
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Takeshi Hatta | -- | 1892 | 2022-06-30 12:04:43 | | | |
| 2 | Catherine Yang | Meta information modification | 1892 | 2022-07-04 10:25:38 | | |
Video Upload Options
Blood-feeding arthropods, particularly ticks and mosquitoes are considered the most important vectors of arthropod-borne diseases affecting humans and animals. Vector competence (also termed vector potential) refers to the ability of arthropods to transmit pathogens, which is greatly influenced by the genetic and/or other intrinsic factors of arthropod vectors. Additionally, it is also governed by the factors exerted by hosts themselves during pathogen inoculation, development, and propagation in particular hosts. During an infection, ROS have pivotal roles in the triangular relationship among vectors, pathogens, and hosts and may influence the triad either positively or negatively. A pluripotent molecule isolated from the salivary glands of H. longicornis, called longistatin, plays a central role in the feeding and development of ticks and has been elegantly shown to ameliorate cellular ROS production in human endothelial cells, making it a key molecule in the survival of hard ticks. On the other hand, the acquisition of pathogens into a vector also induces modification of the normal ROS production resulting in oxidative stress to arthropod cells, which ultimately is being utilized by hematophagous arthropods to eliminate invading pathogens.
1. ROS and Arthropod’s Innate Immunity
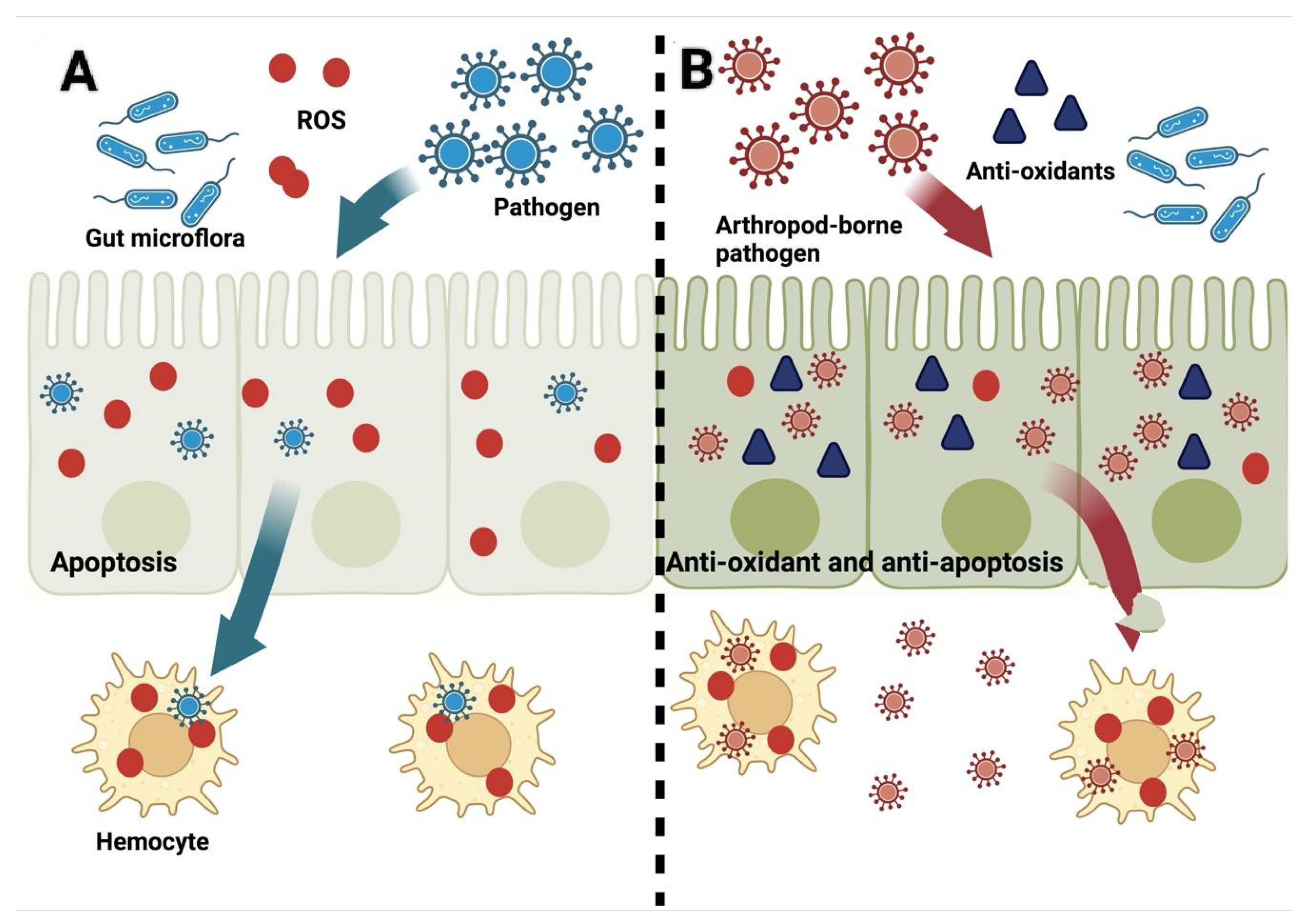
2. ROS after the Establishment of Infection in Hosts
3. ROS and Arthropod Microbiome
References
- Budachetri, K.; Crispell, G.; Karim, S. Amblyomma maculatum SECIS binding protein 2 and putative selenoprotein P are indispensable for pathogen replication and tick fecundity. Insect Biochem. Mol. Biol. 2017, 88, 37–47.
- Ha, E.M.; Oh, C.T.; Ryu, J.H.; Bae, Y.S.; Kang, S.W.; Jang, I.H.; Brey, P.T.; Lee, W.J. An antioxidant system required for host protection against gut infection in Drosophila. Dev. Cell 2005, 8, 125–132.
- Muralidharan, S.; Mandrekar, P. Cellular stress response and innate immune signaling: Integrating pathways in host defense and inflammation. J. Leukoc. Biol. 2013, 94, 1167–1184.
- Molina-Cruz, A.; DeJong, R.J.; Charles, B.; Gupta, L.; Kumar, S.; Jaramillo-Gutierrez, G.; Barillas-Mury, C. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J. Biol. Chem. 2008, 283, 3217–3223.
- Graça-Souza, A.V.; Maya-Monteiro, C.; Paiva-Silva, G.O.; Braz, G.R.C.; Paes, M.C.; Sorgine, M.H.F.; Oliveira, M.F.; Oliveira, P.L. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem. Mol. Biol. 2006, 36, 322–335.
- Kumar, S.; Christophides, G.K.; Cantera, R.; Charles, B.; Han, Y.S.; Meister, S.; Dimopoulos, G.; Kafatos, F.C.; Barillas-Mury, C. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2003, 100, 14139–14144.
- Kalil, S.P.; da Rosa, R.D.; Capelli-Peixoto, J.; Pohl, P.C.; de Oliveira, P.L.; Fogaça, A.C.; Daffre, S. Immune-related redox metabolism of embryonic cells of the tick Rhipicephalus microplus (BME26) in response to infection with Anaplasma marginale. Parasit. Vectors 2017, 10, 613.
- Pereira, L.S.; Oliveira, P.L.; Barja-Fidalgo, C.; Daffre, S. Production of reactive oxygen species by hemocytes from the cattle tick Boophilus microplus. Exp. Parasitol. 2001, 99, 66–72.
- Surachetpong, W.; Pakpour, N.; Cheung, K.W.; Luckhart, S. Reactive oxygen species-dependent cell signaling regulates the mosquito immune response to Plasmodium falciparum. Antioxid. Redox Signal. 2011, 14, 943–955.
- Corby-Harris, V.; Drexler, A.; Watkins de Jong, L.; Antonova, Y.; Pakpour, N.; Ziegler, R.; Ramberg, F.; Lewis, E.E.; Brown, J.M.; Luckhart, S.; et al. Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 2010, 6, e1001003.
- Ashida, H.; Mimuro, H.; Ogawa, M.; Kobayashi, T.; Sanada, T.; Kim, M.; Sasakawa, C. Cell death and infection: A double-edged sword for host and pathogen survival. J. Cell Biol. 2011, 195, 931–942.
- Villar, M.; Ayllón, N.; Alberdi, P.; Moreno, A.; Moreno, M.; Tobes, R.; Mateos-Hernández, L.; Weisheit, S.; Bell-Sakyi, L.; de la Fuente, J. Integrated Metabolomics, Transcriptomics and Proteomics Identifies Metabolic Pathways Affected by Anaplasma phagocytophilum Infection in Tick Cells. Mol. Cell Proteom. 2015, 14, 3154–3172.
- de la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front. Cell Infect. Microbiol. 2017, 7, 114.
- Hajdušek, O.; Síma, R.; Ayllón, N.; Jalovecká, M.; Perner, J.; de la Fuente, J.; Kopáček, P. Interaction of the tick immune system with transmitted pathogens. Front. Cell Infect. Microbiol. 2013, 3, 26.
- Villar, M.; Popara, M.; Bonzón-Kulichenko, E.; Ayllón, N.; Vázquez, J.; de la Fuente, J. Characterization of the tick-pathogen interface by quantitative proteomics. Ticks Tick Borne Dis. 2012, 3, 154–158.
- Chen, T.H.; Wu, Y.J.; Hou, J.N.; Chiang, Y.H.; Cheng, C.C.; Sifiyatun, E.; Chiu, C.H.; Wang, L.C.; Chen, W.J. A novel p53 paralogue mediates antioxidant defense of mosquito cells to survive dengue virus replication. Virology 2018, 519, 156–169.
- Chen, T.H.; Lo, Y.P.; Yang, C.F.; Chen, W.J. Additive protection by antioxidant and apoptosis-inhibiting effects on mosquito cells with dengue 2 virus infection. PLoS Negl. Trop. Dis. 2012, 6, e1613.
- Chen, T.H.; Chiang, Y.H.; Hou, J.N.; Cheng, C.C.; Sofiyatun, E.; Chiu, C.H.; Chen, W.J. XBP1-Mediated BiP/GRP78 Upregulation Copes with Oxidative Stress in Mosquito Cells during Dengue 2 Virus Infection. Biomed Res. Int. 2017, 2017, 3519158.
- Oliveira, J.H.M.; Talyuli, O.A.C.; Goncalves, R.L.S.; Paiva-Silva, G.O.; Sorgine, M.H.F.; Alvarenga, P.H.; Oliveira, P.L. Catalase protects Aedes aegypti from oxidative stress and increases midgut infection prevalence of dengue but not Zika. PLoS Negl. Trop. Dis. 2017, 11, e0005525.
- Chen, T.H.; Tang, P.; Yang, C.F.; Kao, L.H.; Lo, Y.P.; Chuang, C.K.; Shih, Y.T.; Chen, W.J. Antioxidant defense is one of the mechanisms by which mosquito cells survive dengue 2 viral infection. Virology 2011, 410, 410–417.
- Basu, M.; Courtney, S.C.; Brinton, M.A. Arsenite-induced stress granule formation is inhibited by elevated levels of reduced glutathione in West Nile virus-infected cells. PLoS Pathog. 2017, 13, e1006240.
- Kuzmenko, Y.V.; Smirnova, O.A.; Ivanov, A.V.; Starodubova, E.S.; Karpov, V.L. Nonstructural Protein 1 of Tick-Borne Encephalitis Virus Induces Oxidative Stress and Activates Antioxidant Defense by the Nrf2/ARE Pathway. Intervirology 2016, 59, 111–117.
- Hernandez, E.P.; Talactac, M.R.; Vitor, R.J.S.; Yoshii, K.; Tanaka, T. An Ixodes scapularis glutathione S-transferase plays a role in cell survival and viability during Langat virus infection of a tick cell line. Acta Trop. 2021, 214, 105763.
- Grabowski, J.M.; Perera, R.; Roumani, A.M.; Hedrick, V.E.; Inerowicz, H.D.; Hill, C.A.; Kuhn, R.J. Changes in the Proteome of Langat-Infected Ixodes scapularis ISE6 Cells: Metabolic Pathways Associated with Flavivirus Infection. PLoS Negl. Trop. Dis. 2016, 10, e0004180.
- Grabowski, J.M.; Gulia-Nuss, M.; Kuhn, R.J.; Hill, C.A. RNAi reveals proteins for metabolism and protein processing associated with Langat virus infection in Ixodes scapularis (black-legged tick) ISE6 cells. Parasit. Vectors 2017, 10, 24.
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074.
- Busby, A.T.; Ayllón, N.; Kocan, K.M.; Blouin, E.F.; de la Fuente, G.; Galindo, R.C.; Villar, M.; de la Fuente, J. Expression of heat shock proteins and subolesin affects stress responses, Anaplasma phagocytophilum infection and questing behaviour in the tick, Ixodes scapularis. Med. Vet. Entomol. 2012, 26, 92–102.
- Villar, M.; Ayllón, N.; Busby, A.T.; Galindo, R.C.; Blouin, E.F.; Kocan, K.M.; Bonzón-Kulichenko, E.; Zivkovic, Z.; Almazán, C.; Torina, A.; et al. Expression of Heat Shock and Other Stress Response Proteins in Ticks and Cultured Tick Cells in Response to Anaplasma spp. Infection and Heat Shock. Int. J. Proteom. 2010, 2010, 657261.
- Alberdi, P.; Cabezas-Cruz, A.; Prados, P.E.; Rayo, M.V.; Artigas-Jerónimo, S.; de la Fuente, J. The redox metabolic pathways function to limit Anaplasma phagocytophilum infection and multiplication while preserving fitness in tick vector cells. Sci. Rep. 2019, 9, 13236.
- Reeves, M.A.; Hoffmann, P.R. The human selenoproteome: Recent insights into functions and regulation. Cell Mol. Life Sci. 2009, 66, 2457–2478.
- Narasimhan, S.; Sukumaran, B.; Bozdogan, U.; Thomas, V.; Liang, X.; De Ponte, K.; Marcantonio, N.; Koski, R.A.; Anderson, J.F.; Kantor, F.; et al. A tick antioxidant facilitates the Lyme disease agent’s successful migration from the mammalian host to the arthropod vector. Cell Host Microbe 2007, 2, 7–18.
- Kocan, K.M.; Zivkovic, Z.; Blouin, E.F.; Naranjo, V.; Almazán, C.; Mitra, R.; de la Fuente, J. Silencing of genes involved in Anaplasma marginale-tick interactions affects the pathogen developmental cycle in Dermacentor variabilis. BMC Dev. Biol. 2009, 9, 42.
- Galay, R.L.; Umemiya-Shirafuji, R.; Mochizuki, M.; Fujisaki, K.; Tanaka, T. Iron metabolism in hard ticks (Acari: Ixodidae): The antidote to their toxic diet. Parasitol. Int. 2015, 64, 182–189.
- Kahlon, A.; Ojogun, N.; Ragland, S.A.; Seidman, D.; Troese, M.J.; Ottens, A.K.; Mastronunzio, J.E.; Truchan, H.K.; Walker, N.J.; Borjesson, D.L.; et al. Anaplasma phagocytophilum Asp14 is an invasin that interacts with mammalian host cells via its C terminus to facilitate infection. Infect. Immun. 2013, 81, 65–79.
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118.
- Kinross, J.M.; Darzi, A.W.; Nicholson, J.K. Gut microbiome-host interactions in health and disease. Genome Med. 2011, 3, 14.
- Chaitanya, R.K.; Shashank, K.; Sridevi, P. Oxidative stress in invertebrate systems. In Free Radicals and Diseases; Ahmad, R., Ed.; InTech: London, UK, 2016.
- Budachetri, K.; Browning, R.E.; Adamson, S.W.; Dowd, S.E.; Chao, C.-C.; Ching, W.M.; Karim, S. An insight into the microbiome of the Amblyomma maculatum (Acari: Ixodidae). J. Med. Entomol. 2014, 51, 119–129.
- Cirimotich, C.M.; Ramirez, J.L.; Dimopoulos, G. Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe 2011, 10, 307–310.
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 2011, 332, 855–858.
- Budachetri, K.; Kumar, D.; Crispell, G.; Beck, C.; Dasch, G.; Karim, S. The tick endosymbiont Candidatus Midichloria mitochondrii and selenoproteins are essential for the growth of Rickettsia parkeri in the Gulf Coast tick vector. Microbiome 2018, 6, 141.
- Crispell, G.; Budachetri, K.; Karim, S. Rickettsia parkeri colonization in Amblyomma maculatum: The role of superoxide dismutases. Parasit. Vectors 2016, 9, 291.
- Kumar, D.; Budachetri, K.; Meyers, V.C.; Karim, S. Assessment of tick antioxidant responses to exogenous oxidative stressors and insight into the role of catalase in the reproductive fitness of the Gulf Coast tick, Amblyomma maculatum. Insect Mol. Biol. 2016, 25, 283–294.
- Lee, W.J. Bacterial-modulated signaling pathways in gut homeostasis. Sci. Signal. 2008, 1, pe24.
- Champion, C.J.; Xu, J. The impact of metagenomic interplay on the mosquito redox homeostasis. Free Radic. Biol. Med. 2017, 105, 79–85.
- Romoli, O.; Gendrin, M. The tripartite interactions between the mosquito, its microbiota and Plasmodium. Parasit. Vectors 2018, 11, 200.




