electrodes are better activated in alkaline solutions, they cannot be used in in vivo studies, they have surface contamination from anions such as phosphates and chlorides, and their selectivity is significantly smaller than Pt-based electrodes. Scandurra et al. prepared a graphene paper-based electrode using the dewetting technique. In this case, an 8-nm-thick Au layer was sputtered onto graphene paper before being dewetted with a laser. Dewetting with a laser-produced smaller AuNPs on the electrode surface. The sensor’s sensitivity was 1240 µA mM−1 cm−2 [30].
Combining the advantages of another transition-metal oxide palladium (Pd) and CNT with graphene nanoplates, Kiattisak et al. modified glassy carbon (GCE) using a nanocomposite of multi-walled carbon nanotubes wrapped with palladium nanoparticle-graphene nanoplatelets (PdNPs-GNPs/MWCNTs). This modified GCE was coupled with a flow injection amperometric detector where this sensor exhibits a detection limit of around 0.008 mM. The following reactions can be used to describe the mechanism of glucose oxidation by Pd nanoparticles.
Pd + Glucose → Pd-Glucose ads + H+ + e−
Pd (OH)2 + glucose → Pd + gluconolactone + H2O
Pd + 2OH− → Pd (OH)2 + 2e−
Electrode materials such as Au and Pt are suitable for glucose detection, but they are costly. As a result, non-precious transition metals such as nickel (Ni) and copper (Cu) and their oxides have been studied. Despite the wide range of catalytic materials available for glucose sensors, nickel (Ni)-based nanomaterials have attracted researchers’ interest due to their high catalytic activity for glucose oxidation in alkaline medium, resulting in NiOOH and Ni(OH)2 species. Despite their high electrocatalytic activity, surface fouling from glucose oxidation compromises their stability. As a result, a conductive nanostructured substrate is required to improve electron transport and electrode stability against Ni glucose oxidation. Considering graphene’s excellent properties as a catalyst support material, Jothi et al. fabricated a non-enzymatic glucose sensor based on graphene nanoribbon/graphene sheet/nickel nanoparticles to prevent re-stacking and increase surface area. This Ni-based hybrid has a larger specific surface area, more active sites, and better electrical conductivity, allowing for easy ion transport and unrestricted OH- diffusion throughout the electrochemical process. The mechanism of glucose oxidation by Ni nanoparticles can be explained using the reactions listed below [31].
NiO(OH) + glucose → Ni(OH)2 + gluconolactone
Gluconolactone → gluconic acid
Among these Ni-based materials, Ni-based porous materials have a large surface area, and more active sites have been used as sensitive materials for glucose sensors. To exhibit the full potential of porous Ni materials, different kinds of substrates such as Ni foil, SPEC, and Cu foil have been used. Still, the electron transferability of porous Ni is still weak. Exploiting the properties of graphene, where graphene provides anchoring, conducting and separating actions, Ren et al. prepared a porous Ni over exfoliated graphene by using the hydrogen bubble method over the Cu foil substrate. This porous sensor shows high sensitivity due to the high electron transfer ability between the active reaction centre and electrode induced by the graphene [32]. Another Ni/graphene hybrid material was reported by Lavanya et al. where this hybrid material was synthesized using a simple in situ chemical reduction method. To begin, Ni2+ was adsorbed onto the GOR and GOS surfaces via the electrostatic interaction between functional groups (epoxy, carboxyl, and hydroxyl groups) and the Ni2+ on the GOR and GOS surfaces. The Ni(OH)2 developed in GOS after adding NaOH precipitant was then co-reduced with the help of hydrazine hydrate. The reducing agent contributes to the reduction of GOR and GOS, the conversion of Ni2+ ions to Ni0 nanoparticles, and the development of Ni nanoparticles in the GS/GNR network throughout this process. This sensor shows a detection limit of around 2.5 nM, and this superior catalytic performance of this sensor was due to the synergistic action of Ni nanoparticle and GS/GNR hybrid network. The Ni nanoparticles were uniformly dispersed throughout the GS/GNR hybrid to increase electrical conductivity, a greater surface area with more active sites, and unrestricted flow of OH- ions during the electrochemical process. The incorporated Ni nanoparticles are an effective catalytic active material for direct glucose oxidation, which improves electron transfer and results in a high lower detection limit, selectivity, and good sensitivity for glucose detection. Here during the co-reduction of GOR, GOS and Ni(OH)2, the abundant functional groups in the basal and edges of GOR and GOS offer additional anchoring sites for the incorporation of more Ni nanoparticles, i.e., catalytic site, and increase the rate of electron transfer between the electrode and glucose and thereby enhancing the performance of sensor [31].
According to recent research, bimetallic materials, particularly bimetal alloys, outperform monometallic counterparts in catalytic performance. Several studies on the performance of bimetallic materials for glucose sensing have been published. However, its performance can be improved by using a support material such as graphene. Deng et al. created a NiFe alloy nanoparticle/graphene oxide hybrid (NiFe/GO) based on this [33]. Rukiye et al. described another Ni-based bimetallic material in which the working electrode was decorated with monodisperse platinum–nickel nanocomposites-decorated on reduced graphene oxide (Pt/Ni@ rGO) manufactured utilizing a novel ultrasonic hydroxide aided reduction technique. This sensor shows excellent electrochemical activity with a sensitivity of around 171.92 μA mM−1 cm2 and LOD of 6.3 μM [34].
Among the transition metallic nanoparticles, metal sulfides, which are found as minerals in nature, have higher cycle stability than conducting polymers. Furthermore, because of the numerous sorts of structures, they have a lot of research potential in glucose sensors. Among these, CuS has attracted a lot of attention due to its exceptional fundamental property diversity, such as attractive photovoltaic capabilities, strong thermal stability, great transport properties, and so on. Yan et al. prepared CuS nanoflakes reduced graphene oxide (rGO/CuSNFs) nanocomposite to avoid its coagulation using a surfactant-free method. This sensor shows excellent sensitivity and a low-detection limit [35]. On the other hand, aggregation and low dispersion remain major issues in the synthesis of MNPs on graphene support, resulting in poor electrocatalytic performance and low stability.
Intrinsically conducting polymers (CPs) are one of the most relevant and extensively used materials for sensor modification due to their unique chemical and physical properties, such as adjustable architecture, adaptability, versatility, room stability, and sensitivity to surface changes in electrochemical activity with minor changes in its surface. Polypyrroles, polyanilines, and polythiophenes have received a lot of attention because of their good film-forming properties, electrical semiconductivity, great transparency in the visible range, and exceptional thermal and environmental stability [36]. CPs have recently been hybridized or mixed with graphene-based materials. When the materials are linked, each component’s combined optical, electrical, thermal, mechanical, chemical, or electrochemical capabilities can be exploited for chemical and biological sensing [37]. For glucose measurements, a composite comprising (PANI/PDPA) along with graphene nanosheets synthesized in a liquid–liquid (CHCl3/HCl) interface individual by Muthushankar et al. During the polymerization procedure, the PANI and PDPA chains grew nicely and were evenly distributed over the graphene nanosheets. The improved electro-catalytic activity of Gra-PANI-co-PDPA-ME towards glucose was achieved with greater sensitivity of 0.51 µA/µM at 5 s. The presence of aniline, graphene and diphenoquinone diamine (DPDI2+) together with their synergistic relationship improves electron transport for glucose oxidation. Interference tests further confirm that the constructed sensor is best suited for glucose sensing performance [38]. Silver (Ag) nanoparticles are considered a promising candidate because of their excellent stability and biocompatibility. Utilizing the advantages of this, Ag nanoparticle and carbon-based nanocomposite having excellent electrical conductivity and stability, Deshmukh et al. demonstrated an Ag-PANI/rGO nanocomposite using a simple hydrothermal method. This composite demonstrated a detection limit of 0.79 µM with a quick response time. They proved the viability of using Ag-PANI/rGO nanocomposites to detect glucose in real-world samples of organic fluids (milk, apple juice, mango juice, orange juice, and Coke). Here the introduction of rGO into PANI polymer improves the operational stability and electron transfer rate. This sensor shows stability up to 30 days [39]. Poly(3,4-ethylenedioxythiophene) (PEDOT) is another candidate that has piqued the interest of this group of CPs. PEDOT has been extensively used as an electrode material among CPs due to its low oxidation potential, good stability and mid-band gap energy. Considering the favorable properties of this polymer, a non-enzymatic glucose sensor based on an Au electrode was modified utilizing electroreduced graphene oxide (ERGO) and layered PEDOT by Mesut et al. The thin films of PEDOT–ERGO were made using a simple electrochemical approach based on progressively layering electrodeposition on an Au electrode, which was employed as a non-enzymatic glucose sensor in the study. The PEDOT–ERGO nanocomposite modified Au electrodes (Au–PEDOT–ERGO) were employed as electrocatalysts for voltammetry and amperometry glucose detection. This sensor exhibits a LOD of around 0.12 μM with a sensitivity of 696.9 μA mM−1 cm−2 [40].
2.2. MXene
MXene is suitable for building high-performance electrochemical glucose sensors because it has high hydrophilicity due to surface termination groups (O, OH, and F), great electrical conductivities, excellent ion intercalation behavior, facile functionalization, and dependable large-scale manufacture. Several high-quality reviews on MXenes have been published to date, suggesting the applicability of MXene in sensor fabrication [23][41][42][43]. However, no review focused on MXene-based electrochemical glucose sensors is available so far. Table 3 shows the properties of MXene facilitate in glucose sensing
2.2.1. Enzymatic Glucose Sensors Based on MXene
MXenes, similar to other 2D materials, can include additional materials such as metal nanoparticles, enzymes, CPs, and metal oxides, resulting in improved structural and electrical characteristics [44]. For example, without the need for external reducing agents, one-step hybridization of Au, Ag, and Pd nanoparticles from their respective aqueous solutions onto the surface of MXene was performed and employed as a substrate for SERS. Ti3C2 was the first MXene to be employed in constructing electrochemical glucose sensors, and Rakhi and her colleagues produced the first electrochemical sensor based on Ti3C2 in 2016. A nanocomposite of gold nanoparticles (Au) and MXene (Ti3C2Tx) for glucose detection has been reported. After in situ reductions of chloroauric acid with sodium borohydride, they deposited Au nanoparticles on the surface of MXene, and the nanocomposite was subsequently dispersed in Nafion. This composite was then drop casted on a glassy carbon electrode before being immobilized with glucose oxidase (GOx) to produce Au/GOx//MXene/Nafion/GCE. Au nanoparticles were critical in increasing the electron exchange between the electrode and the active center of GOx. After a first morphological examination, the biosensor was assessed using CV and amperometry. The glucose-sensing mechanism is based on the following Equations (6) and (7). GOx is composed of two extremely similar protein subunits and one coenzyme molecule, flavin adenine dinucleotide (FAD). FAD is reduced to FADH2 during the transfer process with 2e− and 2H+ because it is present in the active site of the GOx. The biochemical reaction between GOx and glucose culminates in the conversion of glucose to glucono-D-lactone and the reduction of FAD to FADH2. FADH2 was then oxidized by dissolved oxygen to create H2O2 and form FAD.
GOx (FAD) + Glucose → GOx (FADH2) + glucono-D-lactone
GOx (FADH2) + O2 → GOx (FAD)+ H2O2
MXene, as well as Au nanoparticles, can both contribute to improved electron transfer kinetics between the electrode and active redox centers of the enzyme, while the large surface area with the distinctive layered architecture of Au/MXene nanocomposite effectively accommodates the enzyme. The amperometric response was shown to be linear over a concentration range of 0.1 to 18 μM, with a LOD as low as 5.9 μM. This sensor also shows long-term stability. Furthermore, in the presence of AA, UA and DA, this suggested biosensor is highly specific for glucose. The use of enzymes for glucose detection ensures a strong electrochemical response, particularly in complicated matrices. However, there are significant drawbacks to using enzymes, including the risk of enzyme inactivation, the reproducibility of enzyme immobilization, and the increased cost of analysis. Hence, the development of non-enzymatic sensors is becoming increasingly popular [45]. Using a mixing–drying process, researchers improved biosensing capabilities by combining MXene nanosheets with hydrophilic groups with the properties of graphene sheets to offer new functions. This three-dimensional (3D) porous hybrid film had a more open structure, which allowed glucose oxidase to enter through the inner pores, improving the hybrid film’s stable immobilization and retention of GOx. The constructed biosensor outperformed conventional 3D porous materials with sensitivities of 20.16 mM (LOD 0.13 mM) and 12.10 mM (LOD) 0.10 mM) in O2− saturated phosphate-buffered solution (PBS) and air-saturated PBS, respectively. This 3D porous film results in higher stability for GOX immobilization. This is due to (i) the enhanced hydrophilic property of this hybrid MG film, (ii) the open surface facilitates more access to GOx, and (iii) the excess 3D pores and hydrophilic wall of film this film provides a favorable microenvironment for GOX to stay in the pores and avoid re-dissolution in PBS [46].
In practical applications of glucose biosensing, the harmful intermediate product, H2O2, formed during enzymatic glucose oxidation usually hinders the action of GOx. Wu, M. et al. created a hybrid Ti3C2/poly-L-lysine (PLL)/glucose oxidase (GOx) nanohybrid that could catalyze the cascade processes of glucose oxidation and the intermediate H2O2 breakdown to address this issue. The PLL-modified MXene possesses a positive charge and exhibits excellent GOx loading capacity where the amine-groups in PLL are able to form a crosslink with the physically adsorbed enzyme to form MXene nanosheets covered with GOx/PLL. The Ti3C2 MXene was shown to be capable of catalyzing the breakdown reaction of H2O2, and when combined with the GOx, a cascade reaction in glucose decomposition was created. Ti3C2/PLL/GOx nanoreactors with higher catalytic activity were placed on glassy carbon electrodes to create a 2.6 μM LOD glucose biosensor [47].
2.2.2. Non-Enzymatic Glucose Sensors Based on MXene
Li et al., for the first time, reported a non-enzymatic glucose sensor based on MXene/Nickel–Cobalt layered double hydroxide (NiCo-LDH). They synthesized MXene over NiCo-LDH using a simple hydrothermal method. Because MXene is negatively charged, it can absorb cations and provide nucleation sites to form nanoparticles. The redox reaction in the following equation produces hydroxyl ions, which react with Co2+ and Ni2+ to make hydroxide monomers on the MXene sheet, and the monomers then react with others to produce primary particles in the hydrothermal reaction. In the meantime, some divalent cobalt is oxidized to trivalent cobalt. As the number of initial particles increased, they progressively collected and transformed into nanosheets coated on MXene.
4CH3OH + NO3− → 4HCHO + NH3 + OH− + 2H2O
This glucose sensor has a broad linearity range (0.002 mM–4.096 mM), a LOD of around 0.53 µM, with a quick response time (3 s) at a working potential of 0.45 V (vs. SCE). Additionally, good selectivity, stability, and repeatability were obtained [48]. A non-enzymatic glucose sensor based on the MXene–Cu2O hybrid was recently studied by Gopal et al. This sensor has a linear range of 0.01–30 mM and an LOD of 2.83 µM [49].
2.3. TMD Based Electrochemical Glucose Sensors
Two-dimensional TMD nanosheets are appealing as electrochemical glucose sensors because of their high conductivity, large surface area, high signal/noise ratio and quick electron transfer kinetics and, most importantly, their practicality for forming composites.
2.3.1. Enzymatic Glucose Sensors Based on TMD
Other metal NPs, such as Au NPs, are utilized to alter MoS2 in addition to Ni and Cu NPs in enzymatic glucose sensors [50]. The use of AuNPs accelerates electron transport from the electrode to the immobilized enzyme. This enables GOx electrochemistry without the usage of an electron mediator. Thus, the GC modified with Au NPs and MoS2 was studied by Su et al. and showed enhanced electrocatalytic activity [51]. Another electrochemical glucose sensor was fabricated by Parlak et al. using a MoS2/Au NPs hybrid. Here, enzymes aided in directly transferring ions to the modified MoS2 surface, increasing its bioelectroactivity for redox processes. Because of their open 3D architectures with a significant interlayer space and surface area for enzyme immobilization, these electrodes demonstrated remarkable sensitivity to glucose. As a result, fast mass transport was accelerated, and glucose diffused quickly across the electrode surface. Despite the fact that the study revealed bioelectrocatalytic reactions of glucose, suggesting substantial improvements in biocatalysis at a MoS2-based enzymatic nanointerface, real-sample analysis and interferences were not performed, which could have demonstrated selectivity and further justified the analytical functionality of the biosensor. The constructed MoS2 enzymatic electrode, on the other hand, revealed a new potential for creating a unique 3D sensing substrate [52].
Because H2O2 is a consequence of many oxidative biological activities, measuring it with MoS2 electrodes allows for the identification of many more tiny molecules by integrating the sensor into lab-on-chip devices for intracellular detection. For instance, a glucose sensor based on MoS2 demonstrated strong activity towards H2O2. GOx was immobilized over the surface and used to detect glucose. The current response of this biosensor grew as the concentration of glucose climbed from 2.0 to 16.0 mM. Despite the fact that glucose oxidation consumes O2, the biosensor’s overall current response increased because both O2 and H2O2 contributed to the increase in reduction current. To explore the large surface area and enhanced electrical conductivity of 3D porous graphene aerogel, Jeong et al. fabricated a flow-injection biosensor device. They incorporated 2D MoS2 with 3D graphene aerogel using a facile hydrothermal approach to enhance the electrochemical sensing performance. Here the different porous structures of 3D MGA provide rapid, efficient pathways for electrons and ions, demonstrating excellent electrochemical performances. The following qualities contribute to the superior performance of these 3D MGA-based biosensors. At the interface, the interconnected network of MoS2 exposes extensive basal planes against the electrolyte solution, which enhances the H2O2 reduction activity of MoS2 nanosheets using oxygen substitution at exposed Mo edges. Thus, showing excellent selectivity and sensitivity [53][54].
2.4. Layered Double Hydroxides (LDHs)
Nanostructures, having a large surface area and a rough surface, may be capable of transmitting faraday currents for oxidation while reducing the impact of external interfering molecules, such as UA, AA, and AP. LDHs are a type of two-dimensional material with the structural formula [M1−x II M xIII(OH)2](An−)x/n.mH2O, where MII and MIII are divalent and trivalent metals, respectively, and An− signifies the anions between the interlayer spaces. The properties required for glucose sensing are shown in Table 3.
2.4.1. Enzymatic Glucose Sensors Based on LDH
Enzyme-based biohybrid materials, which are made by immobilising various oxidoreductases in the LDH host structure, have been used to detect target analytes such as hydrogen peroxide, phenol derivatives, glucose and other inhibitors or enzyme substrates utilizing amperometric transduction methods, primarily chronoamperometry. There have been very few publications on LDH-based enzymatic glucose sensors. Because of its stability, glucose oxidase (GOx) is frequently used as a model to examine various biosensor setups. The major findings from investigations using glucose biosensors based on the immobilization of GOx in LDH matrices are presented here. Physical adsorption over the electrode surfaces produced by gradual evaporation of colloidal suspensions of mixes of LDH and GOx has been the most extensively utilized method for GOx immobilization. The entrapped biomolecules are cross-linked with the help of glutaraldehyde in the vicinity of bovine serum albumin to prevent the enzyme from being released. Glutaraldehyde was not used since composite materials based on chitosan or alginate with Zn/Al-Cl LDH were used instead. This method of deposition allows a substantial and known quantity of enzyme to be entrapped in the clay layer, assuring great response sensitivity. In this regard, Colambari et al. synthesized Mg/Al LDHs containing ferrocene sulfonate or ferrocene carboxylate (Fc-COOH) as interlayer anions using the coprecipitation method, and they built glucose sensors based on the immobilization of GOx in a glutaraldehyde and BSA network. MnO2 nanoparticles were effectively integrated into the outer protective glutaraldehyde/BSA membrane to improve the selectivity of this GOx/Fc-COOH LDH/GC biosensor, allowing oxidizable interferents (e.g., acetaminophen, urate, and ascorbic acid) present in the sample solution to be analyzed to be peroxidised before reaching the electrode surface [55]. Shan and colleagues created a biosensor that is extremely sensitive to glucose utilizing Zn/Cr LDH. Ferricinium derivatives reoxidised FADH2 to FAD, which is followed by reoxidation of ferrocene (Fc) to Fc+ directly at the electrode, enabling glucose measurement under anaerobic circumstances in this biosensor [56]. The biosensor design reported by Shan et al. is based on a “ping-pong” mechanism for transmitting electrons from glucose to the mediator [57]. Mousty et al. developed another glucose sensor based on redox-active Zn/Cr LDH intercalated in the interlayer domain and contained (3-sulfopropyl) ferrocene carboxylate. Chronoamperometry at 0.5 V was used to examine the biosensor’s performance for glucose measurement under anaerobic conditions, and the sensitivity was 65 µA mM−1 cm−2 in the concentration range of 10–25 µM [58]. GOx was immobilized at the surface of the electrode by entrapment in inert LDH matrices (i.e., NiAl-NO3, ZnAl-Cl,) and ZnAl–Cl/biopolymers (chitosan or alginate) [59] composites with the goal of generating glucose amperometric electrochemical sensors. The majority of these LDH/GOx biosensors were made by solvent casting a predetermined amount of LDH and GOx combination onto a glassy carbon electrode or Pt surface. In these investigations, GOx was immobilized on LDH particles through adsorption, and then glutaraldehyde cross-linking was used to inhibit enzyme leaching. Tonelli’s group demonstrated yet another way of GOx immobilization by trapping GOx during the electrogeneration of NiAl LDH films over the Pt electrodes [60]. Farhat et al. recently published an amperometric biosensor based on Co3Mn–CO3 layered double hydroxide/GOx with biopolymer carrageenan. They used a coprecipitation approach to make this Co3Mn–CO3 LDH and then impregnated this LDH composite over a carbon felt to make this 3D porous sensor [60]. Zhang et al. revealed the first-ever direct electron transfer of GOx immobilized in Mg/Al hydrotalcite nanosheets, creating new avenues for the development of third-generation GOx glucose sensors employing LDHs as the immobilising matrix [61].
2.4.2. Non-Enzymatic Glucose Sensors Based on LDH
Through coprecipitation and electrodeposition, nickel and cobalt inculcated LDHs were produced and used for non-enzymatic glucose sensing [62]. Using the electro-oxidation processes of Ni ions, the LDH nanostructures outperformed GOD-based biosensors for glucose sensing even in the absence of GOD catalase. Furthermore, simple LDH materials were fabricated, such as porous structured NiFe LDH nanolayers grown over the nickel foam by the simple hydrothermal method and used for glucose detection [63]. Similarly, Zhao et al. fabricated a non-enzymatic glucose sensor using a multi-component sensing system where the electrode is made up of NiCo-LDH and cobalt copper carbonate hydroxide (CCCH) nanorods over a Cu foam. Here the large surface area and excellent conductivity of the Cu foam help in enhancing the electrocatalytic properties. This multicomponent hierarchical nanostructure features a large accessible surface area, as well as effective ion diffusion and electron transfer, thus enhancing the sensing properties of NiCo-LDH [64].
Hai et al. fabricated a NiAl LDH, loaded carbon microcylinder (CMC) composite using microelectromechanical systems (C-MEMS). When combined with electrocatalytic Ni centres, the suggested method demonstrated good performance due to improved conductivity [65]. Furthermore, several LDH nanoarchitectures intercalated with valuable noble metals, such as Au, NPs incorporating NiAl LDH SWCNTs–rGO hybrids exhibited stronger electrocatalytic capabilities for glucose oxidation than others. Good conductivity, generated from electron tunneling junctions and 3D CNTs/rGO networks, is thought to be the source of these features [66].
When LDH-based catalysts are integrated with conductive carbon supports such as conductive polymers, CNTs, and graphene, the extraordinary properties of materials based on carbon, such as greater active sites, better consistency, and larger surface to volume ratio, can help improve the catalytic activity and stability of LDH layers. Inserting graphene between the LDH interlayers improves conductivity and glucose detection with high sensitivity while also boosting resistance to poisoning in chloride ion solutions. NiCo LDH-rGO [67] nanoribbons and NiFe LDH-rGO nanohybrids are used in this context [68] and were synthesized using electrochemical coprecipitation and deposition, respectively, and their catalytic properties were investigated. The electrodes with the nanostructures had a significant diffusion coefficient and excellent electrochemical catalytic activity towards glucose. Shahrokhian and group developed a core-shell structure to improve the electrocatalytic properties of CoNi-LDHs, notably in terms of shape, by growing CoNi-LDHs NS over a nanoporous tubular Cu(OH)2 utilizing a green, simple, and extremely controllable direct three-step in situ process over a GC. The step involves (1) copper film electrodeposition on GCE, (2) copper conversion to Cu(OH)2 nanotubes, and (3) CoNi-LDH electrodeposition over Cu(OH)2NTs/GCE. The rate-determining steps of the entire electrocatalytic reaction are represented by the equation below, which involves the diffusion of glucose molecules to the metal sites in the core–shell structure, where the OH− travels from the alkaline solution and then permeates into the CoNi-LDH interlayers.
LDH (OH−)− MIII + Glucose → LDH COIII + Gluconolactone
2CuOOH + 2e− + Glucose → 2CuO + 2OH− + Gluconolactone
This highly porous core-shell nanostructure adheres strongly to the conductive substrate, allowing for electrical and physical contact between the GCE surface and the active components, improving the overall stability and conductivity of the modified electrode. The commercialization capability of this sensor was also evaluated by growing this 3D hierarchical structure over a screen-printed electrode [69].
2.5. Other 2D Materials
Balasubramanian et al. synthesized a cobalt vacancy-rich Co(OH)2 ultrathin nanosheet over a screen-printed electrode and demonstrated its catalytic activity. A practical, simple, and in situ technique for obtaining cobalt hydroxide nanosheets with numerous cobalt vacancies is described here. The cobalt defects significantly enhance the charge transfer rates and increase the number of electroactive sites, resulting in outstanding glucose and L-cysteine oxidation performance. This VCo-Co(OH)2 electrode has a low detection limit of roughly 295 nM over a dynamic range of 0.4 M–8.23 mM [70]. Sahoo et al. studied the catalytic activity of non-faceted and faceted crystal cupric oxide (CuO) nanoribbons using a microwave and hydrothermal method. Here the non-enzymatic glucose sensor made-up of both non-faceted and faceted CuO crystals shows enhancement in amperometric oxidation current that is proportional to the glucose concentration. Their study shows that the glucose sensitivity of faceted CuO is higher than that of non-faceted crystal CuO due to the 2D thin and polygonal shape of this facet plane CuO. Here the facet plane of CuO, such as (100) and (110) planes, play a prominent role in improving the catalytic activity due to their higher surface energy. The faceted ends give more surface area and more electroactive species and are advantageous for electrolyte ion transferring and exchanging activities. The catalytic activity with glucose is as follows:
2CuO(OH)) + glucose → 2CuO + gluconolactone + H2O
Gluconolactone → Gluconic acid
The activation of these CuO nanostructures causes the amperometric current to increase with increasing glucose concentration. Here the hopping free-charges in the facet plane of CuO show a significantly lower detection limit of around 58 µM [71].
Other elements of group VA (also known as “pnictogens” or the “nitrogen” group) can likewise have a layered structure. These elements are similar to graphene: phosphorene, bismuthene, antimonene, and arsenene. Ling chia and group, for the first time, explored the use of pnictogens for non-enzymatic glucose detection. Because pnictogen nanosheets have strong carrier mobilities, they may aid in promoting fast electron transfer kinetics for glucose oxidation. In addition, pnictogens have excess lone-paired electrons on the surface and interact with glucose at high pH with good adsorption capabilities; thus, it could also aid in facilitating the adsorption mechanism by bringing glucose molecules into close vicinity with the electrocatalytic Au@Ag nanorods and enhance the sensing capabilities of both pnictogens and Au@AgNR. The study theoretically proves and opens up new vistas for the potential use of pnictogens-based composites in the future development of electrochemical sensors for the pharmaceutical, biomedical, and food industries [72].
References
- Reddy, V.S.; Agarwal, B.; Ye, Z.; Zhang, C.; Roy, K.; Chinnappan, A.; Narayan, R.J.; Ramakrishna, S.; Ghosh, R. Recent Advancement in Biofluid-Based Glucose Sensors Using Invasive, Minimally Invasive, and Non-Invasive Technologies: A Review. Nanomaterials 2022, 12, 1082.
- Sharma, P. Over 95,000 Children Suffer from Type-1 Diabetes: ICMR. Available online: https://www.livemint.com/science/health/over-95-000-children-suffer-from-type-1-diabetes-icmr-11654542643638.html (accessed on 16 June 2022).
- Witkowska Nery, E.; Kundys, M.; Jeleń, P.S.; Jönsson-Niedziółka, M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal. Chem. 2016, 88, 11271–11282.
- Nava, C.; Modiano Hedenmalm, A.; Borys, F.; Hooft, L.; Bruschettini, M.; Jenniskens, K. Accuracy of Continuous Glucose Monitoring in Preterm Infants: A Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e045335.
- Reghunath, R.; devi, K.; Singh, K.K. Recent Advances in Graphene Based Electrochemical Glucose Sensor. Nano Struct. Nano Objects 2021, 26, 100750.
- Danielsson, B.; Hedberg, U.; Rank, M.; Xie, B. Recent Investigations on Calorimetric Biosensors. Sens. Actuators B Chem. 1992, 6, 138–142.
- Chen, Y.-T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing Using Magnetic Particle Detection Techniques. Sensors 2017, 17, 2300.
- Damborský, P.; Švitel, J.; Katrlík, J. Optical Biosensors. Essays Biochem. 2016, 60, 91–100.
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11, 448.
- Zaidi, S.A.; Shin, J.H. Recent Developments in Nanostructure Based Electrochemical Glucose Sensors. Talanta 2016, 149, 30–42.
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical Glucose Sensors in Diabetes Management: An Updated Review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709.
- Castle, J.R.; Kenneth Ward, W. Amperometric Glucose Sensors: Sources of Error and Potential Benefit of Redundancy. J. Diabetes Sci. Technol. 2010, 4, 221–225.
- Soldatkin, A.P.; El’skaya, A.V.; Shul’ga, A.A.; Jdanova, A.S.; Dzyadevich, S.V.; Jaffrezic-Renault, N.; Martelet, C.; Clechet, P. Glucose Sensitive Conductometric Biosensor with Additional Nafion Membrane: Reduction of Influence of Buffer Capacity on the Sensor Response and Extension of Its Dynamic Range. Anal. Chim. Acta 1994, 288, 197–203.
- Walker, N.L.; Roshkolaeva, A.B.; Chapoval, A.I.; Dick, J.E. Recent Advances in Potentiometric Biosensing. Curr. Opin. Electrochem. 2021, 28, 100735.
- Khan, R.; Radoi, A.; Rashid, S.; Hayat, A.; Vasilescu, A.; Andreescu, S. Two-Dimensional Nanostructures for Electrochemical Biosensor. Sensors 2021, 21, 3369.
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505.
- Wang, J. Glucose Biosensors: 40 Years of Advances and Challenges. Electroanalysis 2001, 13, 983–988.
- Zhang, C.; Zhang, Z.; Yang, Q.; Chen, W. Graphene-Based Electrochemical Glucose Sensors: Fabrication and Sensing Properties. Electroanalysis 2018, 30, 2504–2524.
- Wu, I.-C.; Weng, Y.-H.; Lu, M.-Y.; Jen, C.-P.; Fedorov, V.E.; Chen, W.C.; Wu, M.T.; Kuo, C.-T.; Wang, H.-C. Nano-Structure ZnO/Cu2O Photoelectrochemical and Self-Powered Biosensor for Esophageal Cancer Cell Detection. Opt. Express OE 2017, 25, 7689–7706.
- Shu, J.; Tang, D. Recent Advances in Photoelectrochemical Sensing: From Engineered Photoactive Materials to Sensing Devices and Detection Modes. Anal. Chem. 2020, 92, 363–377.
- Graphene Market & 2D Materials Assessment 2021–2031. 2020. Available online: https://www.idtechex.com/zh/research-report/graphene-market-and-2d-materials-assessment-2021-2031/789 (accessed on 11 May 2022).
- Team Develops Novel Chemical Glucose Sensing Method Based on Boronic Acids and Graphene Foam. Available online: https://phys.org/news/2022-02-team-chemical-glucose-method-based.html (accessed on 16 June 2022).
- Wu, X.; Ma, P.; Sun, Y.; Du, F.; Song, D.; Xu, G. Application of MXene in Electrochemical Sensors: A Review. Electroanalysis 2021, 33, 1827–1851.
- Aziz, A.; Asif, M.; Ashraf, G.; Azeem, M.; Majeed, I.; Ajmal, M.; Wang, J.; Liu, H. Advancements in Electrochemical Sensing of Hydrogen Peroxide, Glucose and Dopamine by Using 2D Nanoarchitectures of Layered Double Hydroxides or Metal Dichalcogenides. A Review. Microchim. Acta 2019, 186, 671.
- Ping, J.; Fan, Z.; Sindoro, M.; Ying, Y.; Zhang, H. Recent Advances in Sensing Applications of Two-Dimensional Transition Metal Dichalcogenide Nanosheets and Their Composites. Adv. Funct. Mater. 2017, 27, 1605817.
- Baek, S.H.; Roh, J.; Park, C.Y.; Kim, M.W.; Shi, R.; Kailasa, S.K.; Park, T.J. Cu-Nanoflower Decorated Gold Nanoparticles-Graphene Oxide Nanofiber as Electrochemical Biosensor for Glucose Detection. Mater. Sci. Eng. C 2020, 107, 110273.
- Mao, Q.; Jing, W.; Zhou, F.; Liu, S.; Gao, W.; Wei, Z.; Jiang, Z. Depositing Reduced Graphene Oxide on ZnO Nanorods to Improve the Performance of Enzymatic Glucose Sensors. Mater. Sci. Semicond. Process. 2021, 121, 105391.
- Hossain, M.F.; Slaughter, G. PtNPs Decorated Chemically Derived Graphene and Carbon Nanotubes for Sensitive and Selective Glucose Biosensing. J. Electroanal. Chem. 2020, 861, 113990.
- Sakr, M.A.; Elgammal, K.; Delin, A.; Serry, M. Performance-Enhanced Non-Enzymatic Glucose Sensor Based on Graphene-Heterostructure. Sensors 2020, 20, 145.
- Scandurra, A.; Ruffino, F.; Sanzaro, S.; Grimaldi, M.G. Laser and Thermal Dewetting of Gold Layer onto Graphene Paper for Non-Enzymatic Electrochemical Detection of Glucose and Fructose. Sens. Actuators B Chem. 2019, 301, 127113.
- Jothi, L.; Jayakumar, N.; Jaganathan, S.K.; Nageswaran, G. Ultrasensitive and Selective Non-Enzymatic Electrochemical Glucose Sensor Based on Hybrid Material of Graphene Nanosheets/Graphene Nanoribbons/Nickel Nanoparticle. Mater. Res. Bull. 2018, 98, 300–307.
- Ren, Z.; Mao, H.; Luo, H.; Liu, Y. Glucose Sensor Based on Porous Ni by Using a Graphene Bottom Layer Combined with a Ni Middle Layer. Carbon 2019, 149, 609–617.
- Deng, Z.-P.; Sun, Y.; Wang, Y.-C.; Gao, J.-D. A NiFe Alloy Reduced on Graphene Oxide for Electrochemical Nonenzymatic Glucose Sensing. Sensors 2018, 18, 3972.
- Ayranci, R.; Demirkan, B.; Sen, B.; Şavk, A.; Ak, M.; Şen, F. Use of the Monodisperse Pt/ Nanocomposite Synthesized by Ultrasonic Hydroxide Assisted Reduction Method in Electrochemical Nonenzymatic Glucose Detection. Mater. Sci. Eng. C 2019, 99, 951–956.
- Yan, X.; Gu, Y.; Li, C.; Zheng, B.; Li, Y.; Zhang, T.; Zhang, Z.; Yang, M. A Non-Enzymatic Glucose Sensor Based on the CuS Nanoflakes–Reduced Graphene Oxide Nanocomposite. Anal. Methods 2018, 10, 381–388.
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials 2021, 11, 252.
- Lei, W.; Si, W.; Xu, Y.; Gu, Z.; Hao, Q. Conducting Polymer Composites with Graphene for Use in Chemical Sensors and Biosensors. Microchim. Acta 2014, 181, 707–722.
- Muthusankar, E.; Ragupathy, D. Graphene/Poly(Aniline-Co-Diphenylamine) Nanohybrid for Ultrasensitive Electrochemical Glucose Sensor. Nano Struct. Nano Objects 2019, 20, 100390.
- Deshmukh, M.A.; Kang, B.-C.; Ha, T.-J. Non-Enzymatic Electrochemical Glucose Sensors Based on Polyaniline/Reduced-Graphene-Oxide Nanocomposites Functionalized with Silver Nanoparticles. J. Mater. Chem. C 2020, 8, 5112–5123.
- Eryiğit, M.; Çepni, E.; Kurt Urhan, B.; Öztürk Doğan, H.; Öznülüer Özer, T. Nonenzymatic Glucose Sensor Based on Poly(3,4-Ethylene Dioxythiophene)/Electroreduced Graphene Oxide Modified Gold Electrode. Synth. Met. 2020, 268, 116488.
- Sivasankarapillai, V.S.; Sharma, T.S.K.; Hwa, K.-Y.; Wabaidur, S.M.; Angaiah, S.; Dhanusuraman, R. MXene Based Sensing Materials: Current Status and Future Perspectives. ES Energy Environ. 2022, 15, 4–14.
- Kalambate, P.K.; Gadhari, N.S.; Li, X.; Rao, Z.; Navale, S.T.; Shen, Y.; Patil, V.R.; Huang, Y. Recent Advances in MXene–Based Electrochemical Sensors and Biosensors. TrAC Trends Anal. Chem. 2019, 120, 115643.
- Mathew, M.; Rout, C.S. Electrochemical Biosensors Based on Ti3C2Tx MXene: Future Perspectives for on-Site Analysis. Curr. Opin. Electrochem. 2021, 30, 100782.
- Zhu, J.; Ha, E.; Zhao, G.; Zhou, Y.; Huang, D.; Yue, G.; Hu, L.; Sun, N.; Wang, Y.; Lee, L.Y.S.; et al. Recent Advance in MXenes: A Promising 2D Material for Catalysis, Sensor and Chemical Adsorption. Coord. Chem. Rev. 2017, 352, 306–327.
- Rakhi, R.B.; Nayak, P.; Xia, C.; Alshareef, H.N. Novel Amperometric Glucose Biosensor Based on MXene Nanocomposite. Sci. Rep. 2016, 6, 36422.
- Gu, H.; Xing, Y.; Xiong, P.; Tang, H.; Li, C.; Chen, S.; Zeng, R.; Han, K.; Shi, G. Three-Dimensional Porous Ti3C2Tx MXene–Graphene Hybrid Films for Glucose Biosensing. ACS Appl. Nano Mater. 2019, 2, 6537–6545.
- Wu, M.; Zhang, Q.; Fang, Y.; Deng, C.; Zhou, F.; Zhang, Y.; Wang, X.; Tang, Y.; Wang, Y. Polylysine-Modified MXene Nanosheets with Highly Loaded Glucose Oxidase as Cascade Nanoreactor for Glucose Decomposition and Electrochemical Sensing. J. Colloid Interface Sci. 2021, 586, 20–29.
- Li, M.; Fang, L.; Zhou, H.; Wu, F.; Lu, Y.; Luo, H.; Zhang, Y.; Hu, B. Three-Dimensional Porous MXene/NiCo-LDH Composite for High Performance Non-Enzymatic Glucose Sensor. Appl. Surf. Sci. 2019, 495, 143554.
- Gopal, T.S.; Jeong, S.K.; Alrebdi, T.A.; Pandiaraj, S.; Alodhayb, A.; Muthuramamoorthy, M. Andrews Nirmala Grace MXene-Based Composite Electrodes for Efficient Electrochemical Sensing of Glucose by Non-Enzymatic Method. Mater. Today Chem. 2022, 24, 100891.
- Mphuthi, N.; Sikhwivhilu, L.; Ray, S.S. Functionalization of 2D MoS2 Nanosheets with Various Metal and Metal Oxide Nanostructures: Their Properties and Application in Electrochemical Sensors. Biosensors 2022, 12, 386.
- SpringerLink. Direct Electrochemistry of Glucose Oxidase and a Biosensor for Glucose Based on a Glass Carbon Electrode Modified with MoS2 Nanosheets Decorated with Gold Nanoparticles. Available online: https://link.springer.com/article/10.1007/s00604-014-1178-9 (accessed on 23 April 2022).
- Parlak, O.; ncel, A.; Uzun, L.; Turner, A.P.F.; Tiwari, A. Biosens. Bioelectron 2017, 89, 545–550.
- Jeong, J.-M.; Yang, M.; Kim, D.S.; Lee, T.J.; Choi, B.G.; Kim, D.H. High Performance Electrochemical Glucose Sensor Based on Three-Dimensional MoS2/Graphene Aerogel. J. Colloid Interface Sci. 2017, 506, 379–385.
- Huang, J.; Dong, Z.; Li, Y.; Li, J.; Tang, W.; Yang, H.; Wang, J.; Bao, Y.; Jin, J.; Li, R. MoS2 nanosheet functionalized with Cu nanoparticles and its application for glucose detection. Mater. Res. Bull. 2013, 48, 4544–4547.
- Colombari, M.; Ballarin, B.; Carpani, I.; Guadagnini, L.; Mignani, A.; Scavetta, E.; Tonelli, D. Glucose Biosensors Based on Electrodes Modified with Ferrocene Derivatives Intercalated into Mg/Al Layered Double Hydroxides. Electroanalysis 2007, 19, 2321–2327.
- Shan, D.; Cosnier, S.; Mousty, C. HRP Wiring by Redox Active Layered Double Hydroxides: Application to the Mediated H2O2 Detection. Anal. Lett. 2003, 36, 909–922.
- Shan, D.; Yao, W.; Xue, H. Electrochemical Study of Ferrocenemethanol-Modified Layered Double Hydroxides Composite Matrix: Application to Glucose Amperometric Biosensor. Biosens. Bioelectron. 2007, 23, 432–437.
- Mousty, C.; Prevot, V. Hybrid and Biohybrid Layered Double Hydroxides for Electrochemical Analysis. Anal. Bioanal. Chem. 2013, 405, 3513–3524.
- Shi, Q.; Han, E.; Shan, D.; Yao, W.; Xue, H. Development of a High Analytical Performance Amperometric Glucose Biosensor Based on Glucose Oxidase Immobilized in a Composite Matrix: Layered Double Hydroxides/Chitosan. Bioprocess Biosyst. Eng. 2008, 31, 519–526.
- Tonelli, D.; Scavetta, E.; Giorgetti, M. Layered-Double-Hydroxide-Modified Electrodes: Electroanalytical Applications. Anal. Bioanal. Chem. 2013, 405, 603–615.
- Zhang, Y.; Chen, X.; Wang, J.; Yang, W. The Direct Electrochemistry of Glucose Oxidase Based on Layered Double-Hydroxide Nanosheets. Electrochem. Solid State Lett. 2008, 11, F19.
- Scavetta, E.; Ballarin, B.; Gazzano, M.; Tonelli, D. Electrochemical Behaviour of Thin Films of Co/Al Layered Double Hydroxide Prepared by Electrodeposition. Electrochim. Acta 2009, 54, 1027–1033.
- Lu, Y.; Jiang, B.; Fang, L.; Fan, S.; Wu, F.; Hu, B.; Meng, F. Highly Sensitive Nonenzymatic Glucose Sensor Based on 3D Ultrathin NiFe Layered Double Hydroxide Nanosheets. Electroanalysis 2017, 29, 1755–1761.
- Zhao, Z.; Sun, Y.; Song, J.; Li, Y.; Xie, Y.; Cui, H.; Gong, W.; Hu, J.; Chen, Y. Highly Sensitive Nonenzymetic Glucose Sensing Based on Multicomponent Hierarchical NiCo-LDH/CCCH/CuF Nanostructures. Sens. Actuators B Chem. 2021, 326, 128811.
- Hai, B.; Zou, Y.-Q.; Guo, G.-B.; Wang, Z.-D.; Liu, Y.-Y.; Wang, X.-X.; Yan, H.; Ma, L.-T.; Bai, Y.-C. A Novel Strategy to Prepare LDH Networks Loaded Carbon Structure by C-MEMS Techniques for Glucose Detection. Chin. Chem. Lett. 2017, 28, 149–152.
- Fu, S.; Fan, G.; Yang, L.; Li, F. Non-Enzymatic Glucose Sensor Based on Au Nanoparticles Decorated Ternary Ni-Al Layered Double Hydroxide/Single-Walled Carbon Nanotubes/Graphene Nanocomposite. Electrochim. Acta 2015, 152, 146–154.
- Hai, B.; Wei, H. Facile Preparation of Three-Dimensional NiO Nanosheets Array on Carbon Sphere and Its High-Performance Nonenzymatic Glucose Detection. IOP Conf. Ser. Earth Environ. Sci. 2019, 300, 052037.
- Asadian, E.; Shahrokhian, S.; Iraji Zad, A. Highly Sensitive Nonenzymetic Glucose Sensing Platform Based on MOF-Derived NiCo LDH Nanosheets/Graphene Nanoribbons Composite. J. Electroanal. Chem. 2018, 808, 114–123.
- Shahrokhian, S.; Sanati, E.K.; Hosseini, H. Advanced On-Site Glucose Sensing Platform Based on a New Architecture of Free-Standing Hollow Cu(OH)2 Nanotubes Decorated with CoNi-LDH Nanosheets on Graphite Screen-Printed Electrode. Nanoscale 2019, 11, 12655–12671.
- Balasubramanian, P.; He, S.-B.; Deng, H.-H.; Peng, H.-P.; Chen, W. Defects Engineered 2D Ultrathin Cobalt Hydroxide Nanosheets as Highly Efficient Electrocatalyst for Non-Enzymatic Electrochemical Sensing of Glucose and l-Cysteine. Sens. Actuators B Chem. 2020, 320, 128374.
- Sahoo, R.K.; Das, A.; Samantaray, K.; Singh, S.K.; Mane, R.S.; Shin, H.-C.; Yun, J.M.; Kim, K.H. Electrochemical Glucose Sensing Characteristics of Two-Dimensional Faceted and Non-Faceted CuO Nanoribbons. CrystEngComm 2019, 21, 1607–1616.
- Chia, H.L.; Mayorga-Martinez, C.C.; Gusmão, R.; Novotny, F.; Webster, R.D.; Pumera, M. A Highly Sensitive Enzyme-Less Glucose Sensor Based on Pnictogens and Silver Shell–Gold Core Nanorod Composites. Chem. Commun. 2020, 56, 7909–7912.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
11 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




