Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michaela Wenzel | -- | 2719 | 2022-07-08 13:29:05 | | | |
| 2 | Rita Xu | Meta information modification | 2719 | 2022-07-11 04:08:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sidarta, M.; Baruah, L.; Wenzel, M. Bacterial Mechanosensitive Channels. Encyclopedia. Available online: https://encyclopedia.pub/entry/24950 (accessed on 02 March 2026).
Sidarta M, Baruah L, Wenzel M. Bacterial Mechanosensitive Channels. Encyclopedia. Available at: https://encyclopedia.pub/entry/24950. Accessed March 02, 2026.
Sidarta, Margareth, Luna Baruah, Michaela Wenzel. "Bacterial Mechanosensitive Channels" Encyclopedia, https://encyclopedia.pub/entry/24950 (accessed March 02, 2026).
Sidarta, M., Baruah, L., & Wenzel, M. (2022, July 08). Bacterial Mechanosensitive Channels. In Encyclopedia. https://encyclopedia.pub/entry/24950
Sidarta, Margareth, et al. "Bacterial Mechanosensitive Channels." Encyclopedia. Web. 08 July, 2022.
Copy Citation
Bacteria accumulate osmolytes to prevent cell dehydration during hyperosmotic stress. A sudden change to a hypotonic environment leads to a rapid water influx, causing swelling of the protoplast. To prevent cell lysis through osmotic bursting, mechanosensitive channels detect changes in turgor pressure and act as emergency-release valves for the ions and osmolytes, restoring the osmotic balance.
mechanosensitive channels
1. Introduction
Mechanosensitive channels are integral membrane proteins that are present in the membranes of bacteria, archaea, and eukaryotes [1][2]. These channels are closed under normal conditions and open in response to mechanical-membrane stretch [3]. This mechanosensitive response plays a role in a variety of biological functions such as hearing, touch, and cardiovascular regulation [4]. In bacteria, the role of mechanosensitive channels is that of an emergency-release valve. In response to a sudden shift from high to low osmolarity, as, e.g., experienced by soil bacteria after heavy rainfall, which leads to an influx of water, mechanosensitive channels release osmolytes to relieve increased turgor pressure and prevent cells from bursting (Figure 1) [5][6].
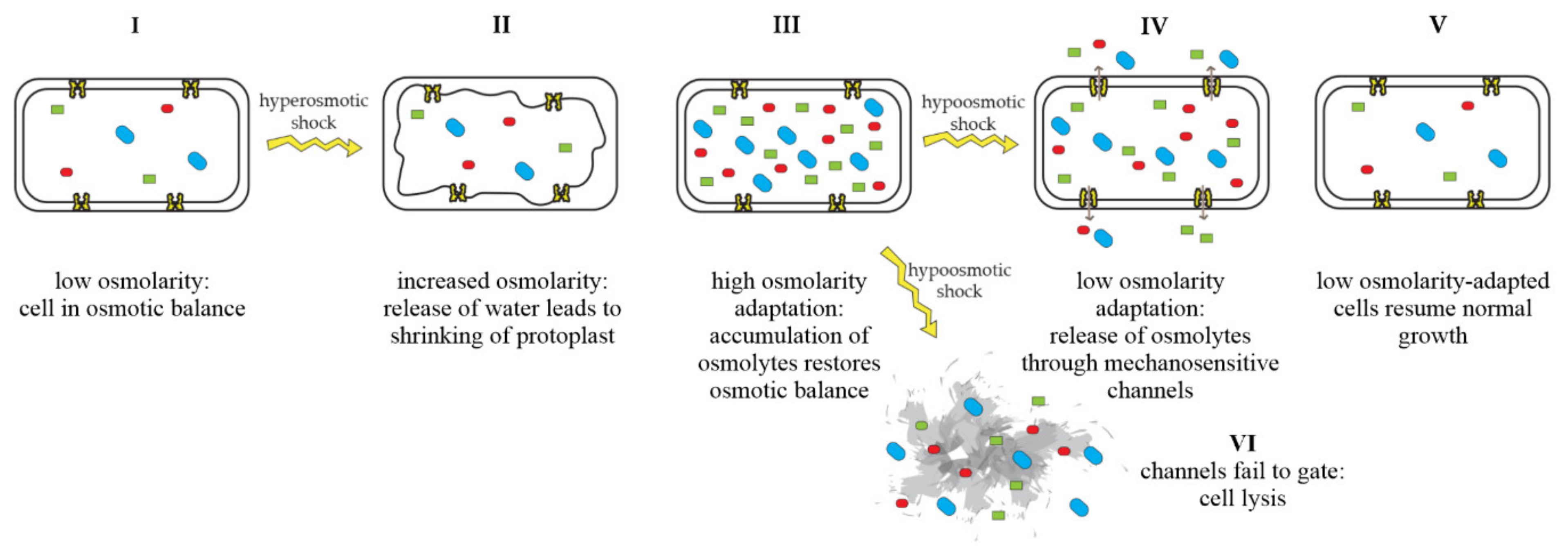
Figure 1. Adaptation of bacterial cells to changing osmotic pressure. A sudden increase in osmolarity leads to loss of water and shrinking of the protoplast, counteracted by the accumulation of compatible solutes, which raise intracellular-solute concentrations and restore the cell’s osmotic balance (I–III). A sudden decrease in osmolarity leads to an influx of water, resulting in increased turgor pressure, which is relieved by the opening of mechanosensitive channels and subsequent release of osmolytes into the environment (IV), restoring osmotic balance (V). If mechanosensitive channels fail to gate, cells lyse (VI). Figure adapted from Booth et al. [7].
Osmolytes, also referred to as osmoprotectants, are highly soluble organic compounds that do not interfere with cellular processes. Amino acids and derivatives (glutamate, glutamine, aspartate, proline, betaines), sugars (sucrose, trehalose), polyols (glycerol, arabitol, inositol), and many other compounds can assume the role of osmoprotectants [8][9][10]. In response to hypotonic challenges, Bacterial Mechanosensitive channels have been shown to release osmolytes, such as glycine betaine, glutamate, and trehalose [11][12][13].
Bacterial Mechanosensitive channels are often viewed from an environmental or biotechnological perspective, e.g., enhanced glutamate secretion in the industrial amino-acid producer Corynebacterium glutamicum. Yet, they have also been reported to play a role in bacterial pathogenesis [14][15][16][17][18] and appear to be of importance for the susceptibility of different bacterial species to antimicrobials [19][20][21]. Interestingly, some studies have shown that mechanosensitive channels increase the potency of certain antibiotics, by serving as their entry gates into bacterial cells [20][21], while others have observed that bacteria adapt to antibiotic-induced membrane stress by opening mechanosensitive channels that contribute to antibiotic tolerance [12][22].
2. Structure and Gating of Bacterial Mechanosensitive Channels
A bacterial cell typically harbors multiple mechanosensitive channels with different properties that allow a nuanced temporal response to various levels of hypoosmotic stress. These different properties are related to conductance, gating kinetics, sensitivity to membrane tension, and structure of the channel proteins. Based on these properties, mechanosensitive channels are typically divided into three classes: MscL (mechanosensitive channel of large conductance), MscS (mechanosensitive channel of small conductance), and MscM (mechanosensitive channel of mini conductance) [3][23]. However, in Escherichia coli, an additional class of mechanosensitive channel has been described. This class, named MscK (mechanosensitive channel K+), is structurally similar to MscS, but its gating requires lower membrane tension and high extracellular concentrations of potassium [24].
Both MscL- and MscS-type channels have been studied extensively, and crystal structures are available [25][26]. MscL-family proteins are moderately to highly conserved, whereas MscS-family proteins exhibit a broader structural diversity [2]. Typically, microorganisms possess only one MscL but multiple MscS homologs [27].
There are two model organisms that have been used predominantly to study bacterial mechanosensitive channels, the standard Gram-negative model E. coli and the ubiquitous Gram-positive soil bacterium Bacillus subtilis. However, C. glutamicum and its relative Mycobacterium tuberculosis have also been used as models to examine the structural and functional aspects of mechanosensitive channels.
A plethora of studies have been performed on these channels and many excellent reviews are available on their structure, gating mechanisms, and function in osmoadaptation [3][6][23][27][28][29][30][31][32].
2.1. Structural Determinants of MscL and MscS Gating
Several crystal structures are available for mechanosensitive channels. Two of these have become major models to study the structures and molecular-gating mechanisms of mechanosensitive channels, M. tuberculosis MscL (Mt-MscL, PDB: 2OAR) and E. coli MscS (Ec-MscS, PDB: 6PWP) (Figure 2) [33][34].
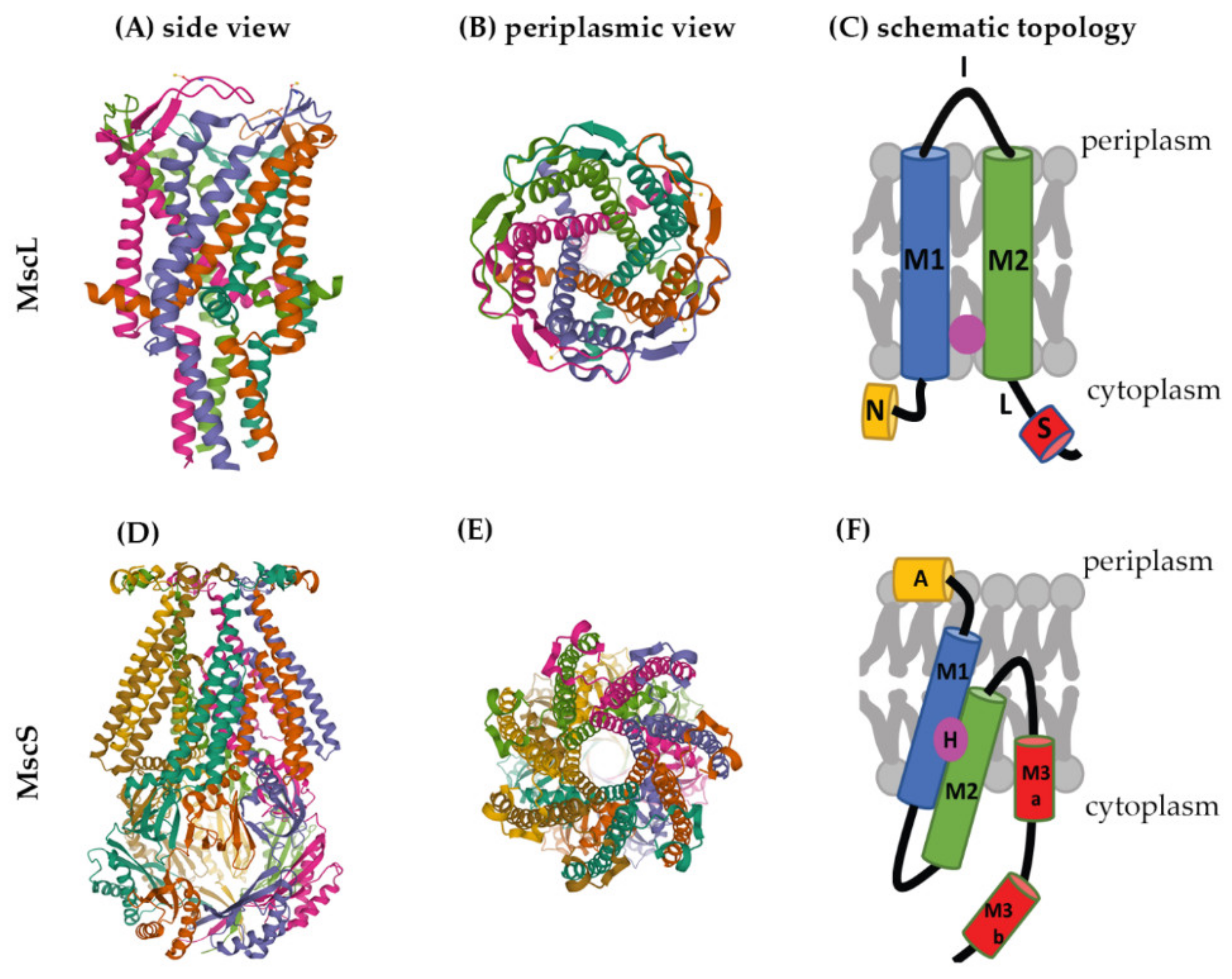
Figure 2. Structures of MscL and MscS. The structure of Mt-MscL (A–C) was derived from X-ray crystallography (PDB: 2OAR) [33]. (A) Side view, (B) periplasmic view, (C) schematic topological depiction of an MscL monomer. The structure of Ec-MscS (D–F) was derived from cryo-electron microscopy in lipid nanodiscs (PDB: 6PWP) [34]. (D) Side view, (E) periplasmic view, (F) schematic topological depictions of an MscS monomer. Schematic topological depictions of MscL and MscS were adapted from Pivetti et al. and Reddy et al. [1][34], respectively (relative sizes of domains not to scale). A: Anchor, N: N-terminal α-helix, M1, M2, M3a, and M3b: transmembrane domains, I: periplasmic loop, L: linker, S: short C-terminal region, H: hook lipid.
MscL channels form homopentamers, as shown for Mt-MscL in Figure 2A,B [33]. Each subunit of this pentamer has an N-terminal α-helix along the membrane (N), two α-helical transmembrane domains (M1 and M2), a periplasmic loop (I) that connects M1 and M2, and a short C-terminal region (S) linked to M2 via a flexible linker (L) (Figure 2C) [1][25][33][35].
M1 and M2 form the transmembrane channel, while the C-terminal region forms a cytoplasmic bundle below the channel pore and has been proposed to maintain the closed state of the channel [1][7]. The N-terminus of MscL acts as a “slide helix” that runs along the cytoplasmic membrane and stabilizes the M1 domain [35].
Recently, a hydrophobic nanopocket in the transmembrane region of MscL, situated between M1 and M2, sitting close to the surface of the inner membrane leaflet, has been identified and proposed to be essential for channel gating [36][37]. In the current model, this nanopocket is in contact with membrane-lipid fatty-acid chains, which act as negative modulators and prevent channel gating. Removal of lipid chains from the nanopocket through a membrane stretch is proposed to activate MscL [36].
MscS is a homoheptamer with a large cytoplasmic domain (Figure 2D,E) [3][38][39]. This cytoplasmic domain is believed to act as a molecular sieve that balances the passage of positive and negative osmolytes as well as ensures a net-neutral efflux in order to conserve the transmembrane potential [40]. The cytoplasmic domain has also been suggested to be a sensor for the excessive cytoplasmic crowding involved in preventing cytoplasm over-draining [41].
Each subunit of the MscS heptamer has three membrane-spanning helices: M1, M2, and M3 (Figure 2D–F). M1 and M2 form a sensor for membrane tension. M3 consists of two parts: the hydrophobic M3a that lines the pore and the amphipathic M3b situated at the membrane-cytoplasm interface [26]. An N-terminal domain, called Anchor (A), has recently been identified by cryo-electron microscopy (Figure 2F). This anchor is situated at the outer-leaflet interface and was shown to be essential for channel gating [34]. The new cryo-electron-microscopy structure also revealed the presence of a ‘hook’ lipid (H) that hooks to the top of each M2–M3 hairpin. This hook lipid is believed to facilitate force transition from the lipid bilayer to the transmembrane domains of the channel (Figure 3). However, it is yet unclear whether the hook lipid is removed from its binding pocket during membrane stretch or not [34].
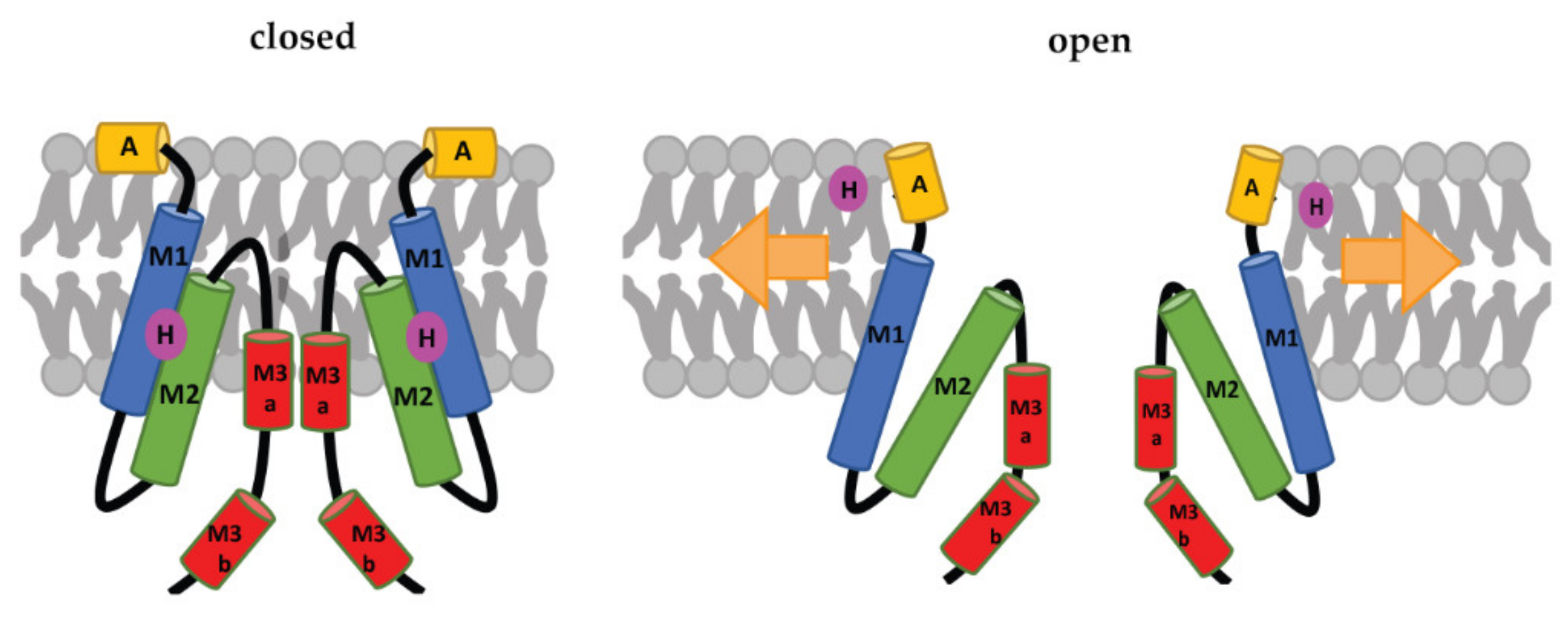
Figure 3. Schematic representation of the MscS-gating mechanism proposed by Reddy et al. [34]. Arrows represent ‘force-from-lipids’. The relative sizes of the domains are not to scale. A: Anchor, M1, M2, M3a, and M3b: transmembrane domains, H: hook lipid. The figure shows a model, in which the hook lipid moves out of its binding pocket upon membrane stretch and corresponding channel opening. An alternative model, where the hook lipid stays situated between M1 and M2, was not yet ruled out [34].
2.2. Importance of Membrane Lipids for the Gating Mechanism
The paradigm of the “force-from-lipid” model, in which, in the simplest of terms, a membrane stretch pulls the channel apart to open, has been long established [42][43]. In this model, the lipid bilayer influences channel gating by directly interacting with mechanosensitive channels or by modulating the global properties of the cell membrane, such as membrane thickness, membrane fluidity, membrane curvature, hydrophobic interactions, and many more [42][44][45].
In recent years, the roles of specific lipids and lipid–protein interaction sites, such as the hydrophobic lipid chain-binding pocket of MscL and the hook lipid binding to MscS, have been revealed. A recent cryo-electron-microscopy study has examined Ec-MscS in different membrane environments, mimicking stretched and unstretched conditions as well as supporting the diverse and complex roles of lipids in the gating of MscS [46]. This includes pore lipids, which prevent the passage of molecules through the channel in the closed state, gatekeeper lipids that stabilize the closed conformation of the channel and dissociate during membrane stretch, and pocket lipids that sit in pockets between subunits and are pulled out under membrane tension, transferring the force of the membrane stretch to the channel protein [46].
Not only can specific membrane lipids in the immediate proximity of the channels play a role in gating but also the overall membrane composition and organization critically influence the behavior of mechanosensitive channels. Since they respond to membrane stretch, factors such as overall membrane thickness and fluidity are the first to come to mind. Water influx into bacterial cells during hypoosmotic shock will increase the turgor pressure against the cell wall. This will stretch the bilayer, concomitantly leading to membrane thinning, fluidization, and increased membrane tension [47].
In fact, Ec-MscL was reported to be sensitive to lipid-bilayer thickness in vitro [48][49]. Thus, a decrease in bilayer thickness led to a lowered gating threshold for MscL, whereas an increase in bilayer thickness led to an increased MscL-gating threshold [49].
In contrast, Ec-MscS was reported to be less sensitive to bilayer thickness [49], indicating a different responsiveness from MscL and MscS. This was supported by another study, which showed that altering fatty-acyl chain length did not notably alter Ec-MscS-gating thresholds [45]. However, a significant shift in tension sensitivity was observed, when more rigid model membranes were used, suggesting that a less-fluid membrane environment, e.g., through enrichment of fully saturated lipids, hampers Ec-MscS gating [45].
Similarly, surface-adhesion forces have been observed to trigger mechanosensitive-channel opening in biofilms of Staphylococcus aureus [50]. Channel gating increased with increased adhesion force. It is believed that, when bacteria attach to a surface during the first stage of biofilm formation, adhesion forces will deform the bacterial cell wall and, consequently, generate sufficient membrane tension to trigger channel opening [51][52]. It is likely that adhesion forces work in concert with other driving forces, such as thickness and fluidity, to modulate channel gating.
3. Role of Mechanosensitive Channels during Infection
While new discoveries are being made regularly about the structural determinants of mechanosensitive-channel gating, their biological role as emergency-release valves that protect bacteria during hypoosmotic shock is long-standing and well-established. Thereby, they are typically viewed in the context of environmental challenges, e.g., the adaptation of soil bacteria such as B. subtilis to heavy rainfalls. However, in recent years, more and more evidence has been discovered about how they also play an important role for pathogenic bacteria during infection. For example, mechanosensitive channels play a role in adapting to osmotic changes occurring upon transitioning from the environment to the host and back [14][17]. They may also play an important role in adapting to changing osmotic conditions within the body, e.g., in bladder infections, where osmolarity can change dramatically depending on the patient’s water intake [53].
3.1. Transition between Host and Environment
Upon transitioning from the environment to the host (and vice versa), pathogens suddenly face a drastic osmotic change and need suitable adaptation mechanisms to survive this challenge. Typically, the human or animal body will constitute a higher osmolarity environment than many natural reservoirs, such as freshwater. For example, Francisella tularensis, a pathogen that causes tularemia disease in mammals [54][55][56], needs an MscS-like channel (Ft-MscS) to survive the transition from its mammalian host to freshwater [17]. Similarly, Campylobacter jejunii, a major cause of bacterial gastroenteritis in humans [57], needs MscS homologs to survive the hypoosmotic stress that occurs during transmission from the digestive tract of the host to the environment [14]. In both cases, mechanosensitive channels are necessary to maintain natural bacterial reservoirs and, thus, play a role in the environmental persistence and transmission of these pathogens. F. tularensis has been known to cause waterborne-tularemia outbreaks in several countries [54][55][56], and Campylobacter spp. are on the WHO’s list of antibiotic-resistant bacteria, against which new antibiotic treatments are most urgently needed [58]. Thus, understanding the role of mechanosensitive channels in transitioning from host to environment and their relevance for maintaining natural reservoirs, allowing transmission and spread of these bacteria, is of high relevance.
In some cases, mechanosensitive channels play a role in transitioning from the environment to the host and enable bacteria to colonize host tissues [15][16][18]. One example for this is Salmonella typhimurium. Studies have shown that the mechanosensitive channel YnaI is required for host-intestinal colonization, and the deletion of the ynaI gene in S. typhimurium leads to an increased internalization in macrophages [15][18]. Another notable example is Neisseria gonorrhoeae, which causes gonorrhea by colonizing the mucosal epithelia of the human urogenital tract. During infection, N. gonorrhoeae may experience fluctuating osmotic conditions, e.g., during the passing of urine. An MscS-like channel (Ng-MscS) has been shown to be essential for osmotic downshock survival in this organism, and the N. gonorrhoeae wild type outcompeted a mutant lacking this channel, with respect to colonization and survival, in a murine vaginal-tract-infection model, putting forward the importance of Ng-MscS in host colonization [16].
3.2. The Urinary Tract as an Osmotically Challenging Environment in the Human Body
The urinary tract is the most prominent example of an environment with drastically fluctuating osmolarity within the human body. Generally, urine is a complex, hypertonic medium with low pH as well as high salt and urea content [59]. In healthy adults, urine typically contains glucose (0.2–0.6 mM), creatine (0.38–55.6 mM), citrate (1.0–2.0 mM), sucrose (70–200 µM), manganese, amino acids, and traces of fatty acids [59]. However, depending on factors such as diet, water intake, frequency of passing urine, and a number of health conditions, the osmolarity of urine can vary considerably. For example, kidney urine usually has higher osmolarity and lower pH compared to bladder urine [59]. This variability poses dramatic osmotic challenges to bacteria colonizing the bladder and urethra. Despite being a generally harsh and challenging environment, uropathogens can survive and even thrive in the urogenital tract. These pathogens utilize urine contents as nutrients and rely on their osmoadaptive mechanisms to survive the stressful osmotic conditions in this environment [59].
Several studies have demonstrated the importance of osmoadaptation mechanisms for uropathogens, yet this has mostly been studied for high-osmolarity-adaptation strategies. For example, Culham et al. have shown that the osmoregulatory proline-transporter ProP has higher activity in the E. coli pyelonephritis isolate HU734 compared to the E. coli lab strain K-12 [53]. Deletion of proP impaired the in vitro growth of E. coli HU734 in human urine but not in a high-osmolarity minimal medium. Moreover, the deletion of ProP also reduced bladder colonization by HU734 [53]. Furthermore, it has been shown that the survival of uropathogenic E. coli in the urinary tract depends on OmpR, which is a part of the EnvZ-OmpR regulatory system that responds to hyperosmotic stress [60].
The osmolarity of urine has been suggested to influence the virulence of uropathogens [61][62]. Thus, the production of virulence factors in Pseudomonas aeruginosa increased when osmolarity was raised from 200 to 300 mOsmol/L [62]. However, a significant decrease in both growth and production of virulence factors was observed with a further increase in osmolarity. Additionally, P. aeruginosa grown in high osmolarity-medium (300 mOsmol/L) was more resistant to phagocytosis and more virulent in a mouse model than the same strain grown in nutrient broth.
These studies show that osmoadaptive mechanisms are crucial for the growth of uropathogens and colonization in the urinary tract. While the importance of hyperosmotic-stress-adaptation strategies in urine is rather clear, virtually nothing is known about the importance of low-osmolarity adaptation measures such as mechanosensitive channels. Yet, considering that the osmolarity of urine can decrease rapidly and considerably, e.g., by imbibing a large amount of water, it is reasonable to assume that mechanosensitive channels may play a role in this environment as well. This notion is supported by the apparent importance of Ng-MscS in N. gonorrhoeae colonization of the urogenital tract [16] and will be an interesting subject for future research.
References
- Pivetti, C.D.; Yen, M.-R.; Miller, S.; Busch, W.; Tseng, Y.-H.; Booth, I.R.; Saier, M.H. Two Families of Mechanosensitive Channel Proteins. Microbiol. Mol. Biol. Rev. 2003, 67, 66–85.
- Booth, I.R.; Miller, S.; Müller, A.; Lehtovirta-Morley, L. The Evolution of Bacterial Mechanosensitive Channels. Cell Calcium 2015, 57, 140–150.
- Kung, C.; Martinac, B.; Sukharev, S. Mechanosensitive Channels in Microbes. Annu. Rev. Microbiol. 2010, 64, 313–329.
- Árnadóttir, J.; Chalfie, M. Eukaryotic Mechanosensitive Channels. Annu. Rev. Biophys. 2010, 39, 111–137.
- Levina, N. Protection of Escherichia coli Cells against Extreme Turgor by Activation of MscS and MscL Mechanosensitive Channels: Identification of Genes Required for MscS Activity. EMBO J. 1999, 18, 1730–1737.
- Booth, I.R.; Blount, P. The MscS and MscL Families of Mechanosensitive Channels Act as Microbial Emergency Release Valves. J. Bacteriol. 2012, 194, 4802–4809.
- Booth, I.R.; Edwards, M.D.; Black, S.; Schumann, U.; Miller, S. Mechanosensitive Channels in Bacteria: Signs of Closure? Nat. Rev. Microbiol. 2007, 5, 431–440.
- Csonka, L.N. Physiological and Genetic Responses of Bacteria to Osmotic Stress. Microbiol. Rev. 1989, 53, 121–147.
- Bougouffa, S.; Radovanovic, A.; Essack, M.; Bajic, V.B. DEOP: A Database on Osmoprotectants and Associated Pathways. Database 2014, 2014, bau100.
- Hoffmann, T.; Bremer, E. Protection of Bacillus subtilis against Cold Stress via Compatible-Solute Acquisition. J. Bacteriol. 2011, 193, 1552–1562.
- Hoffmann, T.; Boiangiu, C.; Moses, S.; Bremer, E. Responses of Bacillus subtilis to Hypotonic Challenges: Physiological Contributions of Mechanosensitive Channels to Cellular Survival. Appl. Environ. Microbiol. 2008, 74, 2454–2460.
- Wenzel, M.; Chiriac, A.I.; Otto, A.; Zweytick, D.; May, C.; Schumacher, C.; Gust, R.; Albada, H.B.; Penkova, M.; Krämer, U.; et al. Small Cationic Antimicrobial Peptides Delocalize Peripheral Membrane Proteins. Proc. Natl. Acad. Sci. USA 2014, 111, E1409–E1418.
- Ajouz, B.; Berrier, C.; Garrigues, A.; Besnard, M.; Ghazi, A. Release of Thioredoxin via the Mechanosensitive Channel MscL during Osmotic Downshock of Escherichia coli Cells. J. Biol. Chem. 1998, 273, 26670–26674.
- Kakuda, T.; Koide, Y.; Sakamoto, A.; Takai, S. Characterization of Two Putative Mechanosensitive Channel Proteins of Campylobacter Jejuni Involved in Protection against Osmotic Downshock. Vet. Microbiol. 2012, 160, 53–60.
- Chaudhuri, R.R.; Morgan, E.; Peters, S.E.; Pleasance, S.J.; Hudson, D.L.; Davies, H.M.; Wang, J.; van Diemen, P.M.; Buckley, A.M.; Bowen, A.J.; et al. Comprehensive Assignment of Roles for Salmonella Typhimurium Genes in Intestinal Colonization of Food-Producing Animals. PLoS Genet. 2013, 9, e1003456.
- Wang, Z.; Wang, X.; Lu, P.; Ni, C.; Li, Y.; van der Veen, S. Identification and Characterization of the Neisseria gonorrhoeae MscS-Like Mechanosensitive Channel. Infect. Immun. 2018, 86, e00090-18.
- Williamson, D.R.; Dewan, K.K.; Patel, T.; Wastella, C.M.; Ning, G.; Kirimanjeswara, G.S. A Single Mechanosensitive Channel Protects Francisella tularensis Subsp. Holarctica from Hypoosmotic Shock and Promotes Survival in the Aquatic Environment. Appl. Environ. Microbiol. 2018, 84, e02203-17.
- Asogwa, M.; Miller, S.; Spano, S.; Stevens, M. Investigating the Role of the Bacterial Mechanosensitive Channel YnaI in Salmonella Pathogenesis. Access Microbiol. 2019, 1, 500.
- Kouwen, T.R.H.M.; Trip, E.N.; Denham, E.L.; Sibbald, M.J.J.B.; Dubois, J.-Y.F.; van Dijl, J.M. The Large Mechanosensitive Channel MscL Determines Bacterial Susceptibility to the Bacteriocin Sublancin 168. Antimicrob. Agents Chemother. 2009, 53, 4702–4711.
- Iscla, I.; Wray, R.; Wei, S.; Posner, B.; Blount, P. Streptomycin Potency Is Dependent on MscL Channel Expression. Nat. Commun. 2014, 5, 4891.
- Wray, R.; Iscla, I.; Gao, Y.; Li, H.; Wang, J.; Blount, P. Dihydrostreptomycin Directly Binds to, Modulates, and Passes through the MscL Channel Pore. PLoS Biol. 2016, 14, e1002473.
- Wenzel, M.; Senges, C.H.R.; Zhang, J.; Suleman, S.; Nguyen, M.; Kumar, P.; Chiriac, A.I.; Stepanek, J.J.; Raatschen, N.; May, C.; et al. Antimicrobial Peptides from the Aurein Family Form Ion-Selective Pores in Bacillus subtilis. ChemBioChem 2015, 16, 1101–1108.
- Haswell, E.S.; Phillips, R.; Rees, D.C. Mechanosensitive Channels: What Can They Do and How Do They Do It? Structure 2011, 19, 1356–1369.
- Li, Y. Ionic Regulation of MscK, a Mechanosensitive Channel from Escherichia coli. EMBO J. 2002, 21, 5323–5330.
- Chang, G.; Spencer, R.H.; Lee, A.T.; Barclay, M.T.; Rees, D.C. Structure of the MscL Homolog from Mycobacterium tuberculosis: A Gated Mechanosensitive Ion Channel. Science 1998, 282, 2220–2226.
- Bass, R.B.; Strop, P.; Barclay, M.; Rees, D.C. Crystal Structure of Escherichia coli MscS, a Voltage-Modulated and Mechanosensitive Channel. Science 2002, 298, 1582–1587.
- Booth, I.R. Bacterial Mechanosensitive Channels: Progress towards an Understanding of Their Roles in Cell Physiology. Curr. Opin. Microbiol. 2014, 18, 16–22.
- Nakayama, Y.; Hashimoto, K.; Sawada, Y.; Sokabe, M.; Kawasaki, H.; Martinac, B. Corynebacterium Glutamicum Mechanosensitive Channels: Towards Unpuzzling “Glutamate Efflux” for Amino Acid Production. Biophys. Rev. 2018, 10, 1359–1369.
- Kawasaki, H.; Martinac, B. Mechanosensitive Channels of Corynebacterium Glutamicum Functioning as Exporters of L-Glutamate and Other Valuable Metabolites. Curr. Opin. Chem. Biol. 2020, 59, 77–83.
- Cox, C.D.; Bavi, N.; Martinac, B. Bacterial Mechanosensors. Annu. Rev. Physiol. 2018, 80, 71–93.
- Rasmussen, T. How Do Mechanosensitive Channels Sense Membrane Tension? Biochem. Soc. Trans. 2016, 44, 1019–1025.
- Iscla, I.; Blount, P. Sensing and Responding to Membrane Tension: The Bacterial MscL Channel as a Model System. Biophys. J. 2012, 103, 169–174.
- Steinbacher, S.; Bass, R.; Strop, P.; Rees, D.C. Structures of the Prokaryotic Mechanosensitive Channels MscL and MscS. In Current Topics in Membranes; Benos, D.J., Simon, S.A., Eds.; Academic Press: San Diego, CA, USA, 2007; Volume 58, pp. 1–24.
- Reddy, B.; Bavi, N.; Lu, A.; Park, Y.; Perozo, E. Molecular Basis of Force-from-Lipids Gating in the Mechanosensitive Channel MscS. eLife 2019, 8, e50486.
- Blount, P.; Iscla, I. Life with Bacterial Mechanosensitive Channels, from Discovery to Physiology to Pharmacological Target. Microbiol. Mol. Biol. Rev. 2020, 84, e00055-19.
- Kapsalis, C.; Wang, B.; el Mkami, H.; Pitt, S.J.; Schnell, J.R.; Smith, T.K.; Lippiat, J.D.; Bode, B.E.; Pliotas, C. Allosteric Activation of an Ion Channel Triggered by Modification of Mechanosensitive Nano-Pockets. Nat. Commun. 2019, 10, 4619.
- Kapsalis, C.; Ma, Y.; Bode, B.E.; Pliotas, C. In-Lipid Structure of Pressure-Sensitive Domains Hints Mechanosensitive Channel Functional Diversity. Biophys. J. 2020, 119, 448–459.
- Naismith, J.H.; Booth, I.R. Bacterial Mechanosensitive Channels—MscS: Evolution’s Solution to Creating Sensitivity in Function. Annu. Rev. Biophys. 2012, 41, 157–177.
- Lu, A.; Park, S.; Reddy, B.; Fei, J.; Perozo, E. Using Fluorescence Microscopy to Characterize the Role of Mechanosensation in Cell Division. Biophys. J. 2020, 118, 529a.
- Gamini, R.; Sotomayor, M.; Chipot, C.; Schulten, K. Cytoplasmic Domain Filter Function in the Mechanosensitive Channel of Small Conductance. Biophys. J. 2011, 101, 80–89.
- Rowe, I.; Anishkin, A.; Kamaraju, K.; Yoshimura, K.; Sukharev, S. The Cytoplasmic Cage Domain of the Mechanosensitive Channel MscS Is a Sensor of Macromolecular Crowding. J. Gen. Physiol. 2014, 143, 543–557.
- Cox, C.D.; Bavi, N.; Martinac, B. Biophysical Principles of Ion-Channel-Mediated Mechanosensory Transduction. Cell Rep. 2019, 29, 1–12.
- Teng, J.; Loukin, S.; Anishkin, A.; Kung, C. The Force-from-Lipid (FFL) Principle of Mechanosensitivity, at Large and in Elements. Pflügers Arch. Eur. J. Physiol. 2015, 467, 27–37.
- Romantsov, T.; Guan, Z.; Wood, J.M. Cardiolipin and the Osmotic Stress Responses of Bacteria. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 2092–2100.
- Xue, F.; Cox, C.D.; Bavi, N.; Rohde, P.R.; Nakayama, Y.; Martinac, B. Membrane Stiffness Is One of the Key Determinants of E. coli MscS Channel Mechanosensitivity. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183203.
- Zhang, Y.; Daday, C.; Gu, R.X.; Cox, C.D.; Martinac, B.; de Groot, B.L.; Walz, T. Visualization of the Mechanosensitive Ion Channel MscS under Membrane Tension. Nature 2021, 590, 509–514.
- Anishkin, A.; Loukin, S.H.; Teng, J.; Kung, C. Feeling the Hidden Mechanical Forces in Lipid Bilayer Is an Original Sense. Proc. Natl. Acad. Sci. USA 2014, 111, 7898–7905.
- Perozo, E.; Kloda, A.; Cortes, D.M.; Martinac, B. Physical Principles Underlying the Transduction of Bilayer Deformation Forces during Mechanosensitive Channel Gating. Nat. Struct. Biol. 2002, 9, 696–703.
- Nomura, T.; Cranfield, C.G.; Deplazes, E.; Owen, D.M.; Macmillan, A.; Battle, A.R.; Constantine, M.; Sokabe, M.; Martinac, B. Differential Effects of Lipids and Lyso-Lipids on the Mechanosensitivity of the Mechanosensitive Channels MscL and MscS. Proc. Natl. Acad. Sci. USA 2012, 109, 8770–8775.
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Role of Adhesion Forces in Mechanosensitive Channel Gating in Staphylococcus aureus Adhering to Surfaces. NPJ Biofilms Microbiomes 2020, 6, 31.
- Li, J.; Busscher, H.J.; Swartjes, J.J.T.M.; Chen, Y.; Harapanahalli, A.K.; Norde, W.; van der Mei, H.C.; Sjollema, J. Residence-Time Dependent Cell Wall Deformation of Different Staphylococcus aureus Strains on Gold Measured Using Surface-Enhanced-Fluorescence. Soft Matter 2014, 10, 7638–7646.
- Gu, J.; Valdevit, A.; Chou, T.M.; Libera, M. Substrate Effects on Cell-Envelope Deformation during Early-Stage: Staphylococcus aureus Biofilm Formation. Soft Matter 2017, 13, 2967–2976.
- Culham, D.E.; Dalgado, C.; Gyles, C.L.; Mamelak, D.; MacLellan, S.; Wood, J.M. Osmoregulatory Transporter ProP Influences Colonization of the Urinary Tract by Escherichia coli. Microbiology 1998, 144, 91–102.
- Maurin, M.; Gyuranecz, M. Tularaemia: Clinical Aspects in Europe. Lancet Infect. Dis. 2016, 16, 113–124.
- Sjöstedt, A. Tularemia: History, Epidemiology, Pathogen Physiology, and Clinical Manifestations. Ann. N. Y. Acad. Sci. 2007, 1105, 1–29.
- Jackson, J.; McGregor, A.; Cooley, L.; Ng, J.; Brown, M.; Ong, C.W.; Darcy, C.; Sintchenko, V. Francisella tularensis Subspecies Holarctica, Tasmania, Australia, 2011. Emerg. Infect. Dis. 2012, 18, 1484–1486.
- Igwaran, A.; Okoh, A.I. Human Campylobacteriosis: A Public Health Concern of Global Importance. Heliyon 2019, 5, e02814.
- Lawe-Davies, O.; Bennett, S.; WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Saudi Med. J. 2017, 38, 444–445.
- Ipe, D.S.; Horton, E.; Ulett, G.C. The Basics of Bacteriuria: Strategies of Microbes for Persistence in Urine. Front. Cell. Infect. Microbiol. 2016, 6, 14.
- Schwan, W.R. Survival of Uropathogenic Escherichia coli in the Murine Urinary Tract Is Dependent on OmpR. Microbiology 2009, 155, 1832–1839.
- Gargan, R.A.; Hamilton-Miller, J.M.; Brumfitt, W. Effect of PH and Osmolality on in Vitro Phagocytosis and Killing by Neutrophils in Urine. Infect. Immun. 1993, 61, 8–12.
- Mittal, R.; Sharma, S.; Chhibber, S.; Harjai, K. Effect of Osmolarity on Virulence of Uropathogenic Pseudomonas aeruginosa. Am. J. Biomed. Sci. 2009, 1, 12–26.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
11 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





