| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexandru Florin Rogobete | -- | 4752 | 2022-07-08 11:26:34 | | | |
| 2 | Catherine Yang | -48 word(s) | 4704 | 2022-07-11 08:49:58 | | | | |
| 3 | Catherine Yang | Meta information modification | 4704 | 2022-07-11 09:46:06 | | |
Video Upload Options
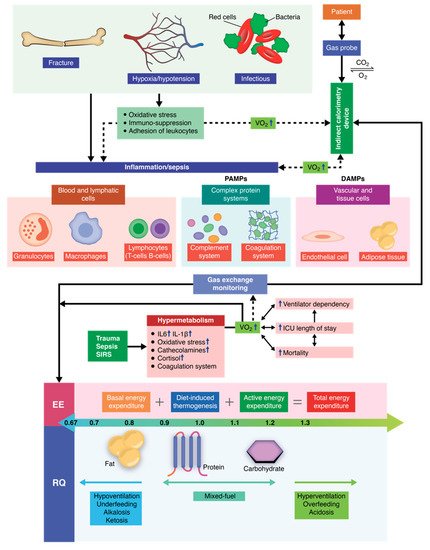
The critically ill polytrauma patient is characterized by a series of metabolic changes induced by inflammation, oxidative stress, sepsis, and primary trauma, as well as associated secondary injuries associated. Metabolic and nutritional dysfunction in the critically ill patient is a complex series of imbalances of biochemical and genetic pathways, as well as the interconnection between them. Therefore, the equation changes in comparison to other critical patients or to healthy individuals, in which cases, mathematical equations can be successfully used to predict the energy requirements. Recent studies have shown that indirect calorimetry is one of the most accurate methods for determining the energy requirements in intubated and mechanically ventilated patients. Current research is oriented towards an individualized therapy depending on the energy consumption (kcal/day) of each patient that also takes into account the clinical dynamics. By using indirect calorimetry, one can measure, in real time, both oxygen consumption and carbon dioxide production. Energy requirements (kcal/day) and the respiratory quotient (RQ) can be determined in real time by integrating these dynamic parameters into electronic algorithms. In this manner, nutritional therapy becomes personalized and caters to the patients’ individual needs, helping patients receive the energy substrates they need at each clinically specific time of treatment.
1. Molecular and Pathophysiological Aspects of Metabolism
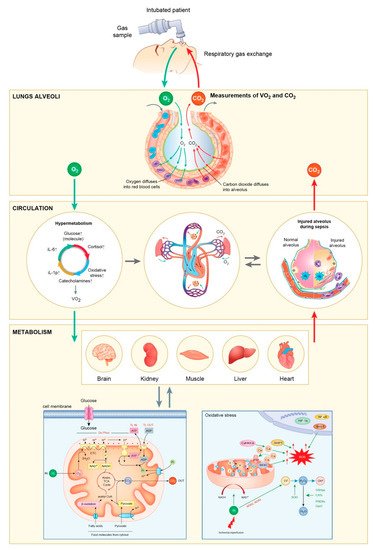
2. Genetic and Epigenetic Expressions Associated with Hypermetabolism
References
- Keel, M.; Trentz, O. Pathophysiology of polytrauma. Injury 2005, 36, 691–709.
- Gu, W.; Jiang, J. Genetic polymorphisms and posttraumatic complications. Comp. Funct. Genom. 2010, 2010.
- Andruszkow, H.; Fischer, J.; Sasse, M.; Brunnemer, U.; Andruszkow, J.H.K.; Gänsslen, A.; Hildebrand, F.; Frink, M. Interleukin-6 as inflammatory marker referring to multiple organ dysfunction syndrome in severely injured children. Scand. J. Trauma Resusc. Emerg. Med. 2014, 22, 16.
- Binkowska, A.M.; Michalak, G.; Słotwiński, R. Current views on the mechanisms of immune responses to trauma and infection. Cent. Eur. J. Immunol. 2015, 40, 206–216.
- Burkhardt, M.; Nienaber, U.; Pizanis, A.; Maegele, M.; Culemann, U.; Bouillon, B.; Flohé, S.; Pohlemann, T.; Paffrath, T. Acute management and outcome of multiple trauma patients with pelvic disruptions. Crit. Care 2012, 16, R163.
- Fujishima, S. Organ dysfunction as a new standard for defining sepsis. Inflamm. Regen. 2016, 36, 24.
- Huber-Lang, M.; Kovtun, A.; Ignatius, A. The role of complement in trauma and fracture healing. Semin. Immunol. 2013, 25, 73–78.
- Crimi, E.; Taccone, F.S.; Infante, T.; Scolletta, S.; Crudele, V.; Napoli, C. Effects of intracellular acidosis on endothelial function: An overview. J. Crit. Care 2012, 27, 108–118.
- Ikezoe, T. Thrombomodulin/activated protein C system in septic disseminated intravascular coagulation. J. Intensive Care 2015, 3, 1.
- Rittirsch, D.; Redl, H.; Huber-Lang, M. Role of complement in multiorgan failure. Clin. Dev. Immunol. 2012, 2012.
- Papurica, M.; Rogobete, A.F.; Sandesc, D.; Dumache, R.; Cradigati, C.A.; Sarandan, M.; Nartita, R.; Popovici, S.E.; Bedreag, O.H. Advances in biomarkers in critical ill polytrauma patients. Clin. Lab. 2016, 62.
- Mokra, D.; Kosutova, P. Biomarkers in acute lung injury. Respir. Physiol. Neurobiol. 2015, 209, 52–58.
- Michopoulos, V.; Norrholm, S.D.; Jovanovic, T. Diagnostic Biomarkers for Posttraumatic Stress Disorder: Promising Horizons from Translational Neuroscience Research. Biol. Psychiatry 2015, 78, 344–353.
- Luca, L.; Rogobete, A.F.; Bedreag, O.H. Oxidative Stress and Antioxidant Therapy in Critically Ill Polytrauma Patients with Severe Head Injury. J. Crit. Care Med. 2015, 1, 83–91.
- Horst, K.; Eschbach, D.; Pfeifer, R.; Hübenthal, S.; Sassen, M.; Steinfeldt, T.; Wulf, H.; Ruchholtz, S.; Pape, H.C.; Hildebrand, F. Local Inflammation in Fracture Hematoma: Results from a Combined Trauma Model in Pigs. Mediat. Inflamm. 2015, 2015.
- Ohri, S.K.; Vohra, H.A.; Whistance, R.; Modi, A. The inflammatory response to miniaturised extracorporeal circulation: A review of the literature. Mediat. Inflamm. 2009, 2009.
- Bedreag, O.H.; Rogobete, A.F.; Sandesc, D.; Cradigati, C.A.; Sarandan, M.; Popovici, S.E.; Dumache, R.; Horhat, F.G.; Vernic, C.; Sima, L.V.; et al. Modulation of the Redox Expression and Inflammation Response in the Crtically Ill Polytrauma Patient with Thoracic Injury. Statistical Correlations between Antioxidant Therapy and Clinical Aspects. Clin. Lab. 2016, 62, 1747–1759.
- Arroyo, V.; García-martinez, R.; Salvatella, X. Review Human serum albumin, systemic inflammation, and cirrhosis. J. Hepatol. 2014, 61, 396–407.
- Budic, I.; Pavlovic, D.; Kocic, G.; Cvetkovic, T.; Simic, D.; Basic, J. Biomarkers of Oxidative Stress and Endothelial Dysfunction After Tourniquet Release in Children. Physiol. Res. 2011, 60, S137–S145.
- Horhat, F.G.; Rogobete, A.F.; Papurica, M.; Sandesc, D.; Tanasescu, S.; Dumitrascu, V.; Licker, M.; Nitu, R.; Cradigati, C.A.; Sarandan, M.; et al. The Use of Lipid Peroxidation Expression as a Biomarker for the Molecular Damage in the Critically Ill Polytrauma Patient. Clin. Lab. 2016, 62, 1601–1607.
- Rogobete, A.F.; Sandesc, D.; Papurica, M.; Stoicescu, E.R.; Popovici, S.E.; Bratu, L.M.; Vernic, C.; Sas, A.M.; Stan, A.T.; Bedreag, O.H. The influence of metabolic imbalances and oxidative stress on the outcome of critically ill polytrauma patients: A review. Burns Trauma 2017, 5, 8.
- Frank, J.; Maier, M.; Koenig, J.; Rose, S.; Bouma, M.; Buurman, W.A.; Marzi, I. Circulating Inflammatory and Metabolic Parameters to Predict Organ Failure after Multiple Trauma. Eur. J. Trauma 2002, 28, 333–339.
- Joseph, B.; Wynne, J.L.; Dudrick, S.J.; Latifi, R. Nutrition in Trauma and Critically Ill Patients. Eur. J. Trauma Emerg. Surg. 2010, 36, 25–30.
- Baartmans, M.G.A.; Van Baar, M.E.; Boxma, H.; Dokter, J.; Tibboel, D.; Nieuwenhuis, M.K. Accuracy of burn size assessment prior to arrival in Dutch Burn centres and its consequences in children: A nationwide evaluation. Injury 2012, 43, 1451–1456.
- Bains, M.; Hall, E.D. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 675–684.
- Hoffer, L.J. Protein and energy provision in critical illness. Am. Soc. Clin. Nutr. 2003, 78, 906–911.
- Dogjani, A.; Zatriqi, S.; Uranues, S.; Latifi, R. Biology-based nutritional support of critically ill and injured patients. Eur. Surg. 2011, 43, 7–12.
- Ramakrishnan, S.; Sulochana, K.N.; Lakshmi, S.; Selvi, R.; Angayarkanni, N. Biochemistry of homocysteine in health and diseases. Indian J. Biochem. Biophys. 2006, 43, 275–283.
- Lenz, A.; Franklin, G.A.; Cheadle, W.G. Systemic inflammation after trauma. Injury 2007, 38, 1336–1345.
- Maegele, M.; Gu, Z.; Huang, Q.; Yang, H. Updated concepts on the pathophysiology and the clinical management of trauma hemorrhage and coagulopathy Trauma-induced Coagulopathy (TIC). Chin. J. Traumatol. 2017, 20, 125–132.
- Afifi, I.; Elazzazy, S.; Abdulrahman, Y.; Latifi, R. Nutrition therapy for critically ill and injured patients. Eur. J. Trauma Emerg. Surg. 2013, 39, 203–213.
- Hartl, W.H.; Jauch, K.W. Metabolic self-destruction in critically ill patients: Origins, mechanisms and therapeutic principles. Nutrition 2014, 30, 261–267.
- Malbrain, M.L.N.G.; Marik, P.E.; Witters, I.; Cordemans, C.; Kirkpatrick, A.W.; Roberts, D.J.; Van Regenmortel, N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol. Intensive Ther. 2014, 46, 361–380.
- Xiu, F.; Stanojcic, M.; Diao, L.; Jeschke, M.G. Stress hyperglycemia, insulin treatment, and innate immune cells. Int. J. Endocrinol. 2014, 2014.
- Grintescu, I.M.; Luca Vasiliu, I.; Cucereanu Badica, I.; Mirea, L.; Pavelescu, D.; Balanescu, A.; Grintescu, I.C. The influence of parenteral glutamine supplementation on glucose homeostasis in critically ill polytrauma patients-A randomized-controlled clinical study. Clin. Nutr. 2015, 34, 377–382.
- Holbein, M.; Béchir, M.; Ludwig, S.; Sommerfeld, J.; Cottini, S.R.; Keel, M.; Stocker, R.; Stover, J.F. Differential influence of arterial blood glucose on cerebral metabolism following severe traumatic brain injury. Crit. Care 2009, 13, R13.
- Mangiola, A.; Vigo, V.; Anile, C.; De Bonis, P.; Marziali, G.; Lofrese, G. Role and Importance of IGF-1 in Traumatic Brain Injuries. BioMed. Res. Int. 2015, 2015.
- Sabour, H.; Norouzi Javidan, A.; Latifi, S.; Larijani, B.; Shidfar, F.; Vafa, M.R.; Heshmat, R.; Emami Razavi, H. Bone biomarkers in patients with chronic traumatic spinal cord injury. Spine J. 2014, 14, 1132–1138.
- Luo, L.; Zhang, S.; Wang, Y.; Rahman, M.; Syk, I.; Zhang, E.; Thorlacius, H. Proinflammatory role of neutrophil extracellular traps in abdominal sepsis. AJP Lung Cell. Mol. Physiol. 2014, 307, L586–L596.
- Qu, C.; Wang, X.-W.; Huang, C.; Qiu, F.; Xiang, X.-Y.; Lu, Z.-Q. High mobility group box 1 gene polymorphism is associated with the risk of postoperative atrial fibrillation after coronary artery bypass surgery. J. Cardiothorac. Surg. 2015, 10, 88.
- Chen, C.B.; Liu, L.S.; Zhou, J.; Wang, X.P.; Han, M.; Jiao, X.Y.; He, X.S.; Yuan, X.P. Up-regulation of HMGB1 exacerbates renal ischemia-reperfusion injury by stimulating inflammatory and immune responses through the TLR4 signaling pathway in mice. Cell. Physiol. Biochem. 2017, 41, 2447–2460.
- Entezari, M.; Javdan, M.; Antoine, D.J.; Morrow, D.M.P.; Sitapara, R.A.; Patel, V.; Wang, M.; Sharma, L.; Gorasiya, S.; Zur, M.; et al. Redox Biology Inhibition of extracellular HMGB1 attenuates hyperoxia-induced in flammatory acute lung injury. Redox Biol. 2014, 2, 314–322.
- Collier, B.R.; Giladi, A.; Dossett, L.A.; Dyer, L.; Fleming, S.B.; Cotton, B.A. Impact of High-Dose Antioxidants on Outcomes in Acutely Injured Patients. J. Parenter. Enter. Nutr. 2008, 32, 384–388.
- Dickerson, R.N.; Van Cleve, J.R.; Swanson, J.M.; Maish, G.O.; Minard, G.; Croce, M.A.; Brown, R.O. Vitamin D deficiency in critically ill patients with traumatic injuries. Burns Trauma 2016, 4, 28.
- Nägeli, M.; Fasshauer, M.; Sommerfeld, J.; Fendel, A.; Brandi, G.; Stover, J.F. Prolonged continuous intravenous infusion of the dipeptide l-alanine-l-glutamine significantly increases plasma glutamine and alanine without elevating brain glutamate in patients with severe traumatic brain injury. Crit. Care 2014, 18, R139.
- Bedreag, O.H.; Papurica, M.; Rogobete, A.F.; Sarandan, M.; Cradigati, C.A.; Vernic, C.; Dumbuleu, C.M.; Nartita, R.; Sandesc, D. New perspectives of volemic resuscitation in polytrauma patients: A review. Burns Trauma 2016, 4, 5.
- Chiarla, C.; Giovannini, I.; Siegel, J.H. The relationship between plasma cholesterol, amino acids and acute phase proteins in sepsis Ã. Amino Acids 2004, 27, 97–100.
- Fraipont, V.; Preiser, J.-C. Energy Estimation and Measurement in Critically Ill Patients. J. Parenter. Enter. Nutr. 2013, 37, 705–713.
- Panitchote, A.; Thiangpak, N.; Hongsprabhas, P.; Hurst, C. Short Communication Energy expenditure in severe sepsis or septic shock in a Thai Medical Intensive Care Unit. Asia Pac. J. Clin. Nutr. 2017, 26, 794–797.
- Weijs, P.J.M.; Vansant, G.A.A.M. Validity of predictive equations for resting energy expenditure in Belgian normal weight to morbid obese women. Clin. Nutr. 2010, 29, 347–351.
- Sundström Rehal, M.; Fiskaare, E.; Tjäder, I.; Norberg, Å.; Rooyackers, O.; Wernerman, J. Measuring energy expenditure in the intensive care unit: A comparison of indirect calorimetry by E-sCOVX and Quark RMR with Deltatrac II in mechanically ventilated critically ill patients. Crit. Care 2016, 20, 54.
- Kross, E.K.; Sena, M.; Schmidt, K.; Stapleton, R.D. A comparison of predictive equations of energy expenditure and measured energy expenditure in critically ill patients. J. Crit. Care 2012, 27, 321.
- Maday, K.R. Energy Estimation in the Critically Ill: A Literature Review. Univ. J. Clin. Med. 2013, 1, 39–43.
- Stapel, S.N.; Weijs, P.J.M.; Girbes, A.R.J.; Oudemans-van Straaten, H.M. Indirect calorimetry in critically ill mechanically ventilated patients: Comparison of E-sCOVX with the deltatrac. Clin. Nutr. 2018, 38, 2155–2160, in press.
- Maraki, M.I.; Panagiotakos, B.; Jansen, L.T. Validity of Predictive Equations for Resting Energy Expenditure in Greek Adults. Ann. Nutr. Metab. 2018, 72, 134–141.
- Rousing, M.L.; Hahn-Pedersen, M.H.; Andreassen, S.; Pielmeier, U.; Preiser, J.-C. Energy expenditure in critically ill patients estimated by population-based equations, indirect calorimetry and CO2-based indirect calorimetry. Ann. Intensive Care 2016, 6, 16.
- Berger, M.M.; Pichard, C. Development and current use of parenteral nutrition in critical care—An opinion paper. Crit. Care 2014, 18, 478.
- Weimann, A.; Kuse, E.R.; Bechstein, W.O.; Neuberger, J.M.; Plauth, M.; Pichlmayr, R. Perioperative parenteral and enteral nutrition for patients undergoing orthotopic liver transplantation. Results of a questionnaire from 16 European transplant units. Transpl. Int. 1998, 11 (Suppl. 1), S289–S291.
- Bauer, J.; Hentschel, R.; Linderkamp, O. Effect of sepsis syndrome on neonatal oxygen consumption and energy expenditure. Pediatrics 2002, 110, e69.
- Van der Kuip, M.; de Meer, K.; Oosterveld, M.J.S.; Lafeber, H.N.; Gemke, R.J.B.J. Simple and accurate assessment of energy expenditure in ventilated paediatric intensive care patients. Clin. Nutr. 2004, 23, 657–663.
- Tobin, M.J. Respiratory Monitoring During Mechanical Ventilation. Mech. Vent. 1990, 6, 679–709.
- Siirala, W.; Noponen, T. Validation of indirect calorimetry for measurement of energy expenditure in healthy volunteers undergoing pressure controlled non-invasive ventilation support. J. Clin. Monit. Comput. 2012, 26, 37–43.
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Best Practice Methods to Apply to Measurement of Resting Metabolic Rate in Adults: A Systematic Review. J. Am. Diet. Assoc. 2006, 106, 881–903.
- Graf, S.; Genton, L.; Oshima, T.; Pichard, C.; Heidegger, C.P. Energy expenditure (EE) in mechanically ventilated patients: Espen equation using different body weights (BW) vs indirect calorimetry (IC). Intensive Care Med. Exp. 2015, 3, A293.
- Meyer, R.; Briassouli, E.; Briassoulis, G.; Habibi, P. Evaluation of the M-COVX metabolic monitor in mechanically ventilated adult patients. e-SPEN 2008, 3, e232–e239.
- Briassoulis, G.; Michaeloudi, E.; Fitrolaki, D.M.; Spanaki, A.M.; Briassouli, E. Influence of different ventilator modes on Vo2 and Vco2 measurements using a compact metabolic monitor. Nutrition 2009, 25, 1106–1114.
- Singer, P.; Pogrebetsky, I.; Attal-Singer, J.; Cohen, J. Comparison of metabolic monitors in critically ill, ventilated patients. Nutrition 2006, 22, 1077–1086.
- Al-dorzi, H.M.; Albarrak, A.; Ferwana, M.; Murad, M.H.; Arabi, Y.M. Lower versus higher dose of enteral caloric intake in adult critically ill patients: A systematic review and meta-analysis. Crit. Care 2016, 20, 358.
- Kreymann, K.G.; Delegge, M.H.; Luft, G.; Heer, G. De Clinical Nutrition ESPEN Opinion paper A nutrition strategy for obese ICU patients with special consideration for the reference of protein. Clin. Nutr. ESPEN 2015, 10, e160–e166.
- Anbar, R.; Theilla, M.; Fisher, H.; Madar, Z.; Cohen, J.; Singer, P. O024 decrease in hospital mortality in tight calorie balance control study: The preliminary results of the ticacos study. Clin. Nutr. Suppl. 2008, 3, 11.
- Inadomi, C.; Terao, Y.; Yamashita, K.; Fukusaki, M.; Takada, M.; Sumikawa, K. Comparison of oxygen consumption calculated by Fick’s principle (using a central venous catheter) and measured by indirect calorimetry. J. Anesth. 2008, 22, 163–166.
- Allingstrup, M.J.; Kondrup, J.; Wiis, J.; Claudius, C.; Pedersen, U.G.; Rasmussen, R.H.; Bjerregaard, M.R.; Steensen, M.; Jensen, T.H.; Lange, T.; et al. Early goal—Directed nutrition versus standard of care in adult intensive care patients: The single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017, 43, 1637–1647.
- Martindale, R.G.; McClave, S.A.; Vanek, V.W.; McCarthy, M.; Roberts, P.; Taylor, B.; Ochoa, J.B.; Napolitano, L.; Cresci, G. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit. Care Med. 2009, 37, 1751–1761.
- Luca, L.; Rogobete, A.F.; Bedreag, O.H.; Sarandan, M.; Cradigati, C.A.; Papurica, M.; Gruneantu, A.; Patrut, R.; Vernic, C.; Dumbuleu, C.M.; et al. Intracranial Pressure Monitoring as a Part of Multimodal Monitoring Management of Patients with Critical Polytrauma: Correlation between Optimised Intensive Therapy According to Intracranial Pressure Parameters and Clinical Picture. Turk. J. Anaesthesiol. Reanim. 2015, 43, 412–417.
- Doley, J.; Phillips, W. Coding for Malnutrition in the Hospital: Does It Change Reimbursement. Nutr. Clin. Pract. 2019.
- Sun, S.; Sursal, T.; Adibnia, Y.; Zhao, C.; Zheng, Y.; Li, H.; Otterbein, L.E.; Hauser, C.J.; Itagaki, K. Mitochondrial DAMPs Increase Endothelial Permeability through Neutrophil Dependent and Independent Pathways. PLoS ONE 2013, 8, e59989.
- Sunderland, P.M.; Heilbrun, M.P. Estimating energy expenditure in traumatic brain injury: Comparison of indirect calorimetry with predictive formulas. Neurosurgery 1992, 31, 243–246.
- Maxwell, J.; Gwardschaladse, C.; Lombardo, G.; Petrone, P.; Policastro, A.; Karev, D.; Prabhakaran, K.; Betancourt, A.; Marini, C.P. The impact of measurement of respiratory quotient by indirect calorimetry on the achievement of nitrogen balance in patients with severe traumatic brain injury. Eur. J. Trauma Emerg. Surg. 2017, 43, 775–782.
- Van Schijndel, R.J.M.S.; Weijs, P.J.M.; Koopmans, R.H.; Sauerwein, H.P.; Beishuizen, A.; Girbes, A.R.J. Optimal nutrition during the period of mechanical ventilation decreases mortality in critically ill, long-term acute female patients: A prospective observational cohort study. Crit. Care 2009, 13, R132.
- Rogobete, A.F.; Sandesc, D.; Bedreag, O.H.; Papurica, M.; Popovici, S.E.; Bratu, T.; Popoiu, C.M.; Nitu, R.; Dragomir, T.; AAbed, H.I.M.; et al. MicroRNA Expression is Associated with Sepsis Disorders in Critically Ill Polytrauma Patients. Cells 2018, 7, 271.
- Pop-Began, V.; Păunescu, V.; Grigorean, V.; Pop-Began, D.; Popescu, C. Molecular mechanisms in the pathogenesis of sepsis. J. Med. Life 2014, 7, 38–41.
- Wienholds, E.; Plasterk, R.H.A. MicroRNA function in animal development. FEBS Lett 2005, 579, 5911–5922.
- Papaioannou, V.E.; Chouvarda, I.G.; Maglaveras, N.K.; Pneumatikos, I.A. Temperature variability analysis using wavelets and multiscale entropy in patients with systemic inflammatory response syndrome, sepsis and septic shock. Crit. Care 2012, 16, R51.
- Suzuki, K.T. Metabolomics of Selenium: Se Metabolites Based on Speciation Studies. J. Health Sci. 2005, 51, 107–114.
- Singer, P.; Pichard, C. Reconciling divergent results of the latest parenteral nutrition studies in the ICU. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 187–193.
- Bar-Or, D.; Bar-Or, R.; Rael, L.T.; Brody, E.N. Oxidative stress in severe acute illness. Redox Biol. 2015, 4, 340–345.
- Melzer, K.; Kayser, B.; Schutz, Y. Respiratory quotient evolution during normal pregnancy: What nutritional or clinical information can we get out of it? Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 176, 5–9.
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 2016, 121, 628–634.
- Jeon, J.; Kym, D.; Cho, Y.S.; Kim, Y.; Yoon, J.; Yim, H.; Hur, J.; Chun, W. Reliability of resting energy expenditure in major burns: Comparison between measured and predictive equations. Clin. Nutr. 2018.
- Zusman, O.; Singer, P. Resting energy expenditure and optimal nutrition in critical care: How to guide our calorie prescriptions. Crit. Care 2017, 21, 128.
- Singer, P.; Cohen, J.; Shalita-chesner, M. The tight calorie control study (TICACOS): A prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011, 37, 601–609.
- Heidegger, C.P.; Berger, M.M.; Graf, S.; Zingg, W.; Darmon, P.; Costanza, M.C.; Thibault, R.; Pichard, C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet 2012, 6736, 1–9.
- Tamura, T.; Yatabe, T.; Yokoyama, M. Energy expenditure measured using indirect calorimetry after elective cardiac surgery in ventilated postoperative patients: A prospective observational study. Clin. Nutr. Exp. 2019, 24, 15–23.
- Dias Rodrigues, J.C.; Lamarca, F.; Lacroix de Oliveira, C.; Cuppari, L.; Lourenço, R.A.; Avesani, C.M. Agreement between prediction equations and indirect calorimetry to estimate resting energy expenditure in elderly patients on hemodialysis. ESPEN J. 2014, 9, e91–e96.
- Valainathan, S.; Boukris, A.; Arapis, K.; Schoch, N.; Goujon, G.; Konstantinou, D.; Bécheur, H.; Pelletier, A.L. Energy expenditure in acute pancreatitis evaluated by the Harris–Benedict equation compared with indirect calorimetry. Clin. Nutr. ESPEN 2019, 33, 57–59.