Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vania Paschoalin | -- | 2617 | 2022-06-30 18:13:42 | | | |
| 2 | Camila Xu | Meta information modification | 2617 | 2022-07-01 02:27:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ferreira, P.G.; Ferreira, V.F.; Silva, F.D.C.D.; Freitas, C.S.; Pereira, P.R.; Paschoalin, V.M.F. Chitosans and Nanochitosans. Encyclopedia. Available online: https://encyclopedia.pub/entry/24702 (accessed on 07 February 2026).
Ferreira PG, Ferreira VF, Silva FDCD, Freitas CS, Pereira PR, Paschoalin VMF. Chitosans and Nanochitosans. Encyclopedia. Available at: https://encyclopedia.pub/entry/24702. Accessed February 07, 2026.
Ferreira, Patricia Garcia, Vitor Francisco Ferreira, Fernando De Carvalho Da Silva, Cyntia Silva Freitas, Patricia Ribeiro Pereira, Vania Margaret Flosi Paschoalin. "Chitosans and Nanochitosans" Encyclopedia, https://encyclopedia.pub/entry/24702 (accessed February 07, 2026).
Ferreira, P.G., Ferreira, V.F., Silva, F.D.C.D., Freitas, C.S., Pereira, P.R., & Paschoalin, V.M.F. (2022, June 30). Chitosans and Nanochitosans. In Encyclopedia. https://encyclopedia.pub/entry/24702
Ferreira, Patricia Garcia, et al. "Chitosans and Nanochitosans." Encyclopedia. Web. 30 June, 2022.
Copy Citation
Chitosan displays a dual function, acting as both an active ingredient and/or carrier for pharmaceutical bioactive molecules and metal ions. Its hydroxyl- and amino-reactive groups and acetylation degree can be used to adjust this biopolymer’s physicochemical and pharmacological properties in different forms, including scaffolds, nanoparticles, fibers, sponges, films, and hydrogels, among others.
chitosan/nanochitosan scaffolds
composites
antimicrobial
biopolymer
1. Chitin and Chitosan: Availability, Preparation, Physicochemical Characteristics, and Novel Pharmaceutical Uses
Chitin and chitosan originating from fungal and marine waste, such as crab or shrimp exoskeletons, and displaying low, medium, or high molecular weights and different deacetylation degrees are marketed by various manufacturers. The global chitin and chitosan market exhibited an annual growth rate (CAGR) of 15.4% between 2016 and 2020 and was valued at USD 42.29 billion in 2020. Estimates indicate that this figure will reach USD 69.297 billion in 2028, increasing at a CAGR of 5.07% from 2021 to 2028 (https://www.verifiedmarketresearch.com/product/chitin-market/, accessed on 17 November 2021). Companies conducting global market research estimate that this growth will be driven by novel biomedical applications, including pharmaceutical thickeners, protein entrapment, and the synthesis of biodegradable composites. However, additives in food and beverages, animal feed, agriculture, and water treatment may also contribute to the estimated increase. Many simple and inexpensive chemical methods for recovering these biopolymers from marine waste have been described in the literature, e.g., demineralization and deproteinization, which can extract chitin and chitosan from fungi and insects mollusk, shrimp, and crustacean shells. Yadav, et al. [1] reviewed the most important aspects of chitin and chitosan obtention methods from marine waste, as well as the preparation of nanostructured materials for commercial purposes. Chitin is obtained by chemical extraction processes, using acids to remove minerals and proteins. However, this method is not ecological and adversely affects chitin’s physical and chemical properties, although it is still the most applied method in large-scale industrial processes. Based on chitin’s similarity to cellulose, chitin nanofibers are produced according to protocols previously established for cellulose hydrolysis. Chitin can be hydrolyzed in acidic media and/or by enzymatic processes that cleave its crystalline structure [2], making subsequent chain deacetylation easy. Chitosans form when at least 50% of available deacetylated residues are obtained. Biodegradable and porous chitin and chitosan nanofibers under 100 nm in diameter display extremely high surface-to-volume ratios, presenting different morphological, optical, and mechanical characteristics. The most common method of obtaining chitin or chitosan nanofibers is through a disintegration process, employing either microwave irradiation or sonication [3][4][5].
Both chitin and chitosan are suitable for processing gels, membranes, nanofibers, spheres, microparticles, nanoparticles, scaffolds, and sponges [6][7][8]. Although nanostructured chitosans are less than 100 nm in size in at least one of their dimensions, they allow for increased interaction between components, making them more appropriate for human tissue recovery and repair engineering [9] as excipients in drug and gene delivery systems [8][10]. Nanochitosans exhibit three-dimensional architectural structures with open and interconnected pores that make them highly functional, resistant, and a suitable facilitator for drug encapsulation. In addition, they can also form inorganic composites that enhance their properties and ensure potential use in biomedicine tissue engineering, bone graft fabrication, and drug encapsulation, as indicated previously. Polymer/metal nanocomposites comprise a class of hybrid materials composed of an organic polymer matrix with dispersed metallic nanoparticles used in various applications, such as biosensors, cancer diagnosis/treatment, cell labeling, drug delivery, molecular imaging, and materials chemistry [11].
Chitosans are biocompatible with human tissues and present several pharmacological characteristics, including antimicrobial, hemostatic, anti-inflammatory, antioxidant, mucoadhesion, analgesic, adsorption-enhancing, antihypertensive, anticholesterolemic, anticancer, and antidiabetic properties. These intrinsic chitosan functionalities can be increased if sophisticated molecules are linked to chitosan scaffolds. In particular, chitosans and/or their counterparts can improve skin health and protection, aid in defense against carcinogenic radiation, oxidative stress, infections, and, eventually, skin cancer treatment. In the following sections, researchers will address the promotion of skin by chitosan repair by interfering with physiopathological conditions through gene expression control, cell adhesion, granulation tissue arrangement, and tissue re-epithelization.
2. Chemical Modifications on Chitin and Chitosan Surfaces: Developing Novel Pharmaceuticals
Chitosan exhibits several inherent pharmacological activities, such as antioxidant, antimicrobial, and antitumoral properties, that can be enhanced and broadened by chemical modifications or physicochemical interactions that contribute to healthy chitosan applications, particularly in benefiting and promoting skin tissue repair and regeneration. In their crystalline forms, the surface of chitosan and/or nanochitosans can be modified with different ligands comprising inorganic ions and hydrophobic or hydrophilic compounds. They can also be functionalized with very sophisticated molecules, such as antibodies, proteins, peptides, polysaccharides, and nucleic acids covalently linked to the primary molecule, or can act as carriers for these molecules through nanoencapsulation. Chitosan efficiency can be improved following nanoencapsulation, as nanochitosans display high surface/volume ratios [12].
Chemical modifications of chitin and chitosan functional groups can alter their physical and mechanical properties [13]. Many chemical reactions can insert functional groups on the surfaces of chitin and chitosan nanofibers to enhance, modify, or expand polymer functionality. Free amine groups are suitable for characteristic amine (NH) reactions that can be modified by acetylation/deacetylation, carboxyalkylation, phthaloylation, naphthaloylation, maleylation, chlorination, oxidation, and graft polymerization ranging from 5 to 8%. Secondary hydroxyl groups (OH) can also be chemically functionalized [14].
Although chitin can be deacetylated by alkaline hydrolysis, other sustainable and ecofriendly methods have been developed to generate chitosan from crustaceous solid residues. The hydrolysis of the N-acetamido groups in chitin N-acetyl-D-glucosamine residues can also be performed by employing recombinant yeast chitin deacetylase (E.C.3.5.1.41), which converts chitin into chitosan in a controlled manner with no harsh secondary residues [2][15]. Recently, chitosan nanofibers were obtained by treating chitin crustaceans with the crude extract of Annona muricata, an edible plant originally from the Antilles [16]. Alternatively, chitin can be acetylated by introducing additional acetyl groups without altering the degree of polymerization, generating biopolymers for different applications. Chitin acetylation is the simplest functionalization to perform, but complete deacetylation is rarely achieved, and some remaining compounds retain some acetamide groups (Figure 1A).
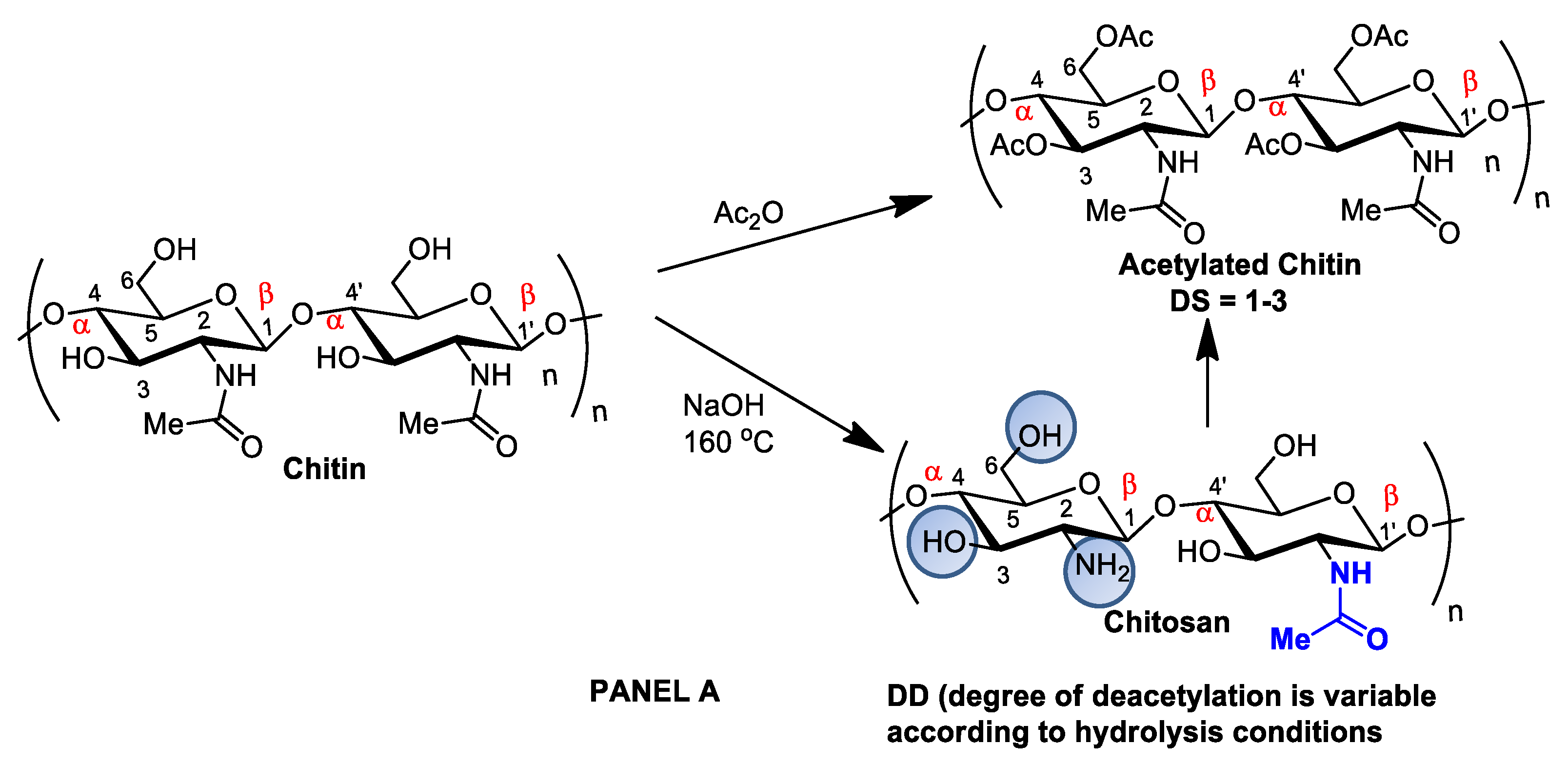
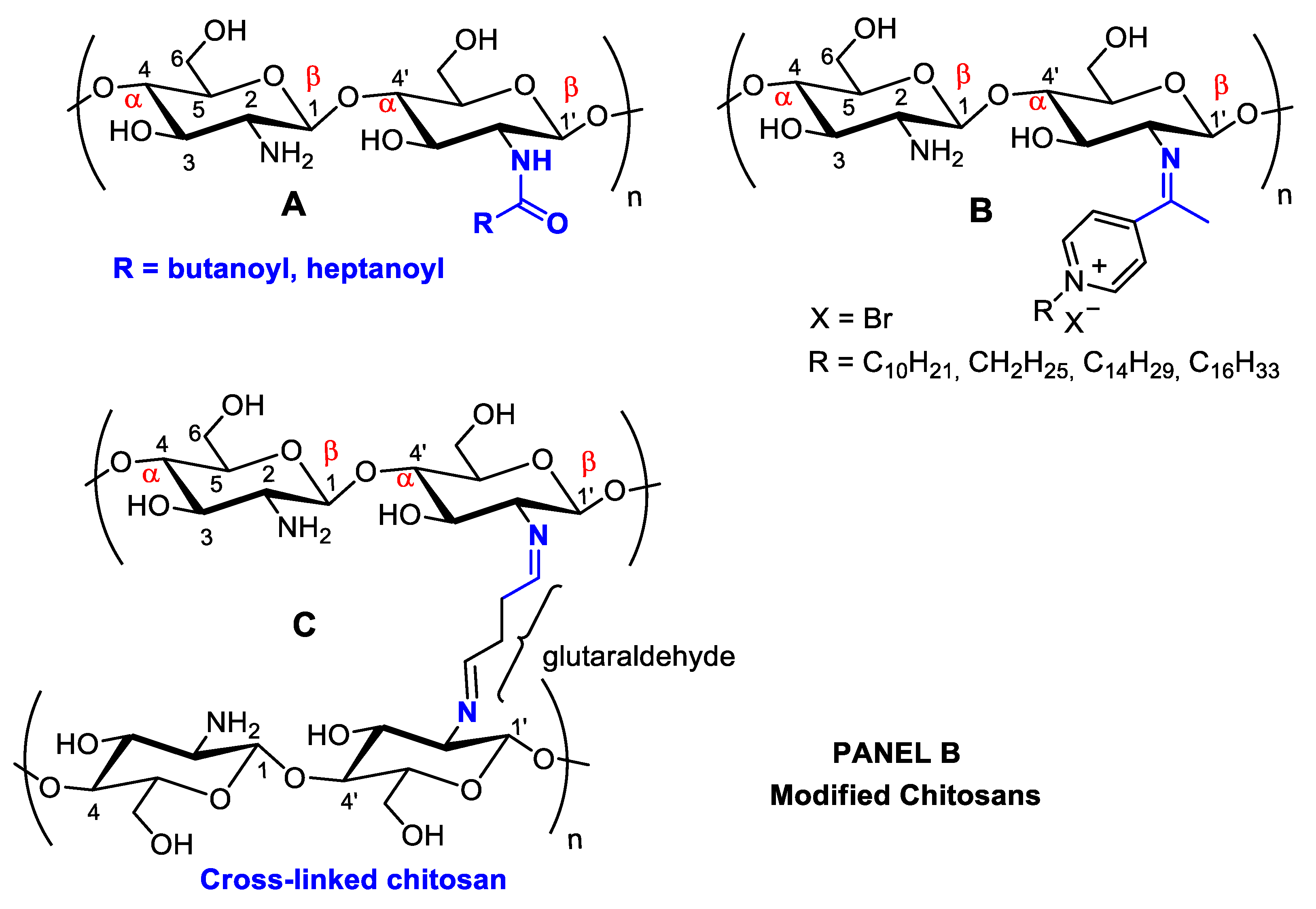 Figure 1. Panel (A): chitin and chitosan acetylation and deacetylation without alteration of the degree of polymerization. Chitin acetylation varies from 1–3 (DS = 1–3). Panel (B): chitosan structural modifications through amino group reactions with butanoyl or hepatanoyl (A), pyridine (B) or chitosan fibers crosslinking with glutaraldehyde (C).
Figure 1. Panel (A): chitin and chitosan acetylation and deacetylation without alteration of the degree of polymerization. Chitin acetylation varies from 1–3 (DS = 1–3). Panel (B): chitosan structural modifications through amino group reactions with butanoyl or hepatanoyl (A), pyridine (B) or chitosan fibers crosslinking with glutaraldehyde (C).The introduction of certain functional groups in the two chitin nanofiber hydroxyl groups can significantly improve their physicochemical properties and expand their use in new applications [17]. Polyacetylation and carboxymethyl acetylation, replacing hydrogens in the amino and hydroxyl groups, confer chitosan hydrophobicity and consequent waterproofing properties, which are very useful for obtaining non-toxic and biodegradable bandage coatings for minor cuts, scrapes, blisters, and burns, associating inherent chitosan healing properties to waterproof protection (Figure 1A—Panel A) [18][19].
However, chitin and chitosan acetylation degree, as well as other physicochemical characteristics, such as molecular mass and moisture content, are associated with fungal and bacteria growth inactivation. Low-molecular-weight chitosan oligomers pass through the cell membrane and alter cell permeability [20]. Nanochitosans have been shown to disassemble bacteria cell membranes and inhibit metabolic and biosynthetic pathways [21].
Antioxidant chitosan properties are ascribed to free amino groups, amino acetyl groups, and hydroxyl groups found in polymer molecules, all of which can react with different reactive oxygen (ROS) and nitrogen (RNS) species (Figure 2). Many studies indicate that chitosan polymers display antioxidant activities. However, the molecular mechanisms of chitosan radical scavenger abilities are still not well understood from a chemical point of view [22][23]. The antioxidant activity of chitosan increases with the degree of acetylation and with decreasing biopolymer molecular weight. It is important to note that shorter chains form fewer intramolecular hydrogen bonds between hydroxyls, resulting in more activated hydroxyl and amino groups, contributing to radical scavenging activity. Chitosan antioxidant activities can be identified by employing a very common antioxidant assay involving 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals. The DPPH radical exhibits a strong absorption band centered at about 520 nm and becomes colorless or pale yellow when neutralized (Figure 2). The ability to scavenge the DPPH radical by chitosan ranges between 30 and 60% [24].
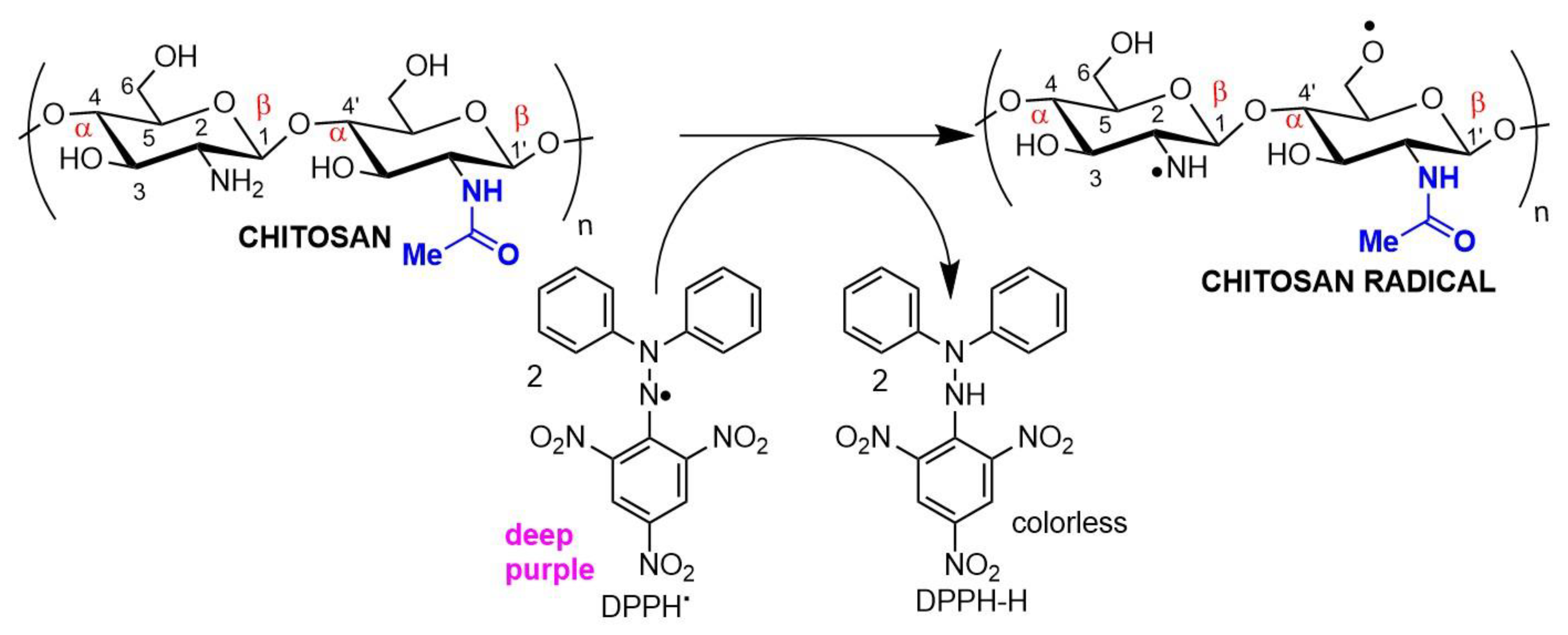 Figure 2. Chitosan radical scavenger activity demonstrated in an antioxidant assay using DPPH (2,2-diphenyl-1-picrylhydrazyl) as the electron acceptor.
Figure 2. Chitosan radical scavenger activity demonstrated in an antioxidant assay using DPPH (2,2-diphenyl-1-picrylhydrazyl) as the electron acceptor.The antioxidant/antimicrobial effects of chitosan and its derivatives can be explored in drug and cosmetic formulations due to their biocompatibility, non-toxic character, and effective human metabolization, enhancing the safety and shelf life of both pharmaceuticals and food products [25].
When associated with resveratrol (3,5,4′-trihydroxystilbene), a natural phytoalexin, chitosan synthesized in cis and trans isomers of grapevines in response to pathogen infection or physical injury can be administered either topically or systemically (Figure 3). Resveratrol owes its antioxidant activity to its phenolic hydroxyl groups and can be used to achieve oxidative skin cell homeostasis following topic use or aid in the fight against chronic and degenerative human physiopathological conditions by promoting organism oxidative balance when administered systemically as a nutraceutical or pharmaceutical [26].
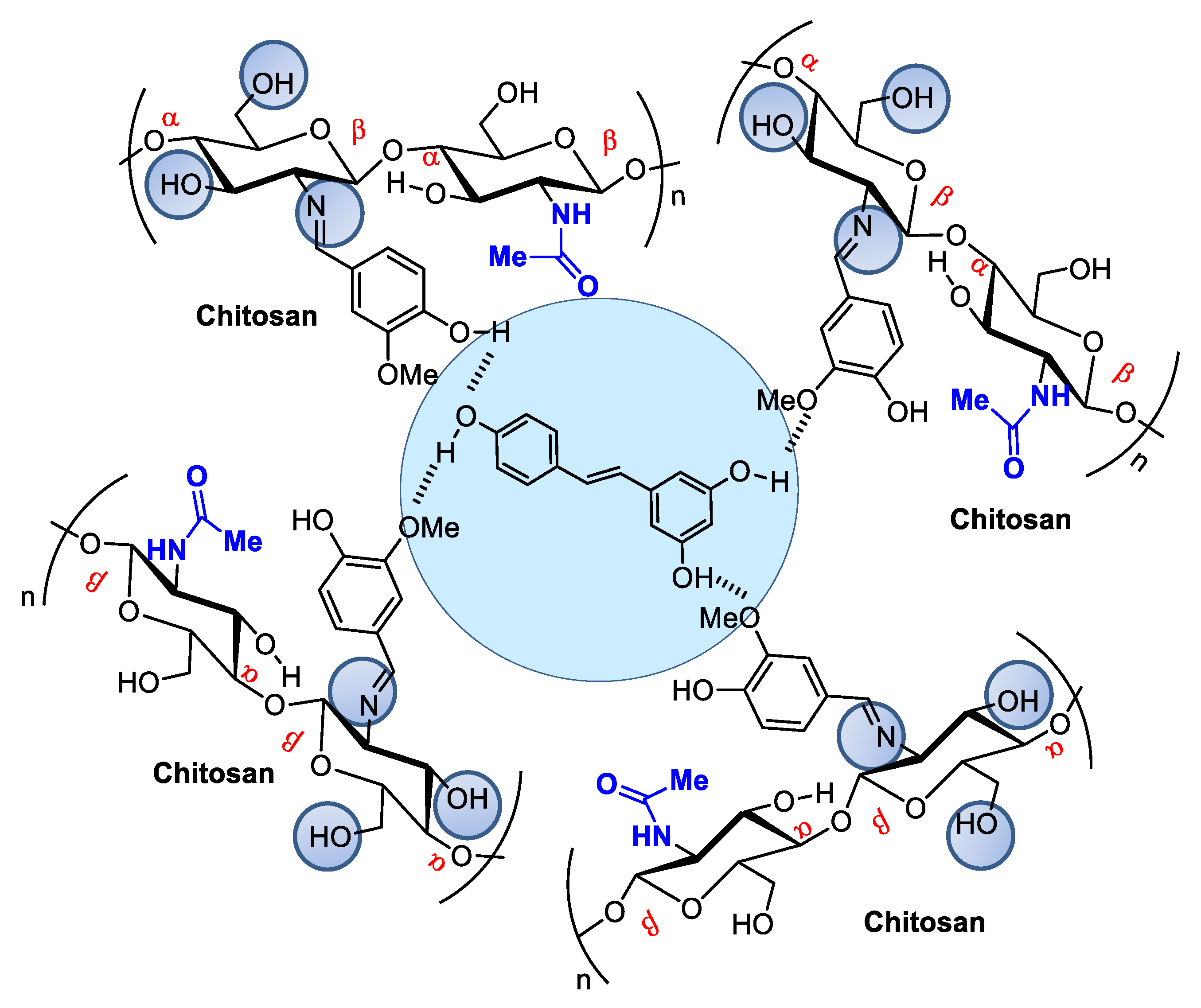 Figure 3. Resveratrol encapsulated in chitosan microspheres crosslinked with vanillin can be administered to modulate metabolic dysfunctions.
Figure 3. Resveratrol encapsulated in chitosan microspheres crosslinked with vanillin can be administered to modulate metabolic dysfunctions.Resveratrol from red wine has been considered promising in preventing human cardiovascular diseases [27] and as an antitumorigenic [28][29] and antiviral [30] compound, among other applications. It also modulates lipid metabolism and inhibits low-density lipoprotein oxidation and platelet aggregation. The potential of this natural product has led to hundreds of studies concerning the search for pharmaceutical strategies for its stabilization and use in food and cosmeceutical supplements. Regarding controlled release, many studies describe resveratrol incorporation into several natural and modified chitosan types in film, nanoparticle, or microsphere form [31][32][33]. For example, Frémont [34] incorporated resveratrol into chitosan microspheres crosslinked with vanillin. The microspheres presented a smooth surface composed of small but irregularly sized particles ranging from 53 to 311 μm. Network crosslinking between chitosan and vanillin occurred via the Schiff base reaction, and resveratrol encapsulation efficiency in the chitosan microsphere reached an efficiency rate of up to 93.68%.
Sodium tripolyphosphate (TPP) salts can also be used to encapsulate resveratrol in microspheres at concentrations that considerably alter its bioavailability, consequently increasing its effectiveness [35].
Chitosans also exhibit well-documented antimicrobial activities against Gram-positive and Gram-negative bacteria and fungi [21][36]. However, due to its low solubility, antimicrobial activity is limited to acidic media, although antimicrobial activity in neutral media can be improved by transforming amino groups into quaternary ammonium salts (NR4 + X−) [37]. Structural chitosan modifications obtained by introducing groups covalently linked to amines or hydroxyls can increase antimicrobial and antioxidant activities compared to native chitosan [38].
Chitosan antimicrobial activity can be customized and enhanced by covalently binding to antibiotics or antifungal agents to treat nosocomial infections. The introduction of radicals containing quaternary ammonium/pyridinium salts used as commercial antimicrobial agents (e.g., dimethyl dodecyl ammonium chloride or bromide and cetylpyridinium chloride) can improve antimicrobial activity (Figure 4). For example, chitosan nanoparticles modified through the formation of a Schiff base in the amino group, forming long-chain pyridinium radicals, exhibited increased antibacterial activity against the Gram-positive species Staphylococcus aureus and Bacillus cereus, as evaluated by MIC (minimum inhibitory concentration) and MIB (minimum bactericidal concentration), as well as mild antioxidant effects (Figure 2) [39].
 Figure 4. Chitosan nanoparticles functionalized by long-chain pyridinium salts to increase antimicrobial effectiveness.
Figure 4. Chitosan nanoparticles functionalized by long-chain pyridinium salts to increase antimicrobial effectiveness.Metallic nanoparticles containing Ag, Ni, Cu, Pt, Co, Pd, or Au can exhibit unusual physical and chemical characteristics distinct from their bulk counterparts or individual metals [40], such as antimycotic, antiparasitic, antineoplastic, insecticidal, and antibiotic properties [41]. For example, Ag-chitosan nanoparticles are promising pharmaceuticals to treat fungal infections caused by Candida spp., with fungal growth inactivation due to cytoplasmic degeneration and membrane and cell wall rupture. Ag-nanochitosans in combination with fluconazole and amphotericin B demonstrated an additive effect. Low nanocomposite cytotoxicity against mammalian cells has been reported in Galleria mellonella larvae, suggesting potential in vivo use. When tested as a topical treatment against murine cutaneous candidiasis, nanocomposites were found to reduce fungal skin loads in a dose-response behavior and favored tissue repair [42].
Molecular chitosan antimicrobial enrollment mechanisms are not yet fully understood. The most accepted mechanism involves the combination of chitosan cationic amine groups and anionic phospholipids on bacterial surfaces, promoting membrane disassembly and cell disintegration (Figure 5) [43]. It has also been proposed that chitosan and its low-molecular-weight derivatives, penetrate the bacterial membrane, thus impairing selective protein biosynthesis [21][44]. Another possibility is that chitosan, employing its exceptional ability to bind metals, interacts with divalent ions in bacterial cell walls to prevent cells from performing their normal functions [45]. Chitosan can also act as an occluding compound, docking to the outer bacteria membrane and preventing nutrient cell uptake and oxygen diffusion (Figure 5) [46]. Many studies have focused on optimizing the antibacterial properties of chitosan through various modifications [39][47]. For example, Liu, et al. [48] reported that surface grafting of gentamicin molecules improved antibacterial chitosan properties [48]. In another study, chitosan films incorporated with Ag nanoparticles exhibited enhanced antimicrobial properties (Figure 5) [49]. Chitosan’s wound-healing properties are enhanced in Ag-chitosan. Chitosan/glycosaminoglycan scaffolds loaded with Ag nanoparticles can also promote wound repair by stimulating fibroblast proliferation [50] and modulating the matrix production of metalloproteinases, preventing degradation of both fiber junctions and peptide growth factors [51]. Other studies report that nanofibers with Ag nanoparticles promote wound healing by activating the TGF β1/Smad signaling pathway [52]. Furthermore, the addition of Ag nanoparticles can effectively and conclusively inhibit an inflammatory response by regulating inflammatory mediators, and Ag-chitosan has been proven to induce a superior anti-inflammatory inhibitory effect than any monomer, significantly reducing interleukin-1 levels [53].
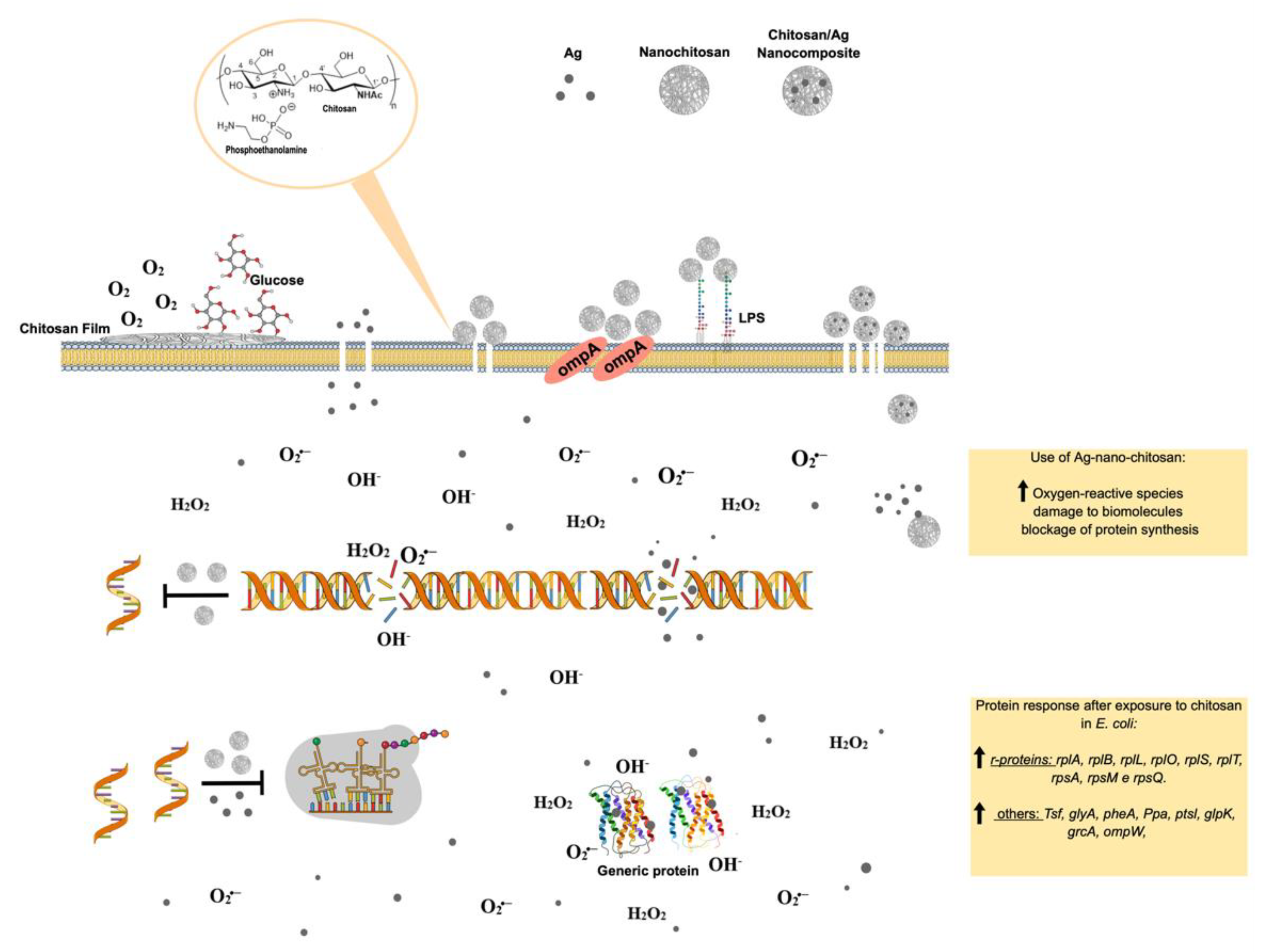 Figure 5. Molecular effects of chitosan nanoparticles on E. coli growth inactivation. Chitosan seems to interact with phospholipids, particularly phosphatidylethanolamine (PE), the most abundant phospholipid in E. coli, triggering the impermeabilization or disassembly of its outer membrane. The impermebialization of bacteria membrane by the nano-chitosan films inhibit glucose and O2 uptake, causing bacteria death. After exposure to nano-chitosan particles, E. coli experiences a differential expression of proteins involved in protein biosynthesis machinery and amino acid and purine nucleotide supply, as already demonstrated [21] and shown in the yellow rectangle at the lower right corner. When E. coli is exposed to Ag-nano-chitosans, an increase in reactive oxygen species is observed, followed by consequent damage to cellular structures, including proteins and nucleic acids. Chitosan and Ag nanoparticles, as well as cellular structures, are not represented in their real scale. This figure was drawn using the infographic maker Mind the Graph, available at https://mindthegraph.com.
Figure 5. Molecular effects of chitosan nanoparticles on E. coli growth inactivation. Chitosan seems to interact with phospholipids, particularly phosphatidylethanolamine (PE), the most abundant phospholipid in E. coli, triggering the impermeabilization or disassembly of its outer membrane. The impermebialization of bacteria membrane by the nano-chitosan films inhibit glucose and O2 uptake, causing bacteria death. After exposure to nano-chitosan particles, E. coli experiences a differential expression of proteins involved in protein biosynthesis machinery and amino acid and purine nucleotide supply, as already demonstrated [21] and shown in the yellow rectangle at the lower right corner. When E. coli is exposed to Ag-nano-chitosans, an increase in reactive oxygen species is observed, followed by consequent damage to cellular structures, including proteins and nucleic acids. Chitosan and Ag nanoparticles, as well as cellular structures, are not represented in their real scale. This figure was drawn using the infographic maker Mind the Graph, available at https://mindthegraph.com.Chitin derivatives exhibiting controlled molecular weight and/or deacetylation degree variations have been reported to display antitumorigenic activity against several cancer cell lines, including human myeloid leukemia HL-60 cells, human melanoma cells (cell lines A375, SKMEL28, and RPMI7951), HeLa cells, human hepatoma, and human monocyte leukemia cells [54][55][56][57][58].
Schneible, et al. [59] studied drug delivery systems employing chitosan hydrogels for combined chemotherapy using doxorubicin and gemcitabine for the synergistic treatment of advanced solid tumors (i.e., breast cancer). Butanoyl and heptanoyl-hydrophobic chains were chosen for chitosan modification to target drug release kinetics to control the hydrogel network structure and, therefore, drug migration (Figure 1A). Specifically, these modifications allow for modulation of the molar ratio and relative drug release kinetics, which has been validated experimentally, allowing for the prediction of behaviors for specific formulations offering synergistic effects. The achieved results demonstrate the potential of this approach to accelerate the use of drug-laden hydrogels for cancer therapy, including chitosan-doxorubicin, chitosan-gentamicin, chitosan-butanoyl, heptanoyl hydrophobic chains, dimethyl dodecyl ammonium chloride, or bromide and cetylpyridinium chloride (Figure 4). Many substituents can be inserted into hydroxyl groups, depending on the intended therapeutic effect. For example, chitosan can be crosslinked using glutaraldehyde (Figure 1B) [60]. The more rigid structure of this polymer crosslinked with a dialdehyde linking the NH2 groups can be used for many different applications compared to isolated chitosan fibers, mainly concerning polymer strength. This material can be coated with other polymers, forming surface films bonded by hydrogen bonds. With the aim of increasing the resistance of crosslinked polymers, Sun, et al. [61] anchored poly(ethylene oxide) presenting different molecular weights when preparing various membrane materials, and the surface of this dense-structure membrane became less porous, decreasing the amount of water absorption and pore size and increasing thermal stability and tensile strength.
References
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8.
- Gomes, L.P.; Andrade, C.T.; Silva, J.T.; Del Aguila, E.M.; Paschoalin, V.M.F. Green Synthesis and Physical and Chemical Characterization of Chitosans with a High Degree of Deacetylation, Produced by a Binary Enzyme System. J. Life Sci. 2014, 8, 276–282.
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318.
- Gomes, L.P.; Souza, H.K.S.; Campiña, J.M.; Andrade, C.T.; Paschoalin, V.M.F.; Silva, A.F.; Gonçalves, M.P. Tweaking the mechanical and structural properties of colloidal chitosans by sonication. Food Hydrocoll. 2016, 56, 29–40.
- EL Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102.
- Tamura, H.; Furuike, T.; Nair, S.V.; Jayakumar, R. Biomedical applications of chitin hydrogel membranes and scaffolds. Carbohydr. Polym. 2011, 84, 820–824.
- Madhumathi, K.; Binulal, N.S.; Nagahama, H.; Tamura, H.; Shalumon, K.T.; Selvamurugan, N.; Nair, S.V.; Jayakumar, R. Preparation and characterization of novel β-chitin–hydroxyapatite composite membranes for tissue engineering applications. Int. J. Biol. Macromol. 2009, 44, 1–5.
- Jayakumar, R.; Reis, R.L.; Mano, J.F. Phosphorous Containing Chitosan Beads for Controlled Oral Drug Delivery. J. Bioact. Compat. Polym. 2006, 21, 327–340.
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632.
- Brasuel, M.; Wise, K. The current state of engineered nanomaterials in consumer goods and waste streams: The need to develop nanoproperty-quantifiable sensors for monitoring engineered nanomaterials. Nanotechnol. Sci. Appl. 2011, 4, 73–86.
- Li, X.; Shan, J.; Zhang, W.; Su, S.; Yuwen, L.; Wang, L. Recent Advances in Synthesis and Biomedical Applications of Two-Dimensional Transition Metal Dichalcogenide Nanosheets. Small 2017, 13, 1602660.
- Mushi, N.E. A review on native well-preserved chitin nanofibrils for materials of high mechanical performance. Int. J. Biol. Macromol. 2021, 178, 591–606.
- Kurita, K. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971.
- Del Aguila, E.M.; Gomes, L.P.; Andrade, C.T.; Silva, J.T.; Paschoalin, V.M.F. Biocatalytic production of chitosan polymers from shrimp shells, using a recombinant enzyme produced by pichia pastoris. Am. J. Mol. Biol. 2012, 2, 341–350.
- Gopal, J.; Muthu, M.; Dhakshanamurthy, T.; Kim, K.J.; Hasan, N.; Kwon, S.J.; Chun, S. Sustainable ecofriendly phytoextract mediated one pot green recovery of chitosan. Sci. Rep. 2019, 9, 1383.
- Franca, E.F.; Freitas, L.C.G.; Lins, R.D. Chitosan molecular structure as a function of N-acetylation. Biopolymers 2011, 95, 448–460.
- Bukzem, A.L.; Signini, R.; dos Santos, D.M.; Lião, L.M.; Ascheri, D.P.R. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol. 2016, 85, 615–624.
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 2013, 91, 452–466.
- Elsoud, M.M.A.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 59.
- Gomes, L.P.; Anjo, S.I.; Manadas, B.; Coelho, A.V.; Paschoalin, V.M.F. Proteomic Analyses Reveal New Insights on the Antimicrobial Mechanisms of Chitosan Biopolymers and Their Nanosized Particles against Escherichia coli. Int. J. Mol. Sci. 2020, 21, 225.
- Ngo, D.-H.; Kim, S.-K. Chapter Two–Antioxidant Effects of Chitin, Chitosan, and Their Derivatives. Adv. Food Nutr. Res. 2014, 73, 15–31.
- Arancibia, M.Y.; Alemán, A.; Calvo, M.M.; López-Caballero, M.E.; Montero, P.; Gómez-Guillén, M.C. Antimicrobial and antioxidant chitosan solutions enriched with active shrimp (Litopenaeus vannamei) waste materials. Food Hydrocoll. 2014, 35, 710–717.
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844.
- Kerch, G. The Potential of Chitosan and Its Derivatives in Prevention and Treatment of Age-Related Diseases. Mar. Drugs 2015, 13, 2158–2182.
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340.
- Fogacci, F.; Tocci, G.; Presta, V.; Fratter, A.; Borghi, C.; Cicero, A.F.G. Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1605–1618.
- Da Costa, D.C.F.; Rangel, L.P.; Martins-Dinis, M.M.D.D.C.; Ferretti, G.D.D.S.; Ferreira, V.F.; Silva, J.L. Anticancer Potential of Resveratrol, β-Lapachone and Their Analogues. Molecules 2020, 25, 893.
- Silva, J.; Costa, D.; Campos, N.; Costa, C.; Rangel, L. Understanding the p53 Aggregation Process through its Activators Resveratrol and PRIMA. FASEB J. 2015, 29, LB151.
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613.
- Ratz-Łyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 2019, 21, 84–90.
- Pastor, C.; Sánchez-González, L.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Physical and antioxidant properties of chitosan and methylcellulose based films containing resveratrol. Food Hydrocoll. 2013, 30, 272–280.
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and in vitro/in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug Deliv. 2016, 23, 971–981.
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673.
- Cho, A.R.; Chun, Y.G.; Kim, B.-K.; Park, D.J. Preparation of Chitosan-TPP Microspheres as Resveratrol Carriers. J. Food Sci. 2014, 79, E568–E576.
- Wei, L.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. The antioxidant and antifungal activity of chitosan derivatives bearing Schiff bases and quaternary ammonium salts. Carbohydr. Polym. 2019, 226, 115256.
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Antimicrobial Activity of Chitosan Derivatives Containing N-Quaternized Moieties in Its Backbone: A Review. Int. J. Mol. Sci. 2014, 15, 20800–20832.
- Ying, G.-Q.; Xiong, W.-Y.; Wang, H.; Sun, Y.; Liu, H.-Z. Preparation, water solubility and antioxidant activity of branched-chain chitosan derivatives. Carbohydr. Polym. 2011, 83, 1787–1796.
- Omidi, S.; Kakanejadifard, A. Modification of chitosan and chitosan nanoparticle by long chain pyridinium compounds: Synthesis, characterization, antibacterial, and antioxidant activities. Carbohydr. Polym. 2019, 208, 477–485.
- Duan, S.; Wang, R. Bimetallic nanostructures with magnetic and noble metals and their physicochemical applications. Prog. Nat. Sci. 2013, 23, 113–126.
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. Rsc Adv. 2014, 4, 3974–3983.
- Bonilla, J.J.A.; Honorato, L.; de Oliveira, D.F.C.; Gonçalves, R.A.; Guimarães, A.; Miranda, K.; Nimrichter, L. Silver chitosan nanocomposites as a potential treatment for superficial candidiasis. Med. Mycol. 2021, 59, 993–1005.
- Ganan, M.; Carrascosa, A.V.; Martínez-Rodríguez, A.J. Antimicrobial Activity of Chitosan against Campylobacter spp. and Other Microorganisms and Its Mechanism of Action. J. Food Prot. 2009, 72, 1735–1738.
- Gomes, L.P.; Andrade, C.T.; Del Aguila, E.M.; Alexander, C.; Paschoalin, V. Assessing the antimicrobial activity of chitosan nanoparticles by fluorescence-labeling. Int. J. Biotechnol. Bioeng. 2018, 12, 111–117.
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized Chitosan as an Antimicrobial Agent: Antimicrobial Activity, Mechanism of Action and Biomedical Applications in Orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869.
- Xing, Y.; Xu, Q.; Li, X.; Chen, C.; Ma, L.; Li, S.; Che, Z.; Lin, H. Chitosan-Based Coating with Antimicrobial Agents: Preparation, Property, Mechanism, and Application Effectiveness on Fruits and Vegetables. Int. J. Polym. Sci. 2016, 2016, 1–24.
- Ly, N.T.K.; Shin, H.; Gupta, K.C.; Kang, I.K.; Yu, W. Bioactive Antibacterial Modification of Orthodontic Microimplants Using Chitosan Biopolymer. Macromol. Res. 2019, 27, 504–510.
- Liu, Y.; Lv, H.; Qin, Y.; Deng, L.; Wang, Y. Modification of chitosan film via surface grafting of gentamicin molecular to improve the antibacterial property. J. Control. Release 2017, 259, e166.
- Dananjaya, S.; Kulatunga, D.; Godahewa, G.; Nikapitiya, C.; Oh, C.; Edussuriya, M.; Lee, J.; De Zoysa, M. Preparation, characterization, and antimicrobial properties of chitosan–silver nanocomposites films against fish pathogenic bacteria and fungi. Indian J. Microbiol. 2017, 57, 427–437.
- Sandri, G.; Miele, D.; Faccendini, A.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Taglietti, A.; Ruggeri, M.; Bruni, G.; Vigani, B.; et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers 2019, 11, 1207.
- Mei, M.L.; Li, Q.; Chu, C.H.; Yiu, C.K.; Lo, E.C. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent. Mater. 2012, 28, 903–908.
- Li, C.-W.; Wang, Q.; Li, J.; Hu, M.; Shi, S.-J.; Li, Z.-W.; Wu, G.-L.; Cui, H.-H.; Li, Y.-Y.; Zhang, Q. Silver nanoparticles/chitosan oligosaccharide/poly (vinyl alcohol) nanofiber promotes wound healing by activating TGFβ1/Smad signaling pathway. Int. J. Nanomed. 2016, 11, 373.
- Oryan, A.; Alemzadeh, E.; Tashkhourian, J.; Ana, S.F.N. Topical delivery of chitosan-capped silver nanoparticles speeds up healing in burn wounds: A preclinical study. Carbohydr. Polym. 2018, 200, 82–92.
- Kim, E.-K.; Je, J.-Y.; Lee, S.-J.; Kim, Y.-S.; Hwang, J.-W.; Sung, S.-H.; Moon, S.-H.; Jeon, B.-T.; Kim, S.-K.; Jeon, Y.-J.; et al. Chitooligosaccharides induce apoptosis in human myeloid leukemia HL-60 cells. Bioorg. Med. Chem. Lett. 2012, 22, 6136–6138.
- Gibot, L.; Chabaud, S.; Bouhout, S.; Bolduc, S.; Auger, F.A.; Moulin, V.J. Anticancer properties of chitosan on human melanoma are cell line dependent. Int. J. Biol. Macromol. 2015, 72, 370–379.
- Kaya, M.; Akyuz, B.; Bulut, E.; Sargin, I.; Tan, G.; Erdonmez, D.; Maheta, M.; Satkauskas, S.; Mickevičius, S. DNA interaction, antitumor and antimicrobial activities of three-dimensional chitosan ring produced from the body segments of a diplopod. Carbohydr. Polym. 2016, 146, 80–89.
- Subhapradha, N.; Shanmugam, A. Fabrication of β-chitosan nanoparticles and its anticancer potential against human hepatoma cells. Int. J. Biol. Macromol. 2017, 94, 194–201.
- Salah, R.; Michaud, P.; Mati, F.; Harrat, Z.; Lounici, H.; Abdi, N.; Drouiche, N.; Mameri, N. Anticancer activity of chemically prepared shrimp low molecular weight chitin evaluation with the human monocyte leukaemia cell line, THP-1. Int. J. Biol. Macromol. 2013, 52, 333–339.
- Schneible, J.D.; Singhal, A.; Lilova, R.L.; Hall, C.K.; Grafmüller, A.; Menegatti, S. Tailoring the Chemical Modification of Chitosan Hydrogels to Fine-Tune the Release of a Synergistic Combination of Chemotherapeutics. Biomacromolecules 2019, 20, 3126–3141.
- Oliveira, M.Z.F.d.S.; Fernandes, T.S.M.; Carvalho, T.V. Síntese e caracterização de beads de quitosana comercial reticulados com glutaraldeído. Matéria 2021, 26.
- Sun, M.; Yuan, L.; Yang, X.; Shao, L. Preparation and Modification of Chitosan-Based Membrane. ES Mater. Manuf. 2020, 9, 40–47.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
01 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




