Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fujie Zhao | -- | 2436 | 2022-07-06 15:50:10 | | | |
| 2 | Camila Xu | Meta information modification | 2436 | 2022-07-07 03:47:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhao, F.; Satyanarayana, G.; Zhang, Z.; Zhao, J.; Ma, X.; Wang, Y. Endothelial Autophagy in Cardiovascular Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/24870 (accessed on 07 February 2026).
Zhao F, Satyanarayana G, Zhang Z, Zhao J, Ma X, Wang Y. Endothelial Autophagy in Cardiovascular Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/24870. Accessed February 07, 2026.
Zhao, Fujie, Ganesh Satyanarayana, Zheng Zhang, Jianli Zhao, Xin-Liang Ma, Yajing Wang. "Endothelial Autophagy in Cardiovascular Disease" Encyclopedia, https://encyclopedia.pub/entry/24870 (accessed February 07, 2026).
Zhao, F., Satyanarayana, G., Zhang, Z., Zhao, J., Ma, X., & Wang, Y. (2022, July 06). Endothelial Autophagy in Cardiovascular Disease. In Encyclopedia. https://encyclopedia.pub/entry/24870
Zhao, Fujie, et al. "Endothelial Autophagy in Cardiovascular Disease." Encyclopedia. Web. 06 July, 2022.
Copy Citation
Autophagy is a highly conserved process in which obsolete and dysfunctional cytoplasmic components (such as unfolded proteins, lipids, and damaged organelles) are degraded and recycled, and infectious organisms are removed by lysosomes. Deficient or uncontrolled activation of endothelial autophagy is associated with the onset and development of diverse cardiovascular diseases (CVDs), including coronary microvascular dysfunction” (CMD).
coronary microvascular dysfunction
endothelial cell
autophagy
1. Brief Overview of Autophagy
Autophagy is a highly conserved process in which obsolete and dysfunctional cytoplasmic components (such as unfolded proteins, lipids, and damaged organelles) are degraded and recycled, and infectious organisms are removed by lysosomes [1][2][3][4]. Autophagy is stimulated by different stresses (i.e., nutrient deprivation and hypoxia) and functions primarily as a cell survival mechanism [5][6][7][8]. However, it switches to promoting cell death under insurmountable lethal stress, which is known as autophagic (type II) cell death [9][10]. Three classes of autophagy are identified as follows: chaperone-required autophagy, microautophagy, and macroautophagy [11][12][13]. Chaperone-required autophagy is the selective degradation of proteins with a KFERQ-like motif. During the process, targeted proteins are transferred to the lysosomes with the company of the chaperone HSC70 and co-chaperones, subsequently internalized into the lysosomes via an interaction with the lysosome-associated membrane protein type 2A (LAMP2A) [14]. In microautophagy, the cargo, alone or in a complex with chaperones, can be directly engulfed by the lysosome and late endosomes through invagination at the lysosomal membrane via electrostatic forces [15][16][17]. Macroautophagy (hereafter referred to as autophagy) is the most well studied and the major type of autophagy. The macroautophagy pathway is characterized by the formation of autophagosomes, within which cytoplasmic components are insulated and subsequently degraded by fusing with the lysosomes [10][18][19][20]. The process of autophagy can be dissected into the following sequential steps: induction of a phagophore assembly site, nucleation of an autophagosome precursor (known as the phagophore), membrane expansion and maturation of the autophagosome (a double membrane vesicle), fusion with the lysosome for degradation, and lastly, recycling the degraded cargo [1][2][21][22].
The process of autophagy is sequentially regulated by multiple mechanisms (Figure 1). (1) First, initiation of the phagophore assembly begins with the formation of a preinitiation complex, which is composed of the unc-51-like kinase (ULK1/2), autophagy-related protein 13 (ATG13), and the non-catalytic focal adhesion kinase-family interacting protein of 200 kD (FIP200) [10][23][24]. The activity of this kinase complex is negatively inhibited by the mammalian target of the rapamycin complex 1 (mTORC1) and the positively activated AMP-activated protein kinase (AMPK) [25][26]. (2) Further nucleation involves the recruitment and activation of the initiation complex, which is composed of the vacuolar protein sorting protein 15 (VPS15), a class III PI3K (VPS34), and Beclin 1 [27][28]. The activity of the initiation complex is downregulated by several independent signaling pathways, such as the PI3K-AKT pathway, and the Bcl2 and Bcl-xL pathway [29][30]. Conversely, starvation or exercise-induced autophagy activation is carried out by the JNK family [31][32]. (3) Next, the phagophore elongation and closure require two distinct but complementary ubiquitin-like protein conjugation systems—the ATG12/ATG5/ATG16L1 complex and the microtubule-associated protein 1-light chain 3 (LC3)-phosphatidylethanolamine (PE) machinery [10]. (4) Finally, the fusion of the autophagosomes with the lysosomes requires the syntaxin17 (Stx17) synaptosome-associated protein 29 (SNAP29), the vesicle-associated membrane protein 8 (VAMP8), and the lysosomal-associated membrane protein 1/2 (LAMP1/2) [33][34][35][36].
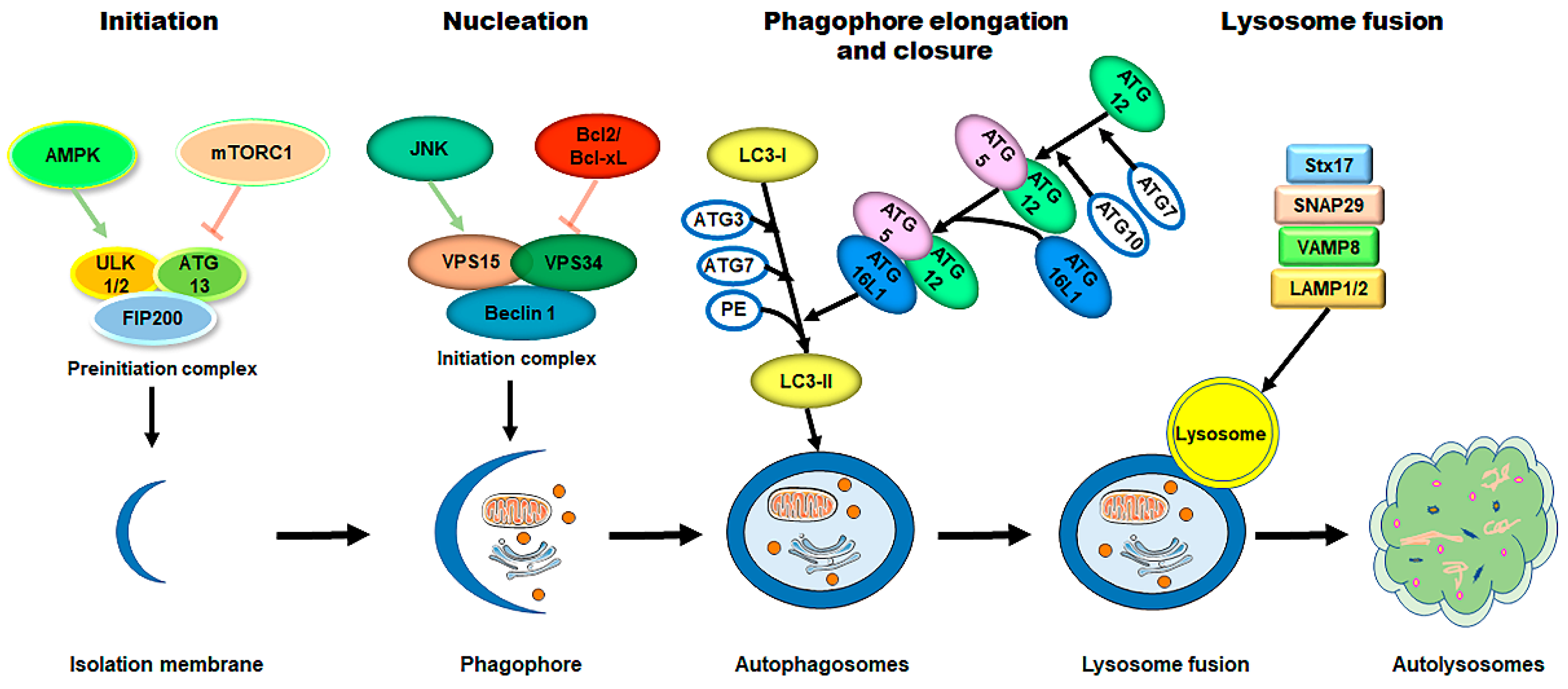 Figure 1. The process of autophagy in mammalian cells. The process of autophagy is sequentially dissected into several phases, including initiation, nucleation, phagophore elongation/closure, and autophagosome–lysosome fusion. (1) First, upon metabolic insults, the AMPK activation and/or mTORC1 inhibition result in the initiation of the preinitiation complex (ULK1/2, ATG13, and FIP200). (2) Further nucleation involves the recruiting and activating of the initiation complex (VPS15, VPS34, and Beclin 1), which is downregulated by the Bcl2/Bcl-xL pathways and upregulated by the JNK family. (3) Next, phagophore elongation and closure require the ATG12/ATG5/ATG16L1 complex and the LC3-PE machinery. (4) Finally, the fusion of the autophagosomes with the lysosomes requires Stx17, SNAP29, VAMP8, and LAMP1/2. Abbreviations: AMPK, AMP-activated protein kinase; mTORC1, mammalian target of rapamycin complex 1; ULK1/2, unc-51-like kinase 1/2; ATG, autophagy-related protein; FIP200, the non-catalytic focal adhesion kinase-family interacting protein of 200 kD; VPS, vacuolar protein sorting; LC3, microtubule-associated protein 1-light chain 3; PE, phosphatidylethanolamine; Stx17, syntaxin 17; SNAP29, synaptosome-associated protein 29; VAMP8, vesicle-associated membrane protein 8; LAMP1/2, lysosomal-associated membrane protein 1/2.
Figure 1. The process of autophagy in mammalian cells. The process of autophagy is sequentially dissected into several phases, including initiation, nucleation, phagophore elongation/closure, and autophagosome–lysosome fusion. (1) First, upon metabolic insults, the AMPK activation and/or mTORC1 inhibition result in the initiation of the preinitiation complex (ULK1/2, ATG13, and FIP200). (2) Further nucleation involves the recruiting and activating of the initiation complex (VPS15, VPS34, and Beclin 1), which is downregulated by the Bcl2/Bcl-xL pathways and upregulated by the JNK family. (3) Next, phagophore elongation and closure require the ATG12/ATG5/ATG16L1 complex and the LC3-PE machinery. (4) Finally, the fusion of the autophagosomes with the lysosomes requires Stx17, SNAP29, VAMP8, and LAMP1/2. Abbreviations: AMPK, AMP-activated protein kinase; mTORC1, mammalian target of rapamycin complex 1; ULK1/2, unc-51-like kinase 1/2; ATG, autophagy-related protein; FIP200, the non-catalytic focal adhesion kinase-family interacting protein of 200 kD; VPS, vacuolar protein sorting; LC3, microtubule-associated protein 1-light chain 3; PE, phosphatidylethanolamine; Stx17, syntaxin 17; SNAP29, synaptosome-associated protein 29; VAMP8, vesicle-associated membrane protein 8; LAMP1/2, lysosomal-associated membrane protein 1/2.The selective sequestration of the dysfunctional mitochondria by the autophagosomes and the subsequent degradation by the lysosomes are termed "mitophagy” [18] (Figure 2). There are two distinct signaling pathways for mitophagy, which are as follows: the phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1)–Parkin-dependent pathway and the mitophagy receptor-required pathway [37][38]. To date, multiple mitophagy receptors have been recognized in mammals, including the BCL2/adenovirus E1B 19kDa-interacting protein 3 (BNIP3) [39], NIP3-like protein X (NIX, also called the BNIP3-like protein (BNIP3L)) [40][41][42], FUN14 domain-containing protein 1 (FUNDC1) [43], B-cell lymphoma-2-like 13 (BCL2L13) [44], and FK506-binding protein 8 (FKBP8) [45].
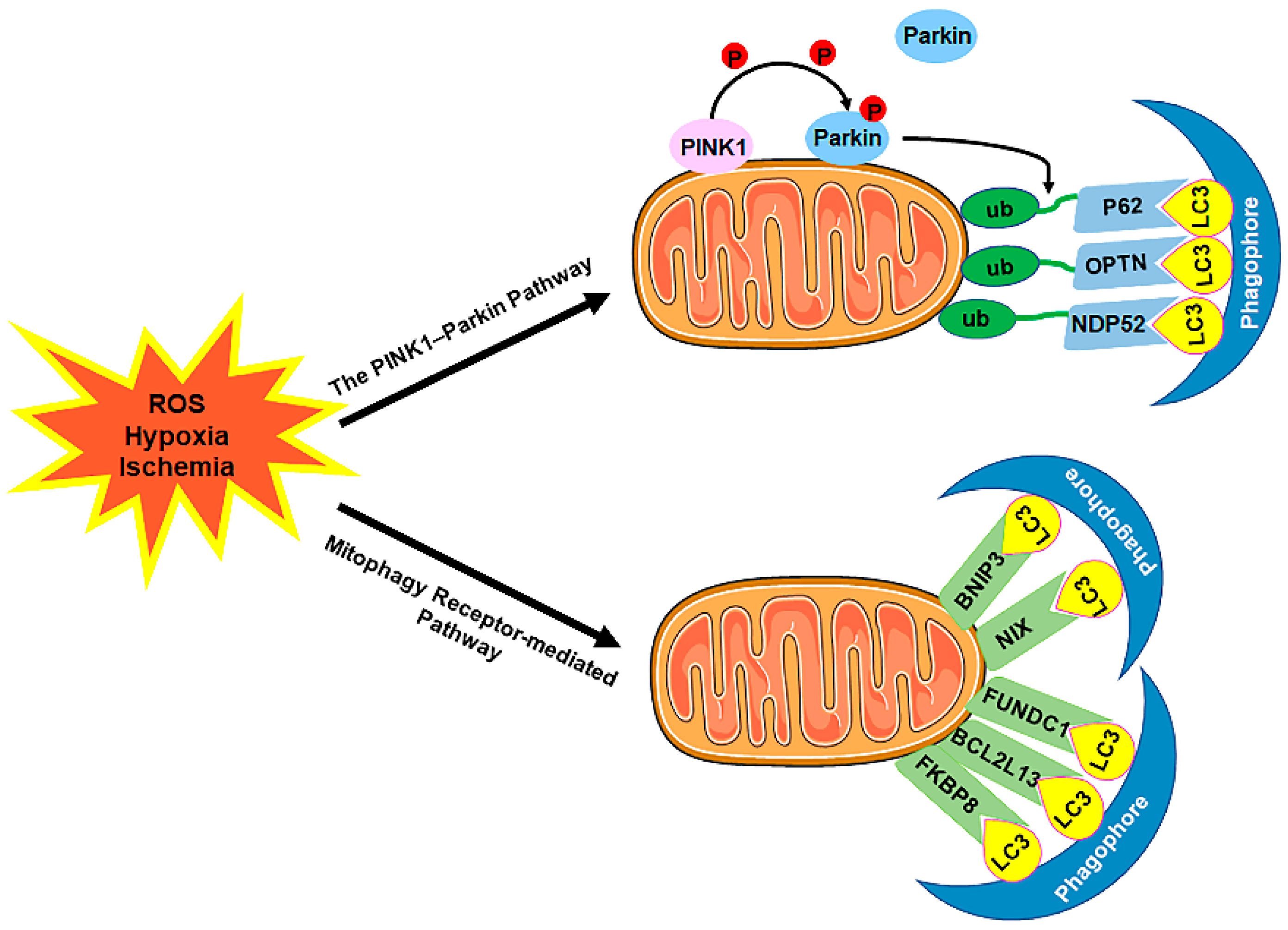 Figure 2. The mechanism of mitophagy. Mitophagy is induced by ROS, hypoxia, ischemia, and other stimuli. There are two distinct signaling pathways for mitophagy, which are as follows: (1) The PINK1–Parkin pathway of mitophagy. Following stress, PINK1 accumulates on the outer membrane of the mitochondria (OMM), promoting Parkin recruitment to ubiquitinate several OMM components. Poly-Ub chains are subsequently recognized by adaptor proteins (p62, OPTN, and NDP52) and further initiate autophagosome formations through binding with LC3. (2) The mitophagy receptor-mediated pathway. The BNIP3, NIX, FUNDC1, BCL2L13, and FKBP8 mitophagy receptors localize to the OMM and directly bind with LC3 to mediate mitochondrial elimination. Abbreviations: PINK1, PTEN-induced putative kinase 1; Parkin, Parkin RBR E3 ubiquitin-protein ligase; ub, ubiquitination; p62/SQSTM1, sequestosome 1; OPTN, optineurin; NDP52/CALCOCO2, calcium binding and coiled-coil domain 2; LC3, microtubule-associated protein 1A/1B-light chain 3; BNIP3, BCL2/adenovirus E1B 19 kDa-interacting protein 3; NIX, NIP3-like protein X; FUNDC1, FUN14 domain-containing 1; BCL2L13, B-cell lymphoma-2-like 13; FKBP8, FK506-binding protein 8.
Figure 2. The mechanism of mitophagy. Mitophagy is induced by ROS, hypoxia, ischemia, and other stimuli. There are two distinct signaling pathways for mitophagy, which are as follows: (1) The PINK1–Parkin pathway of mitophagy. Following stress, PINK1 accumulates on the outer membrane of the mitochondria (OMM), promoting Parkin recruitment to ubiquitinate several OMM components. Poly-Ub chains are subsequently recognized by adaptor proteins (p62, OPTN, and NDP52) and further initiate autophagosome formations through binding with LC3. (2) The mitophagy receptor-mediated pathway. The BNIP3, NIX, FUNDC1, BCL2L13, and FKBP8 mitophagy receptors localize to the OMM and directly bind with LC3 to mediate mitochondrial elimination. Abbreviations: PINK1, PTEN-induced putative kinase 1; Parkin, Parkin RBR E3 ubiquitin-protein ligase; ub, ubiquitination; p62/SQSTM1, sequestosome 1; OPTN, optineurin; NDP52/CALCOCO2, calcium binding and coiled-coil domain 2; LC3, microtubule-associated protein 1A/1B-light chain 3; BNIP3, BCL2/adenovirus E1B 19 kDa-interacting protein 3; NIX, NIP3-like protein X; FUNDC1, FUN14 domain-containing 1; BCL2L13, B-cell lymphoma-2-like 13; FKBP8, FK506-binding protein 8.2. Coronary Endothelial Autophagy in CVDs
In the past, autophagy (including mitophagy) in cardiomyocytes was thought to play a predominant role in heart injuries. With the increasing recognition of the crucial contributions of CMD to CVDs, the role of autophagy (including mitophagy) in non-myocytes, particularly in coronary ECs, has attracted great interest. Emerging evidence unravels that autophagy is required for multiple EC functions, such as secretion of adhesion molecules [46][47], EC nitric oxide synthase (eNOS)-derived NO bioavailability [48][49][50], expression of ET-1 [51][52], ROS production [49][53], and inflammatory cytokines production [49], which participate in a wide range of cellular events, including endothelial proliferation [52], senescence [53][54][55], and apoptosis [54][56][57][58]. EC-intrinsic autophagy is suggested to enable ECs to adjust plastically to various insulting stressors [51][56][59] and leads to autophagic cell death in severely damaged ECs [60][61][62].
2.1. Coronary Endothelial Autophagy in Obstructive CAD (Stable CAD and Acute Coronary Syndromes)
In the hearts of obstructive CAD patients, CMD probably coexists and plays a role in causing myocardial ischemia in regions perfused by arteries both with and without stenosis. Thus, CMD has important diagnostic, prognostic, and management implications [63][64][65][66]. In regions distal to arterial stenosis, the chronic modulation of coronary microcirculation to limited perfusion pressure may negatively impact the microvascular remodeling and the maximal capacity of vasodilation after restoring to normal CBF [67]. The most severe form of CMD is microvascular obstruction (MVO), which refers to a capillary destruction with a no-reflow phenomenon, despite recanalization of the epicardial coronary artery [68][69][70]. The pathogenic mechanisms underlying CMD in obstructive CAD include coronary microvascular EC injuries, which may occur much earlier and with much more severe damage than cardiomyocyte injuries. Emerging evidence shows that autophagy plays a fundamental role in this process [71][72]. However, the underlying mechanisms remain controversial. Upon oxidative stress, Wang et al. reported that microRNA-103 could protect the human coronary artery ECs (HCAECs) against H2O2-induced injuries by preventing the Bcl-2/BNIP3-mediated suppression of end-stage autophagy [73]. Furthermore, by using mice with EC-specific NADPH oxidase 2 (Nox2)/gp91 overexpression, Shafique et al. demonstrated that endogenous ROS oxidative stress protected mouse heart ECs from oxidant-induced cell death by increasing the AMPK-mTOR-mediated autophagy and through improving the AMPK-eNOS-mediated EC-dependent-coronary vasodilatation [74]. Autophagy can also be induced by hypoxia stress [75]. Wang et al. identified that forkhead box O3 alpha (foxO3α)-dependent autophagy aggravated hypoxia-induced rat cardiac microvascular endothelial cell (CMEC) dysfunction and apoptosis [76]. However, according to Sun’s study, the mitophagy induced by the Rcan1-1L (regulator of calcineurin 1-1L) overexpression contributed to cell survival under hypoxic conditions [77].
Notably, the role of endothelial autophagy in hypoxia/reoxygenation (H/R) or ischemia/reperfusion (I/R)-induced injury is much more complex. Generally, autophagy is a protective mechanism against cardiac H/R or I/R injury [78]. Its protective role is driven by inducing the mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway [79] or via activating and promoting the transcription factor EB (TFEB) translocating from the lysosomes to the nuclei [80]. Furthermore, mitophagy was usually referred to as a pro-survival regulator to I/R or H/R injury [81]. Inhibition of the FUNDC1-mediated mitophagy in CMECs by the nuclear receptor subfamily 4 group A member 1 (NR4A1) [82] or receptor-interacting protein kinase 3 (Ripk3) [83] exhibited the disturbed mitochondrial homeostasis, upregulated the expression of EC-derived pro-inflammatory and adhesive factors, enhanced endothelial apoptosis, and provoked CMD in cardiac I/R injuries. In addition, enhancing autophagy through Beclin1 overexpression in CMECs exhibited suppressed NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome activation by promoting tumor necrosis factor-alpha-induced protein 3 (TNFAIP3) [84] and inhibited caspase-4 inflammasome activation [85]. Thus, it resulted in a reduced IL-1β level and an increased animal survival upon myocardial I/R injuries. In addition, the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) overexpression [86] and miR-92a-3p inhibition [87] could protect the CMECs against myocardial I/R injuries by preserving the EC mitophagy. However, hyperautophagy is linked to I/R or H/R injury-induced mitochondria and EC apoptosis. The inhibition of autophagy contributed to the anti-apoptosis effects of glycyrrhizic acid (GA) in H/R-induced CMEC injury [88]. Melatonin was reported to play a beneficial role in CMECs against I/R injury through directly suppressing autophagy via the AMPK/mTOR pathway [89] or by inhibiting the mitophagy-mediated cell death via the dynamin-related protein 1 (Drp1)-voltage-dependent anion channel 1 (VDAC1)-hexokinase 2 (HK2)-mitochondrial permeability transition pore (mPTP)-PINK1/Parkin axis in an AMPKα-dependent manner [90]. Furthermore, neuregulin-1β (Nrg1β) protected the cardiac ECs against I/R injury by preventing ATG5-required autophagy-induced Trx2 (thioredoxin) degradation and rescuing eNOS function via upregulating the Erb-B2 receptor tyrosine kinase 2 (ErbB2) [91].
2.2. Coronary Endothelial Autophagy in HFpEF
HFpEF is recognized as a clinically heterogeneous syndrome, in which patients present classic symptoms and signs of HF but exhibit a normal or near-normal EF [92][93]. CMD is hypothesized to play a fundamental role in the pathophysiological process of HFpEF [94][95][96][97]. Up to 75% of HFpEF patients exhibit impaired CFR in spite of the absence of obstructive CAD [98]. Diabetes mellitus (DM), metabolic syndrome, hypertension, and obesity are prevalent cardiovascular risk factors for HFpEF [92][94][99]; they aggravate cardiac dysfunction and remodeling through CMD [100]. The basic mechanisms that mediate the progression of HFpEF include myocyte hypertrophy, energetic imbalance, mitochondrial dysfunction, EC dysfunction, increased oxidative stress, inflammation, interstitial fibrosis, and damaged angiogenesis [101][102][103][104]. The pathophysiological role of autophagy in HFpEF onset and progression has just begun to be acknowledged. Here, researchers mainly focus on the impact of autophagy on cardiac angiogenesis and fibrosis, two critical events associated with the progression of HFpEF.
2.3. Coronary Endothelial Autophagy in DCM
Clinical studies have shown that CMD is an early feature of DCM (even in patients without obstructive CVDs) [105][106][107] and this impairment is more pronounced in type 2 DM patients [108][109][110][111]. DCM studies in both animals and humans have emphasized the substantial role of the coronary ECs, particularly in the early stages of damage, in promoting ROS generation, and facilitating the recruitment of inflammatory cells. This ultimately resulted in myocardial microvascular rarefaction, diminished angiogenesis, and HFpEF phenotype [112][113][114]. Insulin signaling impairment, hyperglycemia/glucotoxicity, and lipotoxicity are predominant pathophysiological causes of DM-related CMD. Dysregulated autophagy is a key underlying cause in the onset and progression of DCM. A prolonged exposure of a fetal mouse heart to sugars (sucrose or mannitol) could induce severe lysosomal derangements and prominent autophagy in the ECs [115]. Mst1 (mammalian sterile 20-like kinase 1) is a serine/threonine kinase that functions as a negative regulator of autophagy in the heart by enhancing the binding of Beclin1 to Bcl-2 and promoting apoptosis by releasing Bcl-2 from Bax [116][117]. Hu et al. showed that Mst1-enriched exosomes excreted by CMECs were taken up by cardiomyocytes, resulting in inhibited autophagy and ultimately exacerbated high glucose (HG)-induced apoptosis in cardiomyocytes [118]. Meanwhile, Mst1 directly participated in the pathogenesis of CMD by inhibiting autophagy and increasing apoptosis in CMECs [119]. Furthermore, the upregulation of autophagy was reported to rescue HG-induced EC apoptosis through the AKT-mTOR signal pathway [120]. In addition, mitophagy was shown to protect mitochondrial integrity and prevent HG and palmitate acid (HG/PA)-induced EC apoptosis via the PINK1–Parkin pathway [121][122] and hinder HG/PA-induced EC senescence via the AMPK pathway [123]. Furthermore, improving Bnip3-dependent mitophagy could rescue ox-LDL-induced EC damage, resulting in a restored mitochondrial respiration complex activation, reduced ROS production, and an increased EC viability [124]. Interestingly, in certain conditions, the inhibition of autophagy can be protective. The downregulation of autophagy was reported to relieve HG-induced endothelial impairment via the glioma-associated oncogene homolog 1 (GLI1)-dependent-Hedgehog pathway [125].
2.4. Coronary Endothelial Autophagy in Other Heart Diseases
Except for the diseases mentioned above, coronary autophagy was also reported to participate in many other diseases. Kawasaki’s disease (KD) is a systemic febrile vasculitis and can lead to abnormalities of the coronary artery in about 25% of untreated cases, which has been reported as the predominant cause of children’s acquired heart diseases [126][127]. According to Qin’s report, the peripheral blood mononuclear cells (PBMCs) collected from KD patients with fever could induce autophagy in HCAECs, thus, promoting the secretion of chemokines and pro-inflammatory factors [128]. Moreover, ginsenoside Rb1 could effectively alleviate coronary artery lesions in a mouse KD model, possibly by upregulating the AMPK/mTOR/P70S6 pathway-mediated autophagy to prevent EC injury [129]. Additionally, the activation of autophagy was also involved in the anti-inflammatory effects of resveratrol in TNF-α-treated HCAECs [130]. Furthermore, autophagy was found to be upregulated during zebrafish heart regeneration and was positively correlated with the metformin-mediated cardiac regeneration acceleration in zebrafish, including epicardial, endocardial, and vascular endothelial regeneration [131]. Moreover, recent studies show that in aged EC compartments, autophagic activities are compromised [55]. Accordingly, in comparison with ECs in younger mice, ECs from older mice displayed lower levels of vital proautophagic proteins, such as Beclin1 and LC3 [132].
Notably, after coronary angiography, up to 40% of patients with typical clinical manifestations of myocardial ischemia were found with normal or near-normal appearing coronary arteries [133][134][135][136][137][138]. This situation is termed “MVA”, in which CMD is the principal alteration causing symptoms [133]. In particular, MVA is the disease that fosters the concept of CMD and draws people’s attention to the role of CMD in nonobstructive heart diseases for the first time. Unfortunately, to date, there is no animal model for MVA. Mechanistic research is urgently needed.
References
- Yan, Y.; Finkel, T. Autophagy as a regulator of cardiovascular redox homeostasis. Free Radic. Biol. Med. 2017, 109, 108–113.
- Gatica, D.; Chiong, M.; Lavandero, S.; Klionsky, D.J. Molecular mechanisms of autophagy in the cardiovascular system. Circ. Res. 2015, 116, 456–467.
- Schaaf, M.B.; Houbaert, D.; Mece, O.; Agostinis, P. Autophagy in endothelial cells and tumor angiogenesis. Cell Death Differ. 2019, 26, 665–679.
- Scheitlin, C.G.; Nair, D.M.; Crestanello, J.A.; Zweier, J.L.; Alevriadou, B.R. Fluid Mechanical Forces and Endothelial Mitochondria: A Bioengineering Perspective. Cell. Mol. Bioeng. 2014, 7, 483–496.
- Azad, M.B.; Chen, Y.; Henson, E.S.; Cizeau, J.; McMillan-Ward, E.; Israels, S.J.; Gibson, S.B. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 2008, 4, 195–204.
- Chen, Y.; McMillan-Ward, E.; Kong, J.; Israels, S.J.; Gibson, S.B. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008, 15, 171–182.
- Gutierrez, M.G.; Master, S.S.; Singh, S.B.; Taylor, G.A.; Colombo, M.I.; Deretic, V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004, 119, 753–766.
- Yorimitsu, T.; Nair, U.; Yang, Z.; Klionsky, D.J. Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem. 2006, 281, 30299–30304.
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541.
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080.
- Zhang, Y.; Sowers, J.R.; Ren, J. Targeting autophagy in obesity: From pathophysiology to management. Nat. Rev. Endocrinol. 2018, 14, 356–376.
- Zhang, Y.; Whaley-Connell, A.T.; Sowers, J.R.; Ren, J. Autophagy as an emerging target in cardiorenal metabolic disease: From pathophysiology to management. Pharmacol. Ther. 2018, 191, 1–22.
- Ren, J.; Zhang, Y. Targeting Autophagy in Aging and Aging-Related Cardiovascular Diseases. Trends Pharmacol. Sci. 2018, 39, 1064–1076.
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Trends Pharmacol. Sci. 2018, 19, 365–381.
- Abdrakhmanov, A.; Gogvadze, V.; Zhivotovsky, B. To Eat or to Die: Deciphering Selective Forms of Autophagy. Trends Biochem. Sci. 2020, 45, 347–364.
- Li, W.W.; Li, J.; Bao, J.K. Microautophagy: Lesser-known self-eating. Cell. Mol. Life Sci. 2012, 69, 1125–1136.
- Orenstein, S.J.; Cuervo, A.M. Chaperone-mediated autophagy: Molecular mechanisms and physiological relevance. Semin. Cell Dev. Biol. 2010, 21, 719–726.
- Lavandero, S.; Chiong, M.; Rothermel, B.A.; Hill, J.A. Autophagy in cardiovascular biology. J. Clin. Investig. 2015, 125, 55–64.
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873.
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41.
- Farre, J.C.; Subramani, S. Mechanistic insights into selective autophagy pathways: Lessons from yeast. Trends Pharmacol. Sci. 2016, 17, 537–552.
- Klionsky, D.J.; Baehrecke, E.H.; Brumell, J.H.; Chu, C.T.; Codogno, P.; Cuervo, A.M.; Debnath, J.; Deretic, V.; Elazar, Z.; Eskelinen, E.L.; et al. A comprehensive glossary of autophagy-related molecules and processes (2nd edition). Autophagy 2011, 7, 1273–1294.
- Fujioka, Y.; Suzuki, S.W.; Yamamoto, H.; Kondo-Kakuta, C.; Kimura, Y.; Hirano, H.; Akada, R.; Inagaki, F.; Ohsumi, Y.; Noda, N.N. Structural basis of starvation-induced assembly of the autophagy initiation complex. Nat. Struct. Mol. Biol. 2014, 21, 513–521.
- Chang, C.; Jensen, L.E.; Hurley, J.H. Autophagosome biogenesis comes out of the black box. Nat. Cell Biol. 2021, 23, 450–456.
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141.
- Laplante, M.; Sabatini, D.M. mTOR Signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a011593.
- Lamb, C.A.; Yoshimori, T.; Tooze, S.A. The autophagosome: Origins unknown, biogenesis complex. Trends Pharmacol. Sci. 2013, 14, 759–774.
- Hurley, J.H.; Young, L.N. Mechanisms of Autophagy Initiation. Annu. Rev. Biochem. 2017, 86, 225–244.
- Wang, R.C.; Wei, Y.; An, Z.; Zou, Z.; Xiao, G.; Bhagat, G.; White, M.; Reichelt, J.; Levine, B. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 2012, 338, 956–959.
- Maiuri, M.C.; Criollo, A.; Tasdemir, E.; Vicencio, J.M.; Tajeddine, N.; Hickman, J.A.; Geneste, O.; Kroemer, G. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L). Autophagy 2007, 3, 374–376.
- Wei, Y.; Pattingre, S.; Sinha, S.; Bassik, M.; Levine, B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 2008, 30, 678–688.
- He, C.; Bassik, M.C.; Moresi, V.; Sun, K.; Wei, Y.; Zou, Z.; An, Z.; Loh, J.; Fisher, J.; Sun, Q.; et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 2012, 481, 511–515.
- Itakura, E.; Kishi-Itakura, C.; Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 2012, 151, 1256–1269.
- Tanaka, Y.; Guhde, G.; Suter, A.; Eskelinen, E.L.; Hartmann, D.; Lullmann-Rauch, R.; Janssen, P.M.; Blanz, J.; von Figura, K.; Saftig, P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 2000, 406, 902–906.
- Eskelinen, E.L.; Illert, A.L.; Tanaka, Y.; Schwarzmann, G.; Blanz, J.; Von Figura, K.; Saftig, P. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol. Biol. Cell 2002, 13, 3355–3368.
- Eskelinen, E.L.; Schmidt, C.K.; Neu, S.; Willenborg, M.; Fuertes, G.; Salvador, N.; Tanaka, Y.; Lullmann-Rauch, R.; Hartmann, D.; Heeren, J.; et al. Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts. Mol. Biol. Cell 2004, 15, 3132–3145.
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022.
- Gustafsson, A.B.; Dorn, G.W., 2nd. Evolving and Expanding the Roles of Mitophagy as a Homeostatic and Pathogenic Process. Physiol. Rev. 2019, 99, 853–892.
- Hanna, R.A.; Quinsay, M.N.; Orogo, A.M.; Giang, K.; Rikka, S.; Gustafsson, A.B. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J. Biol. Chem. 2012, 287, 19094–19104.
- Diwan, A.; Koesters, A.G.; Odley, A.M.; Pushkaran, S.; Baines, C.P.; Spike, B.T.; Daria, D.; Jegga, A.G.; Geiger, H.; Aronow, B.J.; et al. Unrestrained erythroblast development in Nix-/- mice reveals a mechanism for apoptotic modulation of erythropoiesis. Proc. Natl. Acad. Sci. USA 2007, 104, 6794–6799.
- Sandoval, H.; Thiagarajan, P.; Dasgupta, S.K.; Schumacher, A.; Prchal, J.T.; Chen, M.; Wang, J. Essential role for Nix in autophagic maturation of erythroid cells. Nature 2008, 454, 232–235.
- Schweers, R.L.; Zhang, J.; Randall, M.S.; Loyd, M.R.; Li, W.; Dorsey, F.C.; Kundu, M.; Opferman, J.T.; Cleveland, J.L.; Miller, J.L.; et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19500–19505.
- Liu, L.; Feng, D.; Chen, G.; Chen, M.; Zheng, Q.; Song, P.; Ma, Q.; Zhu, C.; Wang, R.; Qi, W.; et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 2012, 14, 177–185.
- Murakawa, T.; Yamaguchi, O.; Hashimoto, A.; Hikoso, S.; Takeda, T.; Oka, T.; Yasui, H.; Ueda, H.; Akazawa, Y.; Nakayama, H.; et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat. Commun. 2015, 6, 7527.
- Bhujabal, Z.; Birgisdottir, A.B.; Sjottem, E.; Brenne, H.B.; Overvatn, A.; Habisov, S.; Kirkin, V.; Lamark, T.; Johansen, T. FKBP8 recruits LC3A to mediate Parkin-independent mitophagy. EMBO Rep. 2017, 18, 947–961.
- Torisu, T.; Torisu, K.; Lee, I.H.; Liu, J.; Malide, D.; Combs, C.A.; Wu, X.S.; Rovira, I.I.; Fergusson, M.M.; Weigert, R.; et al. Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nat. Med. 2013, 19, 1281–1287.
- Chen, M.L.; Yi, L.; Jin, X.; Liang, X.Y.; Zhou, Y.; Zhang, T.; Xie, Q.; Zhou, X.; Chang, H.; Fu, Y.J.; et al. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy 2013, 9, 2033–2045.
- Shenouda, S.M.; Widlansky, M.E.; Chen, K.; Xu, G.; Holbrook, M.; Tabit, C.E.; Hamburg, N.M.; Frame, A.A.; Caiano, T.L.; Kluge, M.A.; et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011, 124, 444–453.
- Bharath, L.P.; Mueller, R.; Li, Y.; Ruan, T.; Kunz, D.; Goodrich, R.; Mills, T.; Deeter, L.; Sargsyan, A.; Anandh Babu, P.V.; et al. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can. J. Physiol. Pharmacol. 2014, 92, 605–612.
- Abada, A.; Elazar, Z. Getting ready for building: Signaling and autophagosome biogenesis. EMBO Rep. 2014, 15, 839–852.
- Guo, F.; Li, X.; Peng, J.; Tang, Y.; Yang, Q.; Liu, L.; Wang, Z.; Jiang, Z.; Xiao, M.; Ni, C.; et al. Autophagy regulates vascular endothelial cell eNOS and ET-1 expression induced by laminar shear stress in an ex vivo perfused system. Ann. Biomed. Eng. 2014, 42, 1978–1988.
- Hayashi, S.; Sato, N.; Yamamoto, A.; Ikegame, Y.; Nakashima, S.; Ogihara, T.; Morishita, R. Alzheimer disease-associated peptide, amyloid beta40, inhibits vascular regeneration with induction of endothelial autophagy. Arter. Thromb. Vasc. Biol. 2009, 29, 1909–1915.
- Shiroto, T.; Romero, N.; Sugiyama, T.; Sartoretto, J.L.; Kalwa, H.; Yan, Z.; Shimokawa, H.; Michel, T. Caveolin-1 is a critical determinant of autophagy, metabolic switching, and oxidative stress in vascular endothelium. PLoS ONE 2014, 9, e87871.
- Chen, F.; Chen, B.; Xiao, F.Q.; Wu, Y.T.; Wang, R.H.; Sun, Z.W.; Fu, G.S.; Mou, Y.; Tao, W.; Hu, X.S.; et al. Autophagy protects against senescence and apoptosis via the RAS-mitochondria in high-glucose-induced endothelial cells. Cell. Physiol. Biochem. 2014, 33, 1058–1074.
- Menghini, R.; Casagrande, V.; Marino, A.; Marchetti, V.; Cardellini, M.; Stoehr, R.; Rizza, S.; Martelli, E.; Greco, S.; Mauriello, A.; et al. MiR-216a: A link between endothelial dysfunction and autophagy. Cell Death Dis. 2014, 5, e1029.
- Lugus, J.J.; Ngoh, G.A.; Bachschmid, M.M.; Walsh, K. Mitofusins are required for angiogenic function and modulate different signaling pathways in cultured endothelial cells. J. Mol. Cell. Cardiol. 2011, 51, 885–893.
- Uberti, F.; Lattuada, D.; Morsanuto, V.; Nava, U.; Bolis, G.; Vacca, G.; Squarzanti, D.F.; Cisari, C.; Molinari, C. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J. Clin. Endocrinol. Metab. 2014, 99, 1367–1374.
- Peng, N.; Meng, N.; Wang, S.; Zhao, F.; Zhao, J.; Su, L.; Zhang, S.; Zhang, Y.; Zhao, B.; Miao, J. An activator of mTOR inhibits oxLDL-induced autophagy and apoptosis in vascular endothelial cells and restricts atherosclerosis in apolipoprotein E−/− mice. Sci. Rep. 2014, 4, 5519.
- Lu, Q.; Yao, Y.; Hu, Z.; Hu, C.; Song, Q.; Ye, J.; Xu, C.; Wang, A.Z.; Chen, Q.; Wang, Q.K. Angiogenic Factor AGGF1 Activates Autophagy with an Essential Role in Therapeutic Angiogenesis for Heart Disease. PLoS Biol. 2016, 14, e1002529.
- Domigan, C.K.; Warren, C.M.; Antanesian, V.; Happel, K.; Ziyad, S.; Lee, S.; Krall, A.; Duan, L.; Torres-Collado, A.X.; Castellani, L.W.; et al. Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy. J. Cell Sci. 2015, 128, 2236–2248.
- Chau, Y.P.; Lin, S.Y.; Chen, J.H.; Tai, M.H. Endostatin induces autophagic cell death in EAhy926 human endothelial cells. Histol. Histopathol. 2003, 18, 715–726.
- Vion, A.C.; Kheloufi, M.; Hammoutene, A.; Poisson, J.; Lasselin, J.; Devue, C.; Pic, I.; Dupont, N.; Busse, J.; Stark, K.; et al. Autophagy is required for endothelial cell alignment and atheroprotection under physiological blood flow. Proc. Natl. Acad. Sci. USA 2017, 114, E8675–E8684.
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371.
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2625–2641.
- Taqueti, V.R.; Hachamovitch, R.; Murthy, V.L.; Naya, M.; Foster, C.R.; Hainer, J.; Dorbala, S.; Blankstein, R.; Di Carli, M.F. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015, 131, 19–27.
- Serruys, P.W.; di Mario, C.; Piek, J.; Schroeder, E.; Vrints, C.; Probst, P.; de Bruyne, B.; Hanet, C.; Fleck, E.; Haude, M.; et al. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short- and long-term outcomes of coronary balloon angioplasty: The DEBATE Study (Doppler Endpoints Balloon Angioplasty Trial Europe). Circulation 1997, 96, 3369–3377.
- Uren, N.G.; Crake, T.; Lefroy, D.C.; de Silva, R.; Davies, G.J.; Maseri, A. Delayed recovery of coronary resistive vessel function after coronary angioplasty. J. Am. Coll. Cardiol. 1993, 21, 612–621.
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart, A.; et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816.
- Rezkalla, S.H.; Kloner, R.A. Coronary no-reflow phenomenon: From the experimental laboratory to the cardiac catheterization laboratory. Catheter. Cardiovasc. Interv. 2008, 72, 950–957.
- Niccoli, G.; Montone, R.A.; Ibanez, B.; Thiele, H.; Crea, F.; Heusch, G.; Bulluck, H.; Hausenloy, D.J.; Berry, C.; Stiermaier, T.; et al. Optimized Treatment of ST-Elevation Myocardial Infarction. Circ. Res. 2019, 125, 245–258.
- Wang, J.; Toan, S.; Zhou, H. New insights into the role of mitochondria in cardiac microvascular ischemia/reperfusion injury. Angiogenesis 2020, 23, 299–314.
- Chang, X.; Lochner, A.; Wang, H.H.; Wang, S.; Zhu, H.; Ren, J.; Zhou, H. Coronary microvascular injury in myocardial infarction: Perception and knowledge for mitochondrial quality control. Theranostics 2021, 11, 6766–6785.
- Wang, Y.; Song, X.; Li, Z.; Liu, N.; Yan, Y.; Li, T.; Sun, W.; Guan, Y.; Li, M.; Yang, Y.; et al. MicroRNA-103 Protects Coronary Artery Endothelial Cells against H2O2-Induced Oxidative Stress via BNIP3-Mediated End-Stage Autophagy and Antipyroptosis Pathways. Oxidative Med. Cell. Longev. 2020, 2020, 8351342.
- Shafique, E.; Choy, W.C.; Liu, Y.; Feng, J.; Cordeiro, B.; Lyra, A.; Arafah, M.; Yassin-Kassab, A.; Zanetti, A.V.; Clements, R.T.; et al. Oxidative stress improves coronary endothelial function through activation of the pro-survival kinase AMPK. Aging 2013, 5, 515–530.
- Lv, X.; Wang, K.; Tang, W.; Yu, L.; Cao, H.; Chi, W.; Wang, B. miR-34a-5p was involved in chronic intermittent hypoxia-induced autophagy of human coronary artery endothelial cells via Bcl-2/beclin 1 signal transduction pathway. J. Cell. Biochem. 2019, 120, 18871–18882.
- Wang, R.; Yang, Q.; Wang, X.; Wang, W.; Li, J.; Zhu, J.; Liu, X.; Liu, J.; Du, J. FoxO3alpha-mediated autophagy contributes to apoptosis in cardiac microvascular endothelial cells under hypoxia. Microvasc. Res. 2016, 104, 23–31.
- Sun, L.; Hao, Y.; An, R.; Li, H.; Xi, C.; Shen, G. Overexpression of Rcan1-1L inhibits hypoxia-induced cell apoptosis through induction of mitophagy. Mol. Cells 2014, 37, 785–794.
- Chen, J.; Wang, L.; Liu, W.H.; Shi, J.; Zhong, Y.; Liu, S.J.; Liu, S.M. Aspirin protects human coronary artery endothelial cells by inducing autophagy. Physiol. Int. 2020, 107, 294–305.
- Cui, H.; Li, X.; Li, N.; Qi, K.; Li, Q.; Jin, C.; Zhang, Q.; Jiang, L.; Yang, Y. Induction of autophagy by Tongxinluo through the MEK/ERK pathway protects human cardiac microvascular endothelial cells from hypoxia/reoxygenation injury. J. Cardiovasc. Pharmacol. 2014, 64, 180–190.
- Zhang, Y.J.; Zhang, M.; Zhao, X.; Shi, K.; Ye, M.; Tian, J.; Guan, S.; Ying, W.; Qu, X. NAD(+) administration decreases microvascular damage following cardiac ischemia/reperfusion by restoring autophagic flux. Basic Res. Cardiol. 2020, 115, 57.
- Wu, D.; Ji, H.; Du, W.; Ren, L.; Qian, G. Mitophagy alleviates ischemia/reperfusion-induced microvascular damage through improving mitochondrial quality control. Bioengineered 2022, 13, 3596–3607.
- Zhou, H.; Wang, J.; Zhu, P.; Zhu, H.; Toan, S.; Hu, S.; Ren, J.; Chen, Y. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res. Cardiol. 2018, 113, 23.
- Zhou, H.; Zhu, P.; Guo, J.; Hu, N.; Wang, S.; Li, D.; Hu, S.; Ren, J.; Cao, F.; Chen, Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017, 13, 498–507.
- Sun, W.; Dong, S.; Lu, H.; Wang, N.; Zhao, Y.; An, J.; Sun, L.; Lu, D. Beclin-1 overexpression regulates NLRP3 activation by promoting TNFAIP3 in microvascular injury following myocardial reperfusion. Cell. Signal. 2021, 84, 110008.
- Sun, W.; Lu, H.; Dong, S.; Li, R.; Chu, Y.; Wang, N.; Zhao, Y.; Zhang, Y.; Wang, L.; Sun, L.; et al. Beclin1 controls caspase-4 inflammsome activation and pyroptosis in mouse myocardial reperfusion-induced microvascular injury. Cell Commun. Signal. 2021, 19, 107.
- Tan, Y.; Mui, D.; Toan, S.; Zhu, P.; Li, R.; Zhou, H. SERCA Overexpression Improves Mitochondrial Quality Control and Attenuates Cardiac Microvascular Ischemia-Reperfusion Injury. Mol. Ther. Nucleic Acids 2020, 22, 696–707.
- Rogg, E.M.; Abplanalp, W.T.; Bischof, C.; John, D.; Schulz, M.H.; Krishnan, J.; Fischer, A.; Poluzzi, C.; Schaefer, L.; Bonauer, A.; et al. Analysis of Cell Type-Specific Effects of MicroRNA-92a Provides Novel Insights Into Target Regulation and Mechanism of Action. Circulation 2018, 138, 2545–2558.
- Tang, Q.; Cao, Y.; Xiong, W.; Ke, X.; Zhang, J.; Xia, Y.; Liu, D. Glycyrrhizic acid exerts protective effects against hypoxia/reoxygenation-induced human coronary artery endothelial cell damage by regulating mitochondria. Exp. Ther. Med. 2020, 20, 335–342.
- Chen, W.R.; Liu, H.B.; Chen, Y.D.; Sha, Y.; Ma, Q.; Zhu, P.J.; Mu, Y. Melatonin Attenuates Myocardial Ischemia/Reperfusion Injury by Inhibiting Autophagy Via an AMPK/mTOR Signaling Pathway. Cell. Physiol. Biochem. 2018, 47, 2067–2076.
- Zhou, H.; Zhang, Y.; Hu, S.; Shi, C.; Zhu, P.; Ma, Q.; Jin, Q.; Cao, F.; Tian, F.; Chen, Y. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal Res. 2017, 63, e12413.
- Kundumani-Sridharan, V.; Subramani, J.; Owens, C.; Das, K.C. Nrg1beta Released in Remote Ischemic Preconditioning Improves Myocardial Perfusion and Decreases Ischemia/Reperfusion Injury via ErbB2-Mediated Rescue of Endothelial Nitric Oxide Synthase and Abrogation of Trx2 Autophagy. Arter. Thromb. Vasc. Biol. 2021, 41, 2293–2314.
- Redfield, M.M. Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2017, 376, 897.
- Sinha, A.; Rahman, H.; Webb, A.; Shah, A.M.; Perera, D. Untangling the pathophysiologic link between coronary microvascular dysfunction and heart failure with preserved ejection fraction. Eur. Heart J. 2021, 42, 4431–4441.
- Kirkman, D.L.; Bohmke, N.; Billingsley, H.E.; Carbone, S. Sarcopenic Obesity in Heart Failure With Preserved Ejection Fraction. Front. Endocrinol. 2020, 11, 558271.
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559.
- Yang, J.H.; Obokata, M.; Reddy, Y.N.V.; Redfield, M.M.; Lerman, A.; Borlaug, B.A. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 432–441.
- Ahmad, A.; Corban, M.T.; Toya, T.; Verbrugge, F.H.; Sara, J.D.; Lerman, L.O.; Borlaug, B.A.; Lerman, A. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 765–772.
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Ljung Faxen, U.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450.
- Carbone, S.; Lavie, C.J.; Elagizi, A.; Arena, R.; Ventura, H.O. The Impact of Obesity in Heart Failure. Heart Fail. Clin. 2020, 16, 71–80.
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271.
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016, 134, 73–90.
- Hage, C.; Lofgren, L.; Michopoulos, F.; Nilsson, R.; Davidsson, P.; Kumar, C.; Ekstrom, M.; Eriksson, M.J.; Lynga, P.; Persson, B.; et al. Metabolomic Profile in HFpEF vs. HFrEF Patients. J. Card. Fail. 2020, 26, 1050–1059.
- Kassab, G.S.; Lin, D.H.; Fung, Y.C. Morphometry of pig coronary venous system. Am. J. Physiol. Heart Circ. Physiol. 1994, 267, H2100–H2113.
- Avogaro, A.; Fadini, G.P. Microvascular complications in diabetes: A growing concern for cardiologists. Int. J. Cardiol. 2019, 291, 29–35.
- Cosson, E.; Pham, I.; Valensi, P.; Paries, J.; Attali, J.R.; Nitenberg, A. Impaired coronary endothelium-dependent vasodilation is associated with microalbuminuria in patients with type 2 diabetes and angiographically normal coronary arteries. Diabetes Care 2006, 29, 107–112.
- Yokoyama, I.; Momomura, S.; Ohtake, T.; Yonekura, K.; Nishikawa, J.; Sasaki, Y.; Omata, M. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 1997, 30, 1472–1477.
- Sara, J.D.; Taher, R.; Kolluri, N.; Vella, A.; Lerman, L.O.; Lerman, A. Coronary microvascular dysfunction is associated with poor glycemic control amongst female diabetics with chest pain and non-obstructive coronary artery disease. Cardiovasc. Diabetol. 2019, 18, 22.
- Reyes-Soffer, G.; Holleran, S.; Di Tullio, M.R.; Homma, S.; Boden-Albala, B.; Ramakrishnan, R.; Elkind, M.S.; Sacco, R.L.; Ginsberg, H.N. Endothelial function in individuals with coronary artery disease with and without type 2 diabetes mellitus. Metabolism 2010, 59, 1365–1371.
- Kibel, A.; Selthofer-Relatic, K.; Drenjancevic, I.; Bacun, T.; Bosnjak, I.; Kibel, D.; Gros, M. Coronary microvascular dysfunction in diabetes mellitus. J. Int. Med. Res. 2017, 45, 1901–1929.
- Beckman, J.A.; Paneni, F.; Cosentino, F.; Creager, M.A. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II. Eur. Heart J. 2013, 34, 2444–2452.
- Sorop, O.; van den Heuvel, M.; van Ditzhuijzen, N.S.; de Beer, V.J.; Heinonen, I.; van Duin, R.W.; Zhou, Z.; Koopmans, S.J.; Merkus, D.; van der Giessen, W.J.; et al. Coronary microvascular dysfunction after long-term diabetes and hypercholesterolemia. Am. J. Physiol. Circ. Physiol. 2016, 311, H1339–H1351.
- Hinkel, R.; Howe, A.; Renner, S.; Ng, J.; Lee, S.; Klett, K.; Kaczmarek, V.; Moretti, A.; Laugwitz, K.L.; Skroblin, P.; et al. Diabetes Mellitus-Induced Microvascular Destabilization in the Myocardium. J. Am. Coll. Cardiol. 2017, 69, 131–143.
- Toblli, J.E.; Cao, G.; DeRosa, G.; Di Gennaro, F.; Forcada, P. Angiotensin-converting enzyme inhibition and angiogenesis in myocardium of obese Zucker rats. Am. J. Hypertens. 2004, 17, 172–180.
- Houstis, N.E.; Eisman, A.S.; Pappagianopoulos, P.P.; Wooster, L.; Bailey, C.S.; Wagner, P.D.; Lewis, G.D. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Diagnosing and Ranking Its Causes Using Personalized O2 Pathway Analysis. Circulation 2018, 137, 148–161.
- Wildenthal, K.; Dees, J.H.; Buja, L.M. Cardiac lysosomal derangements in mouse heart after long-term exposure to nonmetabolizable sugars. Circ. Res. 1977, 40, 26–35.
- Maejima, Y.; Kyoi, S.; Zhai, P.; Liu, T.; Li, H.; Ivessa, A.; Sciarretta, S.; Del Re, D.P.; Zablocki, D.K.; Hsu, C.P.; et al. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat. Med. 2013, 19, 1478–1488.
- Maejima, Y.; Zablocki, D.; Nah, J.; Sadoshima, J. The role of the Hippo pathway in autophagy in the heart. Cardiovasc Res. 2022, cvac014.
- Hu, J.; Wang, S.; Xiong, Z.; Cheng, Z.; Yang, Z.; Lin, J.; Wang, T.; Feng, X.; Gao, E.; Wang, H.; et al. Exosomal Mst1 transfer from cardiac microvascular endothelial cells to cardiomyocytes deteriorates diabetic cardiomyopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3639–3649.
- Lin, J.; Zhang, L.; Zhang, M.; Hu, J.; Wang, T.; Duan, Y.; Man, W.; Wu, B.; Feng, J.; Sun, L.; et al. Mst1 inhibits CMECs autophagy and participates in the development of diabetic coronary microvascular dysfunction. Sci. Rep. 2016, 6, 34199.
- Zhang, Z.; Zhang, S.; Wang, Y.; Yang, M.; Zhang, N.; Jin, Z.; Ding, L.; Jiang, W.; Yang, J.; Sun, Z.; et al. Autophagy inhibits high glucose induced cardiac microvascular endothelial cells apoptosis by mTOR signal pathway. Apoptosis 2017, 22, 1510–1523.
- Wu, W.; Xu, H.; Wang, Z.; Mao, Y.; Yuan, L.; Luo, W.; Cui, Z.; Cui, T.; Wang, X.L.; Shen, Y.H. PINK1-Parkin-Mediated Mitophagy Protects Mitochondrial Integrity and Prevents Metabolic Stress-Induced Endothelial Injury. PLoS ONE 2015, 10, e0132499.
- Liu, N.; Wu, J.; Zhang, L.; Gao, Z.; Sun, Y.; Yu, M.; Zhao, Y.; Dong, S.; Lu, F.; Zhang, W. Hydrogen Sulphide modulating mitochondrial morphology to promote mitophagy in endothelial cells under high-glucose and high-palmitate. J. Cell. Mol. Med. 2017, 21, 3190–3203.
- Wang, X.; Zhang, J.Q.; Xiu, C.K.; Yang, J.; Fang, J.Y.; Lei, Y. Ginseng-Sanqi-Chuanxiong (GSC) Extracts Ameliorate Diabetes-Induced Endothelial Cell Senescence through Regulating Mitophagy via the AMPK Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 7151946.
- Li, C.; Tan, Y.; Wu, J.; Ma, Q.; Bai, S.; Xia, Z.; Wan, X.; Liang, J. Resveratrol Improves Bnip3-Related Mitophagy and Attenuates High-Fat-Induced Endothelial Dysfunction. Front. Cell Dev. Biol. 2020, 8, 796.
- Niu, C.; Chen, Z.; Kim, K.T.; Sun, J.; Xue, M.; Chen, G.; Li, S.; Shen, Y.; Zhu, Z.; Wang, X.; et al. Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy 2019, 15, 843–870.
- Kitano, N.; Suzuki, H.; Takeuchi, T. Patient Age and the Seasonal Pattern of Onset of Kawasaki’s Disease. N. Engl. J. Med. 2018, 378, 2048–2049.
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999.
- Qin, J.; Zheng, Y.; Ding, Y.; Huang, C.; Hou, M.; Li, M.; Qian, G.; Lv, H. Co-culture of peripheral blood mononuclear cell (PBMC) and human coronary artery endothelial cell (HCAEC) reveals the important role of autophagy implicated in Kawasaki disease. Transl. Pediatr. 2021, 10, 3140–3150.
- Qi, S.H.; Xiao, F.; Wei, B.; Qin, C. Value of ginsenoside Rb1 in alleviating coronary artery lesion in a mouse model of Kawasaki disease. Zhongguo Dang Dai Er Ke Za Zhi 2020, 22, 1034–1040.
- Huang, F.C.; Kuo, H.C.; Huang, Y.H.; Yu, H.R.; Li, S.C.; Kuo, H.C. Anti-inflammatory effect of resveratrol in human coronary arterial endothelial cells via induction of autophagy: Implication for the treatment of Kawasaki disease. BMC Pharmacol. Toxicol. 2017, 18, 3.
- Xie, F.; Xu, S.; Lu, Y.; Wong, K.F.; Sun, L.; Hasan, K.M.M.; Ma, A.C.H.; Tse, G.; Manno, S.H.C.; Tian, L.; et al. Metformin accelerates zebrafish heart regeneration by inducing autophagy. NPJ Regen. Med. 2021, 6, 62.
- LaRocca, T.J.; Henson, G.D.; Thorburn, A.; Sindler, A.L.; Pierce, G.L.; Seals, D.R. Translational evidence that impaired autophagy contributes to arterial ageing. J. Physiol. 2012, 590, 3305–3316.
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study, G. International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20.
- Pepine, C.J.; Anderson, R.D.; Sharaf, B.L.; Reis, S.E.; Smith, K.M.; Handberg, E.M.; Johnson, B.D.; Sopko, G.; Bairey Merz, C.N. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J. Am. Coll. Cardiol. 2010, 55, 2825–2832.
- Murthy, V.L.; Naya, M.; Taqueti, V.R.; Foster, C.R.; Gaber, M.; Hainer, J.; Dorbala, S.; Blankstein, R.; Rimoldi, O.; Camici, P.G.; et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014, 129, 2518–2527.
- Johnson, B.D.; Shaw, L.J.; Pepine, C.J.; Reis, S.E.; Kelsey, S.F.; Sopko, G.; Rogers, W.J.; Mankad, S.; Sharaf, B.L.; Bittner, V.; et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: Results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur. Heart J. 2006, 27, 1408–1415.
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low diagnostic yield of elective coronary angiography. N. Engl. J. Med. 2010, 362, 886–895.
- Kothawade, K.; Bairey Merz, C.N. Microvascular coronary dysfunction in women: Pathophysiology, diagnosis, and management. Curr. Probl. Cardiol. 2011, 36, 291–318.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
861
Revisions:
2 times
(View History)
Update Date:
07 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




