You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesca Forini | -- | 2405 | 2022-07-07 12:56:21 | | | |
| 2 | Amina Yu | + 16 word(s) | 2421 | 2022-07-08 03:13:48 | | | | |
| 3 | Amina Yu | + 2 word(s) | 2423 | 2022-07-08 03:18:39 | | | | |
| 4 | Amina Yu | + 2 word(s) | 2425 | 2022-07-08 03:19:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Forini, F.; Maffei, S.; Nicolini, G.; Canale, P.; Guiducci, L. Gut Microbiota/Sex Hormone in Metabolic and Cardiovascular Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/24906 (accessed on 05 January 2026).
Forini F, Maffei S, Nicolini G, Canale P, Guiducci L. Gut Microbiota/Sex Hormone in Metabolic and Cardiovascular Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/24906. Accessed January 05, 2026.
Forini, Francesca, Silvia Maffei, Giuseppina Nicolini, Paola Canale, Letizia Guiducci. "Gut Microbiota/Sex Hormone in Metabolic and Cardiovascular Disease" Encyclopedia, https://encyclopedia.pub/entry/24906 (accessed January 05, 2026).
Forini, F., Maffei, S., Nicolini, G., Canale, P., & Guiducci, L. (2022, July 07). Gut Microbiota/Sex Hormone in Metabolic and Cardiovascular Disease. In Encyclopedia. https://encyclopedia.pub/entry/24906
Forini, Francesca, et al. "Gut Microbiota/Sex Hormone in Metabolic and Cardiovascular Disease." Encyclopedia. Web. 07 July, 2022.
Copy Citation
Cardiovascular disease (CVD) is responsible for a large incidence of death cases in both men and women worldwide. Even though age-adjusted CVD mortality rates are higher in men compared to premenopausal women, the midlife period, coincident with the menopause transition, leads to a significant increase of coronary heart disease (CHD) risk factors also in women. Many studies have demonstrated the association between specific gut microbiota (GM) signature and several CVD manifestations, highlighting the potential roles of bacteria in the pathogenesis of coronary heart disease, and cardiometabolic (CM) disorders. Unfortunately, most studies linking GM dysbiosis to CVD risk factors and focused on gender difference were performed in preclinical mouse models rather than in humans.
Cardiovascular Disease
Gut Microbiota
Sex hormones
1. Metabolic Syndrome and Diabetes
Although the field is still young, sex differences in the gut microbiota (GM) composition are regarded as key determinants of gender predisposition to metabolic syndrome and diabetes mellitus (DM). Indeed, dyslipidemia, dysglycemia, obesity, and DM may all induce GM alterations and, vice-versa, GM may affect the onset and evolution of metabolic syndrome [1]. For example, in an animal model of high fat diet (HFD)-induced metabolic disorders, body weight gain and insulin resistance were higher in male than in female mice [2]. Antibiotic-pretreatment alleviated diet-induced insulin resistance in male mice while increasing fasting blood glucose in females. The different metabolic responses to high-fat diet (HFD) were paralleled by a remarkable dimorphism in GM composition with a higher abundance of the genera Parabacteroides, Lactobacillus, Bacteroides, and Bifidobacterium observed in females than in males. Importantly, HFD remodeled GM by decreasing the abundance of the protective short chain fatty acid (SCFA)-producing bacteria such as Roseburia and Lachnospiraceae group. Additionally, a gender bias in GM composition following antibiotic pretreatment in HFD mice was observed, thus indicating a sex-dependent sensitivity to antibiotics [2].
On the other hand, androgen deficiency in males can promote metabolic disorders in the presence of an HFD. Androgen deprivation via castration altered fecal microbiota and exacerbated risk factors for CVD, including obesity, fasting glucose, and hepatic triglyceride accumulation [3]. GM depletion through antibiotics treatment alleviated the metabolic alterations induced by hypogonadism [4].
Generally, males are more susceptible than females to impaired glucose metabolism and type 2 diabetes (T2D) [5]. In this context, a recent experimental study revealed that the sex-dependent difference in glucose metabolism, commonly observed in both humans and rodent animals, depends on a different shaping of GM. Mechanistically, this work demonstrated a key role of androgens in deteriorating glucose homeostasis by modulating the GM composition and the circulating levels of glutamine and glutamine/glutamate ratio, thereby contributing to the difference in glucose metabolism between the two sexes [6] (Figure 1).
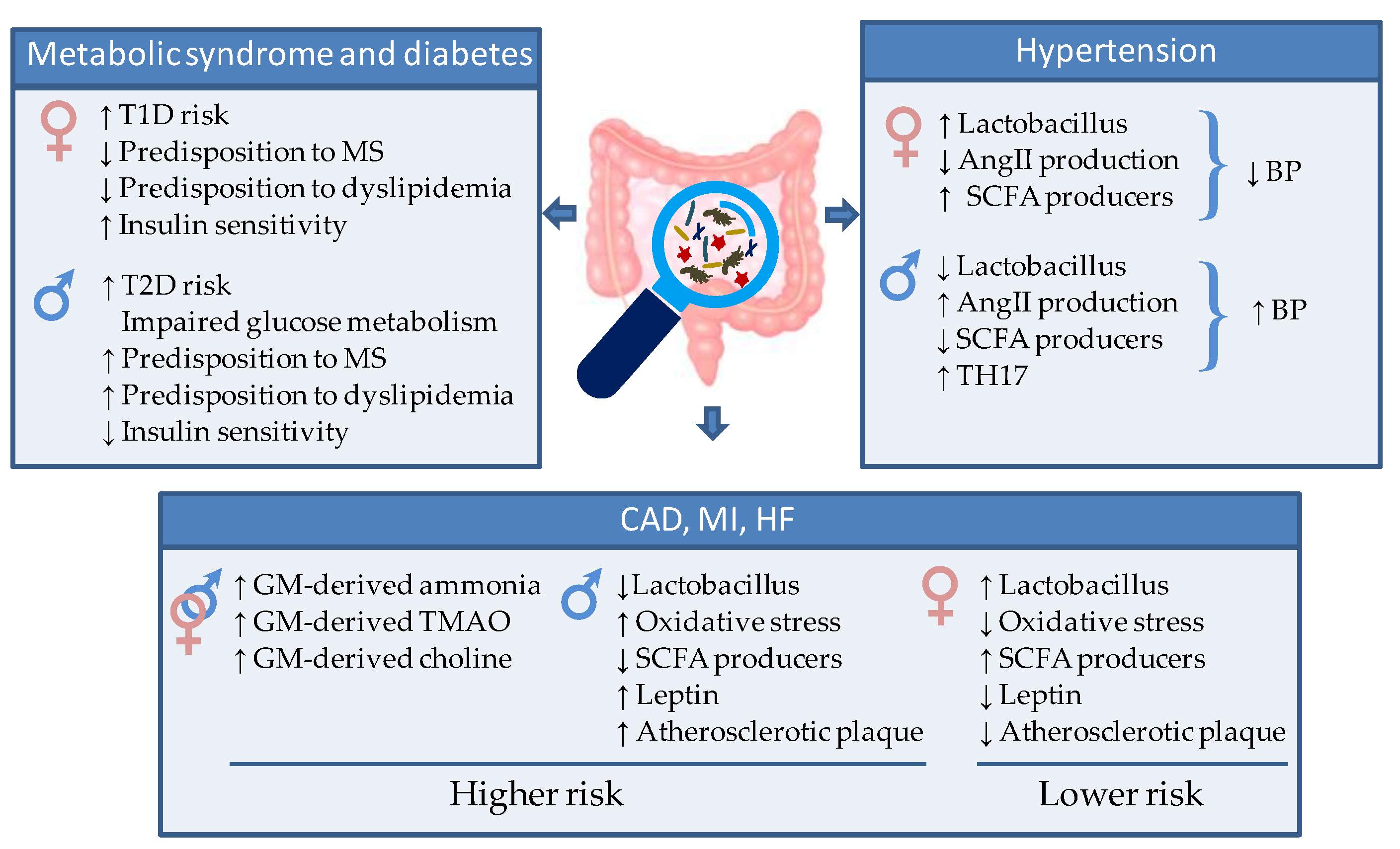
Figure 1. Schematic representation of the gut microbiota-mediated sex differences in cardiometabolic (CM) and cardiovascular (CV) risk and disease. AngII = Angiotensin 2. SCFA = short chain fatty acid, TH17 = T helper 17 cells, TMAO = Trimethylamine/trimethylamine N-oxide, T1D = type 1 diabetes, T2D= type 2 diabetes.
While the obese T2D is more commonly diagnosed in males, the non-obese type I diabetes mellitus (T1D) is observed predominantly in females [7]. It has been shown that fecal microbiota transfer from young male mice to females significantly improved the serum testosterone in the recipients and conferred them an acquired resistance to T1D [8]. This interesting finding suggested that male GM is able to modulate androgen in females and protect them from T1D.
In line with experimental models, in human beings, several studies have indicated differences in the GM as potential determinants of gender predisposition to metabolic syndrome, insulin resistance, and diabetes [9] (Figure 1). For instance, T2D is associated with a reduction of the protective butyrate-producing species, and an increase in Bacteroides–Prevotella species [10][11][12], while transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome [13]. A recent clinical trial (CARDIOPREV NCT00924937) provides evidence of a different GM composition in patients with metabolic syndrome according to gender and a different shaping of GM after 3-year consumption of a Mediterranean or a low-fat diet (LFD). Women evidenced higher levels of Collinsella, Alistipes, Anaerotruncus, and Phascolarctobacterium genera, whereas the abundance of Faecalibacterium and Prevotella genera was higher in men. Moreover, elevated levels of Desulfovibrio, Roseburia, and Holdemania were observed in men than in women after the consumption of the LF diet [9].
Insulin resistance represents an underlying mechanism of the dysmetabolic syndrome frequently associated with GM dysbiosis (Figure 1). In combination with the activity of SH, an altered intestinal bacteria composition could exacerbate insulin resistance via LPS-mediated modulation of the TLR signaling. For example, alteration of TLR2 pathways and activation of TLR4 by estrogens potentiated the serum LPS-induced inflammatory signals in macrophages leading to an impairment of insulin signaling in the muscle, liver, and adipose tissue [14]. The importance of metabolic endotoxemia in the onset of insulin resistance has been demonstrated in TLR knockout animal models. Mice lacking TLR4 were protected against HFD-induced insulin resistance [15]. This mechanism has been confirmed also in TLR2 knockout mice in association with higher proportions of Bacteroidetes and Firmicutes coupled with a lower proportion of Proteobacteria phyla [16]. On the contrary, progesterone, thanks to its anti-inflammatory action, appears to exert a protective effect against the LPS-dependent impairment of insulin signaling [17].
Another route through which the GM influences risk factors in relation to CM disorders is by regulating the homeostasis of the hormones of the gastro-intestinal system. Human studies have demonstrated that GM re-shaping with fermentable fibers increases SCFAs-producers and exerts beneficial effects by modifying the production and plasma levels of enteroendocrine hormones involved in appetite sensation and glucose response (i.e., glucagon-like peptide 1, peptide YY, and Ghrelin) [18][19]. Along the same line, it has recently been demonstrated that probiotics affecting GM composition improve fasting glycaemia, hyperinsulinaemia, insulin resistance index (HOMA-IR), and glycated hemoglobin via gut peptides secretion [20][21].
In addition to influencing the onset and progression of the disease, the GM can affect the response to pharmacological treatments in a gender-specific way. For example, pioglitazone, a peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist used as hypoglycemic drug in the management of T2D, shows a stronger efficacy in female with respect to male mice [22][23]. One of the supposed mechanisms of this gender difference is related to an upregulation of the PPAR-γ receptor mediated by the estrogen 17β-estradiol. This finding is in accordance with the increased susceptibility to dyslipidemia observed in men compared to women [2].
2. Hypertension
Hypertension is a major modifiable risk factor for CVD. GM dysbiosis has been demonstrated both in animal models and in hypertensive populations [24][25]. Compared to individuals with normal blood pressure (BP), essential hypertensive patients show intestinal epithelial barrier dysfunction and altered GM composition [26], which can be prevented with probiotic Lactobacillus or antibiotic treatment [27]. Additionally, the fecal microbiota transplantation (FMT) from hypertensive patients to germ-free mice induced BP elevation, thus demonstrating a direct connection between GM alteration and hypertension [28]. Based on this evidence, an ongoing clinical trial is dedicated to establish the safety and efficacy of bacteriotherapy through FMT from healthy donors to hypertensive patients [29].
GM-host interaction affects BP through multiple routes. One main mechanism involves the crosstalk with the nervous system. For example, enhanced sympathetic drive has been reported to contribute to a shift in gut microbial genera, resulting in increased permeability of gut epithelial barrier, local gut inflammation, and BP elevation [24]. In addition, GM products are implicated in sympathetic activation. In particular, some bacteria of Streptococcus, Escherichia, Lactobacillus, and Bifidobacterium genera can produce neuroactive compounds that affect the vascular tone via modulation of the autonomic nervous system [30]. Alteration in the prevalence of these bacteria contributes to the development of hypertension. [31]. For example, in a rat model, the hypertensive state was paralleled by a reduction in GM Latobacilli and could be ameliorated by exogeneous probiotic administration [32]. The pressure-lowering activity of Lattobacilli is mainly mediated by the secretion of peptides that inhibit angiotensin-converting enzyme, leading to decreased production of angiotensin II, a strong vasoconstrictor [33][34]. It has been demonstrated that women have higher levels of Lactobacilli in the gut [35][36], which may partly explain the lower pressure levels in fertile women compared to age-matched men. Consistently, men show greater blood pressure rises than women in response to angiotensin II [37] (Figure 3).
Bacteria of the Lactobacillus and Bifidobacter genera play a protective role also via the pressure-lowering effects of SCFA metabolites, whose production is inversely associated with hypertensive conditions [38] (Figure 1). A low abundance of the high SCFA-producer Bifidobacteria has been demonstrated in GM of hypertensive rats [39]. On the other hand, administration of antibiotics, high-fiber (prebiotic) diet, or probiotics to increase SCFA-producing bacteria was effective in reducing both systolic and diastolic BP [33][40]. The anti-hypertensive effects of GM-derived SCFA are due in part to their influence on vascular tone and renal sensory nerves [38], and in part to the above-described anti-inflammatory action. Indeed, chronic low-grade inflammation, favored by GM dysbiosis, contributes to a raise in BP [41]. GM-driven proinflammatory state, aggravated by impairment of the renine/angiotensine system and unbalanced salt regulation, can induce endothelial dysfunction ultimately contributing to hypertension. Human studies have demonstrated that the abundance of Faecalibacterium, Bifidobacterium, Ruminococcus, and Prevotella is inversely correlated to different low-grade inflammation markers that have impact on host blood pressure such as high sensitivity C-reactive protein and IL6 [42]. The antihypertensive responses induced by gut-derived SCFA-delivers are partly explained by the anti-inflammatory effects of propionate [43] and may reveal GM contributions to sex differences in hypertension. T helper (TH) 17 cells (TH17), activated by action of certain microbiota strains, are key pro-inflammatory protagonists correlated to hypertensive state [44][45] (Figure 1). Hypertensive male rats present more TH17 cells compared to female rats in association with reduced SCFA-producer Lactobacilli [46][47], thus supporting the relevance of GM/SH axis in setting of hypertension. In the human beings, the reduced GM diversity observed in men can lead to low-grade inflammation contributing to hypertension development. Conversely, estrogens can reduce inflammation concurring to the maintenance of normal BP values during the fertile age.
Finally, arterial stiffness is an independent factor of CV risk particularly relevant to women and is closely related to hypertension. In the post-menopause period, the vascular stiffness increases in parallel with BP. Menni et al. studied the role of GM in arterial stiffness in women, evidencing, for the first time, that GM composition is strongly correlated with levels of arterial stiffness independently of visceral fat and other obesity-related traits, thus suggesting that targeting the microbiome may be a way to treat arterial ageing [48].
Collectively, these data suggest a strong association between GM dysbiosis, hypertension, and gender. However, further clinical studies investigating the impact of the gut microbiome on sex differences in BP and hypertension are necessary.
3. Atherosclerosis, Myocardial Infarction and Heart Failure
Clear sex differences have been documented in the onset and evolution of atherosclerosis and CVD [49] (Figure 1). On average, women show reduced atherosclerotic plaque than men at any age and experience ischemic events, such as myocardial infarction (MI), at a later age. A main mechanism for this sex bias is oxidative stress (OS), which is considered a key trigger of coronary artery disease. Pre-menopause women show lower levels of OS than men, due to the antioxidant properties of estrogens, which up-regulate the expression and protein content of anti-oxidant enzymes, including nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase and angiotensin II [50] (Figure 1). In agreement, a recent proteomic profiling revealed sexual dimorphism of the protein content in human atherosclerotic tissue. The main differences concerned response to oxygen species and inflammatory signaling, along with complement activation, and blood coagulation [51]. Sex-related differences have also been reported in the association between non-calcified or mixed coronary atherosclerotic plaques and plasma fibrinogen, a well-known risk factor for CVD [52].
Several studies confirm the relationship between GM dysbiosis and the pathogenesis of atherosclerosis, MI, coronary artery disease (CAD), and HF in humans.
A major mechanism through which GM dysbiosis favors CVD evolution in patients, is through the production of irritating or noxious molecules. For example, excessive GM-derived ammonia and ammonium hydroxide results in a local pro-inflammatory state that disrupts the intestinal epithelial tight junctions and may propagate at a systemic level (Figure 1). Of note, the identification of bacterial DNA, mostly Proteobacteria, both in the atherosclerotic lesions and in the gut of the same individuals [53] points at a key role of a leaky gut epithelial barrier in the translocation of gut bacteria to the site of plaque formation. Further, high levels of circulating trimethylamine N-oxide (TMAO) produced by GM have been found in association with vulnerable coronary plaque and plaque rupture and represent a relevant long-term risk of incident CV events in patients with acute coronary syndrome [54][55]. In a study of more than 1800 stable cardiac patients undergoing elective coronary angiography, all TMAO-associated metabolites were positively correlated with prevalent CVD and incident CV events. Additionally, a follow-up study showed that TMAO is a predictive biomarker of all-cause mortality or reinfarction at 2 years after MI [36]. Therefore, TMAO can be considered a critical participant in enhanced risk for atherosclerosis and MI (Figure 1).
Another mechanistic link between altered GM diversity and the severity of MI has been reported in rats [56]. High systemic leptin levels represent a well-known risk factor of MI and CVD (Figure 1). Administration of Lactobacillus Plantarum suppresses circulating leptin, and improves left ventricular function, ultimately leading to decreased myocardial infarct size and post ischemic adverse chamber remodeling [49][57]. Interestingly, experimental studies have indicated a greater abundance of Lactobacillus Plantarum in females than in males [58], which can contribute to the gender difference observed in MI and related CVD. Finally, GM has been involved in increased availability of different metabolites of the aromatic amino-acids that are associated with augmented severity of induced MI [59].
HF is a devastating disease with high morbidity and mortality [60]. GM and their dietary-derived metabolites have been implicated in the so-called “gut hypothesis of HF”. In effect, gut dysbiosis, with decreased microbial richness, enhanced inflammation and increased gut permeability, has been observed in conjunction with decreased cardiac output and elevated systemic congestion either in mouse models of pressure overload-induced HF and in HF patients [61][62][63].
Available experimental findings have evidenced a causal role of TMAO in systolic dysfunction and adverse remodeling of HF, including myocardial hypertrophy, wall thinning, ventricular dilation, and increased fibrosis [64][65]. Consistently, clinical studies have confirmed that the GM-derived metabolites TMAO and choline are increased in HF and are associated with poor prognosis even after adjusting for traditional risk factors [66][67][68][69][70]. Collectively, these data suggest a key role of GM and their metabolites in the pathogenesis and progression of HF. In turn, reduced intestinal blood flow, due to heart functional impairment, leads to GM re-shaping with increased bacterial mass and reduced diversity in colonic mucosa, and decreased microbial charge in the stool [63][66].
At present there are no data reporting the effect of GM dysbiosis on the development of HF in relation to gender dimorphism.
References
- Jovel, J.; Dieleman, L.A.; Kao, D.; Mason, A.L. The human gut microbiome in health and disease. In Meta-Genomics. Perspectives, Methods and Applications; Nagarajan, M., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 197–213.
- Peng, C.; Xu, X.; Li, Y.; Li, X.; Yang, X.; Chen, H.; Zhu, Y.; Lu, N.; He, C. Sex-specific association between the gut microbiome and high-fat diet-induced metabolic disorders in mice. Biol. Sex Differ. 2020, 11, 5.
- Harada, N.; Hanaoka, R.; Hanada, K.; Izawa, T.; Inui, H.; Yamaji, R. Hypogonadism alters cecal and fecal microbiota in male mice. Gut Microbes 2016, 7, 533–539.
- Harada, N.; Hanaoka, R.; Horiuchi, H.; Kitakaze, T.; Mitani, T.; Inui, H.; Yamaji, R. Castration influences intestinal microflora and induces abdominal obesity in high-fat diet-fed mice. Sci. Rep. 2016, 6, 23001.
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316.
- Gao, A.; Su, J.; Liu, R.; Zhao, S.; Li, W.; Xu, X.; Li, D.; Shi, J.; Bin Gu, B.; Zhang, J.; et al. Sexual dimorphism in glucose metabolism is shaped by androgen-driven gut microbiome. Nat. Commun. 2021, 12, 7080.
- Li, X.; Cheng, W.; Shang, H.; Wei, H.; Deng, C. The Interplay between Androgen and Gut Microbiota: Is There a Microbiota-Gut-Testis Axis. Reprod. Sci. 2022, 29, 1674–1684.
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339, 1084–1088.
- Santos-Marcos, J.A.; Haro, C.; Vega-Rojas, A.; Alcala-Diaz, J.F.; Molina-Abril, H.; Leon-Acuña, A.; Lopez-Moreno, J.; Landa, B.B.; Tena-Sempere, M.; Perez-Martinez, P.; et al. Sex Differences in the Gut Microbiota as Potential Determinants of Gender Predisposition to Disease. Mol. Nutr. Food Res. 2019, 63, e1800870.
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085.
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60.
- Aw, W.; Fukuda, S. Understanding the role of the gut ecosystem in diabetes mellitus. J. Diabetes Investig. 2018, 9, 5–12.
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7, Erratum in: Gastroenterology 2013, 144, 250.
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Phy. Endocrinol. Metab. 2007, 292, E740–E747.
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid–induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025.
- Caricilli, A.M.; Picardi, P.K.; de Abreu, L.L.; Ueno, M.; Prada, P.O.; Ropelle, E.R.; Hirabara, S.M.; Castoldi, Â.; Vieira, P.; Camara, N.O.; et al. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol. 2011, 9, e1001212.
- García-Gómez, E.; González-Pedrajo, B.; Camacho-Arroyo, I. Role of Sex Steroid Hormones in Bacterial-Host Interactions. BioMed Res. Int. 2013, 2013, 928290.
- Cani, P.D.; Lecourt, E.; Dewulf, E.M.; Sohet, F.M.; Pachikian, B.D.; Naslain, D.; De Backer, F.; Neyrinck, A.M.; Delzenne, N.M. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 2009, 90, 1236–1243.
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754.
- Zhang, W.; Tang, Y.; Huang, J.; Yang, Y.; Yang, Q.; Hu, H. Efficacy of inulin supplementation in improving insulin control, HbA1c and HOMA-IR in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Biochem. Nutr. 2020, 66, 176–183.
- Rao, M.; Gao, C.; Xu, L.; Jiang, L.; Zhu, J.; Chen, G.; Law, B.Y.K.; Xu, Y. Effect of inulin-type carbohydrates on insulin resistance in patients with Type 2 diabetes and obesity: A systematic review and meta-analysis. J. Diabetes Res. 2019, 2019, 5101423.
- Han, L.; Wang, P.; Zhao, G.; Wang, H.; Wang, M.; Chen, J.; Tong, T. Upregulation of SIRT1 by 17β-estradiol depends on ubiquitin-proteasome degradation of PPAR-γ mediated by NEDD4-1. Protein Cell 2013, 4, 310–321.
- Park, H.J.; Park, H.S.; Lee, J.U.; Bothwell, A.L.; Choi, J.M. Gender-specific differences in PPARγ regulation of follicular helper T cell responses with estrogen. Sci Rep. 2016, 6, 28495.
- Santisteban, M.; Qi, Y.; Zubcevic, J.; Kim, S.; Yang, T.; Shenoy, V.; Cole-Jeffrey, C.T.; Lobaton, G.; Stewart, D.C.; Rubiano, A.; et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ. Res. 2017, 120, 312–323.
- Yan, Q.; Gu, Y.; Li, X.; Yang, W.; Jia, L.; Chen, C.; Han, X.; Huang, Y.; Zhao, L.; Li, P.; et al. Alterations of the Gut Microbiome in Hypertension. Front. Cell. Infect. Microbiol. 2017, 7, 381.
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 2018, 132, 701–718.
- Vallianou, N.G.; Geladari, E.; Kounatidis, D. Microbiome and hypertension. J. Cardiovasc. Med. 2020, 21, 83–88.
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14.
- Fan, L.; Ren, J.; Chen, Y.; Wang, Y.; Guo, Z.; Bu, P.; Yang, J.; Ma, W.; Zhu, B.; Zhao, Y.; et al. Effect of fecal microbiota transplantation on primary hypertension and the underlying mechanism of gut microbiome restoration: Protocol of a randomized, blinded, placebo-controlled study. Trials 2022, 23, 178.
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive com-pounds: Microbial endocrinology in the design and use of probiotics. Bioessays 2011, 33, 574–581.
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. Increased Systolic and Diastolic Blood Pressure Is Associated with Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension 2016, 68, 974–981.
- Robles-Vera, I.; Toral, M.; de la Visitación, N.; Sánchez, M.; Romero, M.; Olivares, M.; Jiménez, R.; Duarte, J. The Probiotic Lactobacillus Fermentum Prevents Dysbiosis and Vascular Oxidative Stress in Rats with Hypertension Induced by Chronic Nitric Oxide Blockade. Mol. Nutr. Food Res. 2018, 62, 1800298.
- Palmu, J.; Lahti, L.; Niiranen, T. Targeting Gut Microbiota to Treat Hypertension: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1248.
- Palmu, J.; Salosensaari, A.; Havulinna, A.S.; Cheng, S.; Inouye, M.; Jain, M.; Salido, R.A.; Sanders, K.; Brennan, C.; Humphrey, G.C.; et al. Association Between the Gut Microbiota and Blood Pressure in a Population Cohort of 6953 Individuals. J. Am. Heart Assoc. 2020, 9, e016641.
- Suzuki, T.; Heaney, L.; Jones, D.; Ng, L. Trimethylamine N-oxide and Risk Stratification after Acute Myocardial Infarction. Clin. Chem. 2017, 63, 420–428.
- Suzuki, Y.; Ikeda, K.; Sakuma, K.; Kawai, S.; Sawaki, K.; Asahara, T.; Takahashi, T.; Tsuji, H.; Nomoto, K.; Nagpal, R.; et al. Association between Yogurt Consumption and Intestinal Microbiota in Healthy Young Adults Differs by Host Gender. Front. Microbiol. 2017, 8, 847.
- Gillis, E.E.; Sullivan, J.C. Sex differences in hypertension: Recent advances. Hypertension 2016, 8, 1322–1327.
- Mell, B.; Jala, V.R.; Mathew, A.V.; Byun, J.; Waghulde, H.; Zhang, Y.; Haribabu, B.; Vijay-Kumar, M.; Pennathur, S.; Joe, B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol. Genom. 2015, 47, 187–197.
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension 2015, 65, 1331–1340.
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension 2014, 64, 897–903.
- Beale, A.L.; Kaye, D.M.; Marques, F.Z. The role of the gut microbiome in sex differences in arterial pressure. Biol. Sex Differ. 2019, 10, 22.
- van den Munckhof, I.C.L.; Kurilshikov, A.; Ter Horst, R.; Riksen, N.P.; Joosten, L.A.B.; Zhernakova, A.; Fu, J.; Keating, S.T.; Netea, M.G.; De Graaf, J.; et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: A systematic review of human studies. Obes. Rev. 2018, 19, 1719–1734.
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421.
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intes-tinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498.
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007, 204, 2449–2460.
- Zimmerman, M.A.; Sullivan, J.C. Hypertension: What’s sex got to do with it? Physiology 2013, 28, 234–244.
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589.
- Menni, C.; Lin, C.; Cecelja, M.; Mangino, M.; Matey-Hernandez, M.L.; Keehn, L.; Mohney, R.P.; Steves, C.; Spector, T.D.; Kuo, C.-F.; et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur. Heart J. 2018, 39, 2390–2397.
- Spence, J.D.; Pilote, L. Importance of sex and gender in atherosclerosis and cardiovascular disease. Atherosclerosis 2015, 241, 208–210.
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032.
- Ward, L.J.; Olausson, P.; Li, W.; Yuan, X.-M. Proteomics and multivariate modelling reveal sex-specific alterations in distinct regions of human carotid atheroma. Biol. Sex Differ. 2018, 9, 54.
- Li, T.; Wang, F.; Peng, R.; Pei, S.; Hou, Z.; Lu, B.; Cong, X.; Chen, X. Sex-related differences in the association between plasma fibrinogen and non-calcified or mixed coronary atherosclerotic plaques. Biol. Sex Differ. 2018, 9, 51.
- Koren, O.; Spor, A.; Felin, J.; Fåk, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108, 4592–4598.
- Fu, Q.; Zhao, M.; Wang, D.; Hu, H.; Guo, C.; Chen, W.; Li, Q.; Zheng, L.; Chen, B. Coronary plaque characterization assessed by optical coherence tomography and plasma trimethylamine-n-oxide levels in patients with coronary artery disease. Am. J. Cardiol. 2016, 118, 1311–1315.
- Li, X.S.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Räber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; et al. Gut microbiota-dependent trimethylamine n-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017, 4, 814–824.
- Lam, V.; Su, J.; Hsu, A.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal Microbial Metabolites Are Linked to Severity of Myocardial Infarction in Rats. PLoS ONE 2016, 11, e0160840.
- Gan, X.T.; Ettinger, G.; Huang, C.X.; Burton, J.P.; Haist, J.V.; Rajapurohitam, V.; Sidaway, J.E.; Martin, G.; Gloor, G.B.; Swann, J.R.; et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ. Heart Fail. 2014, 7, 491–499.
- Elderman, M.; Hugenholtz, F.; Belzer, C.; Boekschoten, M.; van Beek, A.; de Haan, B.; Savelkoul, H.; de Vos, P.; Faas, M. Sex and strain dependent differences in mucosal immunology and microbiota composition in mice. Biol. Sex Differ. 2018, 9, 26.
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652.
- Roger, V.L. Epidemiology of Heart Failure: A Contemporary Perspective. Circ. Res. 2021, 128, 1421–1434.
- Boccella, N.; Paolillo, R.; Coretti, L.; D’Apice, S.; Lama, A.; Giugliano, G.; Schiattarella, G.G.; Cuomo, M.; D’Aquino, I.; Cavaliere, G.; et al. Transverse aortic constriction induces gut barrier alterations, microbiota remodeling and systemic inflammation. Sci. Rep. 2021, 11, 7404.
- Luedde, M.; Winkler, T.; Heinsen, F.-A.; Rühlemann, M.C.; Spehlmann, M.E.; Bajrovic, A.; Lieb, W.; Franke, A.; Ott, S.J.; Frey, N. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail. 2017, 4, 97–108.
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F.S. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail. 2016, 4, 220–227.
- Li, Z.; Wu, Z.; Yan, J.; Liu, H.; Liu, Q.; Deng, Y.; Ou, C.; Chen, M. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab. Investig. 2018, 99, 346–357.
- Organ, C.L.; Otsuka, H.; Bhushan, S.; Wang, Z.; Bradley, J.; Trivedi, R.; Polhemus, D.J.; Tang, W.W.; Wu, Y.; Hazen, S.L.; et al. Choline Diet and Its Gut Microbe–Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload–Induced Heart Failure. Circ. Heart Fail. 2016, 9, e002314.
- Tang, W.W.; Wang, Z.; Fan, Y.; Levison, B.; Hazen, J.E.; Donahue, L.M.; Wu, Y.; Hazen, S.L. Prognostic Value of Elevated Levels of Intestinal Microbe-Generated Metabolite Trimethylamine-N-Oxide in Patients With Heart Failure. J. Am. Coll. Cardiol. 2014, 64, 1908–1914.
- Israr, M.Z.; Bernieh, D.; Salzano, A.; Cassambai, S.; Yazaki, Y.; Heaney, L.M.; Jones, D.J.L.; Ng, L.L.; Suzuki, T. Association of gut-related metabolites with outcome in acute heart failure. Am. Heart J. 2021, 234, 71–80.
- Hayashi, T.; Yamashita, T.; Watanabe, H.; Kami, K.; Yoshida, N.; Tabata, T.; Emoto, T.; Sasaki, N.; Mizoguchi, T.; Irino, Y.; et al. Gut Microbiome and Plasma Microbiome-Related Metabolites in Patients with Decompensated and Compensated Heart Failure. Circ. J. 2018, 83, 182–192.
- Cui, X.; Ye, L.; Li, J.; Jin, L.; Wang, W.; Li, S.; Bao, M.; Wu, S.; Li, L.; Geng, B.; et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 2018, 8, 635.
- Schiattarella, G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
618
Revisions:
4 times
(View History)
Update Date:
08 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




