Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Peter Richardson | -- | 2513 | 2022-06-30 17:28:03 | | | |
| 2 | Jason Zhu | -1 word(s) | 2512 | 2022-07-01 03:22:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Richardson, P.; Robinson, B.W.S.; Smith, D.P.; Stebbing, J. Baricitinib in COVID-19 Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/24700 (accessed on 07 February 2026).
Richardson P, Robinson BWS, Smith DP, Stebbing J. Baricitinib in COVID-19 Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/24700. Accessed February 07, 2026.
Richardson, Peter, Bruce W. S. Robinson, Daniel P. Smith, Justin Stebbing. "Baricitinib in COVID-19 Therapy" Encyclopedia, https://encyclopedia.pub/entry/24700 (accessed February 07, 2026).
Richardson, P., Robinson, B.W.S., Smith, D.P., & Stebbing, J. (2022, June 30). Baricitinib in COVID-19 Therapy. In Encyclopedia. https://encyclopedia.pub/entry/24700
Richardson, Peter, et al. "Baricitinib in COVID-19 Therapy." Encyclopedia. Web. 30 June, 2022.
Copy Citation
During the current pandemic, the vast majority of COVID-19 patients experienced mild symptoms, but some had a potentially fatal aberrant hyperinflammatory immune reaction characterized by high levels of IL-6 and other cytokines. Modulation of this immune reaction has proven to be the only method of reducing mortality in severe and critical COVID-19. The anti-inflammatory drug baricitinib (Olumiant) has recently been strongly recommended by the WHO for use in COVID-19 patients because it reduces the risk of progressive disease and death. It is a Janus Kinase (JAK) 1/2 inhibitor approved for rheumatoid arthritis which was suggested in early 2020 as a treatment for COVID-19.
COVID-19
SARS-CoV-2
baricitinib
1. Introduction
The COVID-19 pandemic has caused the death of approximately 6 million people, with a case fatality rate which may be as high as 20% in those over 80 years old [1]. Vaccines have proved to be extremely effective in reducing the damage and hospitalisation caused by this infection, although some patients still need supportive care. As the SARS-CoV-2 virus has continued to evolve, the potential for the virus to escape vaccine and exposure induced immunity remains a threat. In this situation, as at the start of the pandemic when no such vaccines were available, it is important that there exist therapeutics for the treatment of severely ill patients. This described the identification, mechanism of action, and validation of the already approved rheumatology drug baricitinib as a treatment for hospitalised patients with COVID-19. In addition, comparison with other agents demonstrates that this drug is the most potent of the immune modulators in reducing COVID-19 mortality. As a result, it is now strongly recommended for the treatment of COVID-19 by the WHO.
Infection by SARS-CoV-2 is usually via respiratory droplets and, like the related SARS-CoV-1 and MERS viruses, results in a biphasic disease. The first phase shows mild symptoms, e.g., fever, muscle pains, fatigue, headache, diarrhoea, loss of taste and smell, and a cough which may last for up to 2 weeks. This is the experience of most patients, but in some this phase is followed by the onset of breathlessness and pneumonia, often requiring oxygen therapy, and which can also be associated with severe pulmonary and systemic inflammation, similar to a cytokine storm. This involves high levels of circulating cytokines with widespread organ damage, vascular damage/thrombosis, and acute respiratory distress syndrome (ARDS). It is unclear why some people suffer from this hyperinflammatory episode while others do not. Perhaps the most common explanation is that in those experiencing severe disease the response of both the innate and adaptive immune systems is dysregulated [2]. This dysregulated response is associated with ageing of the immune system, obesity [3], and with chronic underlying diseases such as cardiovascular disease, diabetes, COPD [4], and others.
2. The Role of AI in the Repurposing of Baricitinib
In January 2020, when it had become apparent that the new coronavirus was likely to spread worldwide, scientists at BenevolentAI, a London-based AI-enabled drug discovery company, used their AI-enhanced knowledge graph to piece together the mechanisms behind SARS-CoV-2 and then search for approved drugs capable of treating those mechanisms and thereby treat patients with the disease. This knowledge graph combines numerous data sources incorporating information on drugs, drug targets, genes, biological mechanisms, and diseases [5]. The knowledge graph contains machine-read literature covering the extensive collection of biomedical knowledge available (more than 30 M papers are catalogued in PubMed) and the contents of dozens of structured databases. The interrelationships between biomedical concepts in the knowledge graph are enhanced by AI algorithms, which help describe their confidence, causality, and cover gaps in established knowledge. The knowledge graph can be searched or explored by experts using interactive tools, as well as novel proprietary algorithms as described in [5]. To analyse SARS-CoV-2 mechanisms and identify candidate drugs, BenevolentAI scientists adopted an interactive and iterative approach, combining their expertise and interactive tooling with AI-generated biomedical relationships extracted from recent coronavirus literature. The aim was to find an approved drug which could treat the “cytokine storm” responsible for many of the early deaths from COVID-19 [6]. In addition, the drug should also prevent or reduce virus infection, perhaps by inhibiting the infection of cells by the virus which was thought to be via the SARS receptor ACE2.
The BenevolentAI knowledge graph is focused on human biology, so the search in January 2020 was focused on identifying drugs acting on host proteins that were subverted by the virus. In brief, the virus interactome was identified, added to the knowledge graph, and the knowledge graph was queried for anti-inflammatory agents which could counter the cytokine storm and also have antiviral effects. Since virus replication is largely mediated by proteins encoded by the virus, and the knowledge graph is focused on human biology, the search was for those mechanisms and proteins of the host mediating viral infection of cells rather than viral replication. In the search of this SARS-CoV-2 enhanced BenevolentAI knowledge graph for endocytic mechanisms a cluster of protein interactions related to virus entry suggested CME was the likely route of SARS-CoV-2 entry into cells (Figure 1) [6]. A Protein–Protein Interaction (PPI) output that indicated that SARS-CoV-2 may infect cells via CME is shown in Figure 1, where the CME module is identified. This figure illustrates how CME was identified as the probable entry pathway for the SARS-CoV-2 virus. This conclusion was later confirmed [7][8]. The proteins of the CME pathway (AAK1, CLTC, GAK, EPS15, AP2M1 etc.) were enriched in the pink endocytosis cluster of PPIs in Figure 1.
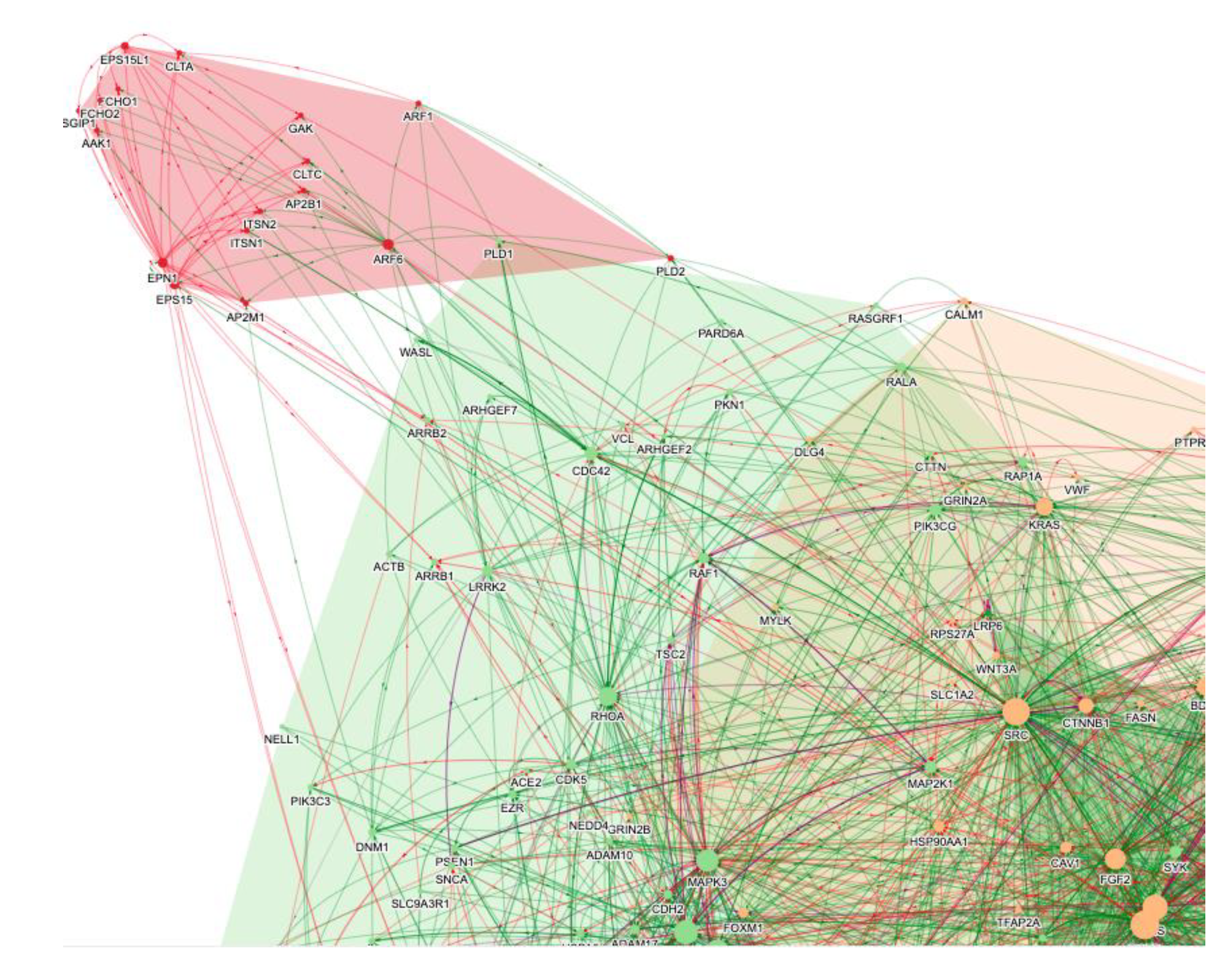
Figure 1. A selection of the PPI networks from a knowledge graph query of “SARS-CoV-2 AND endocytosis”. Specific pathways and processes are grouped in different-coloured clusters (e.g., endocytosis in pink and cytokine signalling in green and orange). Each node reflects one protein, and the edges reflect enhancing (green) or inhibiting (red) protein–protein interactions. The CME module is in pink.
By focusing on anti-inflammatory and viral infection mechanisms as described in [6], the knowledge graph and computational tools revealed approved drugs potentially able to act as both anti-inflammatories and antivirals. The result of this process was the identification of two drugs, baricitinib and fedratinib, approved for inflammatory indications, and ruxolitinib for myeloproliferative diseases. These were predicted inhibitors of JAKs and also of NAKs. Being JAK inhibitors, all three were likely to be effective inhibitors of cytokine signalling and complement activation and neutrophil trapping [9], thereby reducing the inflammatory consequences of the elevated levels of cytokines typically observed in people with COVID-19. Comparison of their pharmacokinetic properties, however, revealed that baricitinib was also predicted to inhibit the NAK enzymes AAK1 and BMP2K at plasma exposures routinely achieved when dosing patients. In contrast, the predicted unbound plasma exposures of ruxolitinib and fedratinib required to inhibit these enzymes (and so CME) greatly exceeded the exposures achieved therapeutically [10]. These drugs were, therefore, unlikely to reduce viral infectivity at tolerated doses, although they might reduce the host inflammatory response through JAK inhibition.
The combination of the oncology therapeutics sunitinib and erlotinib was previously shown to reduce the infectivity of a wide range of viruses, including Hepatitis C virus, Dengue virus, Ebola virus, and respiratory syncytial virus [11][12]. However, sunitinib and erlotinib would be difficult for patients to tolerate at the doses required to inhibit AAK1 and GAK so were not considered further. The high affinity of baricitinib for NAKs, its anti-inflammatory properties, its advantageous pharmacokinetic properties, and mild side effect profile (see later) suggested that it should be used in the treatment of COVID-19 [10][13].
3. Baricitinib in COVID-19 Therapy
After publishing the output of this AI-augmented research, the mechanistic predictions were validated, confirming that baricitinib inhibited signalling by a range of cytokines associated with the COVID-19 hyperinflammation. The anti-inflammatory effects of JAK inhibitors in general are summarised in ref. [9], and the effect of baricitinib confirmed in ref. [14] where the signalling of IL-2, IL-6, IL-10, IFNγ, and GCSF in monocytes, NK, and T cells was demonstrated [14]. Baricitinib also caused a significant reduction in plasma IL-6 in rheumatoid arthritis patients [14][15], these observations together indicating the potential of this drug to inhibit the hyperinflammation associated with COVID-19. In addition, the nM potency of baricitinib on the NAK enzymes was confirmed in [14] and baricitinib was shown to reduce SARS-CoV-2 infection of human liver cells through super resolution microscopy, thereby confirming the predicted antiviral activity of this drug [16]. Perhaps as important was the observation that baricitinib reduced the expression of ISGs associated with platelet activation, suggesting it may reduce the extensive microthrombosis observed in COVID-19.
Baricitinib has other advantages including an oral once per day formulation, a predominantly renal route of clearance and low plasma protein binding. These properties suggested that baricitinib could be readily dosed with the antivirals being tested at the start of the pandemic, since they were largely cleared through liver metabolism. This enabled the testing of a combination with remdesivir in the ACTT-1 trial as well as with drugs being used as standard care.
3.1. Observational Clinical Trials
The first clinical test of baricitinib in COVID-19 was in four patients in Milan, all of whom recovered well [14]. Importantly, these patients underwent seroconversion while taking baricitinib, suggesting that this immunomodulator was unlikely to compromise the endogenous fight against infection. Almost simultaneously in Northern Italy, other hospitals were demonstrating that baricitinib appeared to reduce COVID-19 mortality. These and other small trials showed significant reductions in mortality and/or an improvement in lung function [17][18][19][20]. The next phase of validation was in propensity matched trials in Italy and Spain, which showed a substantial reduction in mortality associated with baricitinib treatment [16]. In 83 patients with moderate-severe SARS-CoV-2 pneumonia and including an aged cohort, baricitinib in the presence of the Standard of Care (SoC, which at the time included hydroxychloroquine, Kaletra, as well as glucocorticoids) caused a 71% reduction in mortality, with few drug-induced adverse events. Similarly, another propensity matched retrospective study showed a 48% reduction of mortality in patients over 70 years old [21].
These data helped to convince the National Institute for Allergy and Infectious Diseases of the NIH that baricitinib should be tested in a randomised trial. Since remdesivir had already shown a positive outcome in the ACTT-1 trial, (i.e., an increased rate of recovery with a 25% reduction of mortality by day 29 [22]), baricitinib was first tested in combination with remdesivir, comparing with the effect of remdesivir alone (ACTT-2). This was despite the fact that there has been some concern about the efficacy of remdesivir resulting in the WHO later not recommending its use in severe COVID-19 (a meta-analysis of four RCTs suggested no effect on mortality [23]).
3.2. Randomised Clinical Trials
The outcome of the ACTT-2 trial with over 1000 patients was a significant reduction in mortality and accelerated recovery in patients treated with baricitinib plus remdesivir compared with those treated with remdesivir alone. These effects were seen most strongly in those requiring supplemental oxygen (ordinal group 5) or non-invasive ventilation (ordinal group 6) at baseline. Both groups showed a 40% reduction in mortality [24]. Based on this and the aforementioned smaller non-randomised trials, the FDA issued an EUA authorising the use of this combination for the treatment of hospitalised COVID patients. Subsequently the CoV-BARRIER randomised trial reported the effect of baricitinib with standard of care (SoC) versus placebo with SoC [25]. Approximately 80% of the over 1500 patients in this trial were treated with dexamethasone, and 20% received remdesivir, as part of the SoC protocols in many institutions. Baricitinib had no effect on disease progression (primary end point) but, consistent with ACTT-2, reduced mortality by 38%. This life-saving effect was especially strong in those in ordinal scales 5 and 6 at baseline and was seen in those taking steroids or remdesivir or neither. Although it was considered possible that later stages of disease would be relatively resistant to such treatments, largely due to the amount of alveolar damage and hyaline membrane formation, in later analysis a 46% reduction in mortality was seen in patients receiving invasive mechanical ventilation (IMV) in this randomised trial [26]. It has been suggested [27] that remdesivir may have some synergistic effects with baricitinib, particularly on duration of disease, although the large life-saving effect of baricitinib is seen in the presence or absence of remdsivir [24][25]. Baricitinib is therefore the first immunomodulator to be shown to reduce COVID-19 mortality in a placebo-controlled trial [27]. Recently the RECOVERY trial examined the efficacy of baricitinib in comparing baricitinib plus SoC versus SoC in more than 8000 patients. In this trial, the mortality was significantly less than that seen in earlier RECOVERY trials, probably reflecting improved care and the widespread use of glucocorticoids (95%), remdesivir (20%), and tocilizumab (32%) in these patients. Despite this, baricitinib significantly reduced mortality (HR 0.87), with an approximate 25% reduction in mortality in those with severe disease (requiring ventilation at baseline) [28]. The effect on mortality was approximately half that seen in the previous trials, almost certainly because of the co-medications, although this interpretation awaits publication of the final data.
Studies of new therapies in at-risk individuals with early stage COVID-19 (prehospitalisation) have been difficult to execute because of the need for strict patient isolation, which precludes careful monitoring. Advanced contactless monitoring methods are being developed to enable such trials to be undertaken [29], including, for example, using combinations of effective oral antivirals such as nirmatrelvir/ritonavir (Paxlovid) with oral agents that limit inflammation-induced damage, such as baricitinib, in those whose risk is high due to disease or age.
It is probable that this approach of targeting the host pathways subverted by the virus could be applied to other infections. For instance, flavivirus (e.g., Dengue) infections also show an early relatively mild disease which, in some cases, is followed by an aberrant hyperinflammation with vasculopathy. The Dengue viruses (and their NS1 proteins) access cells predominantly through CME [30], so anti-inflammatory agents which also inhibit CME (such as baricitinib) could be useful in these infections.
3.3. Baricitinib Safety
Long-term treatment (months to years) with JAK inhibitors is associated with significant side effects in a small number of patients [31]. These include an increased propensity for venous thromboembolisms and Herpes infections. However, in the clinical trials assessed for European Medicines Agency registration (which covered 4214 patient years of dosing), the most significant side-effect seen was a small increase in upper respiratory tract infections (similar to those observed with methotrexate). The incidence of serious infections (including Herpes zoster) over 52 weeks dosing was small (3.2 per 100 patient-years), and similar to placebo [32]. However, even these side effects were considered unlikely given the short duration of dosing required in COVID-19 treatments, and no such safety signals have since been observed in baricitinib COVID-19 trials. There were significantly reduced infections in the baricitinib arm in the ACTT-2 trial [24], while two other RCT reports showed no differences in adverse events between the baricitinib and control arms [25][33]. In the large RECOVERY trial there were no significant increases in thrombosis, secondary infections, or other safety outcomes in those treated with baricitinib and a significant reduction in mortality was observed [28]. Similarly, baricitinib was shown to cause significantly fewer adverse events in the ACTT-4 trial when compared with dexamethasone, both in the presence of remdesivir [34]. These small improvements in safety were also seen in a meta-analysis covering 3564 patients in a mixture of RCT and observational trials where serious adverse events and secondary infections were approximately 20% fewer in those treated with baricitinib [33]. It can therefore be concluded that baricitinib is a safe treatment for those hospitalized with COVID-19.
References
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776.
- Wang, Y.; Perlman, S. COVID-19: Inflammatory Profile. Annu. Rev. Med. 2022, 73, 65–80.
- Vora, S.M.; Lieberman, J.; Wu, H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021, 21, 694–703.
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30.
- Paliwal, S.; de Giorgio, A.; Neil, D.; Michel, J.-B.; Lacoste, A.M. Preclinical validation of therapeutic targets predicted by tensor factorization on heterogeneous graph. Sci. Rep. 2020, 10, 18250.
- Smith, D.P.; Oechsle, O.; Rawling, M.J.; Savory, E.; Lacoste, A.M.B.; Richardson, P.J. Expert-Augmented Computational Drug Repurposing Identified Baricitinib as a Treatment for COVID-19. Front. Pharmacol. 2021, 12, 709856.
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 infects cells following viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021, 296, 100306.
- Gorshkov, K.; Susumu, K.; Jiji Chen, J.; Xu, M.; Pradhan, M.; Zhu, W.; Hu, X.; Breger, J.C.; Wolak, M.; Oh, E. Quantum Dot-Conjugated SARS-CoV-2 Spike Pseudo-Virions Enable Tracking of Angiotensin Converting Enzyme 2 Binding and Endocytosis. ACS Nano 2020, 14, 12234–12247.
- Tanaka, Y.; Luo, Y.; O’Shea, J.J.; Nakayamada, S. Janus kinase-targeting therapies in rheumatology: A mechanisms-based approach. Nat. Rev. Rheumatol. 2022, 5, 1–13.
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020, 20, 400–402.
- Bekerman, E.; Neveu, G.; Shulla, A.; Brannan, J.; Pu, S.Y.; Wang, S.; Xiao, F.; Barouch-Bentov, R.; Bakken, R.R.; Mateo, R.; et al. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J. Clin. Investig. 2017, 127, 1338–1352.
- Pu, S.-Y.; Xiao, F.; Schor, S.; Bekerman, E.; Zanini, F.; Barouch-Bentov, R.; Nagamine, C.M.; Einav, S. Feasibility and biological rationale of repurposing sunitinib and erlotinib for dengue treatment. Antivir. Res. 2018, 155, 67–75.
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Rawling, M.; Savory, E.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, e30–e31.
- Stebbing, J.; Krishnan, V.; de Bono, S.; Ottaviani, S.; Casalini, G.; Richardson, P.J.; Monteil, V.; Lauschke, V.M.; Mirazimi, A.; Youhanna, S.; et al. Sacco Baricitinib Study Group, Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol. Med. 2020, 12, e12697.
- D’Alessandro, M.; Perillo, F.; Metella Refini, R.; Bergantini, L.; Bellisai, F.; Selvi, E.; Cameli, P.; Manganelli, S.; Conticini, E.; Cantarini, L.; et al. Efficacy of baricitinib in treating rheumatoid arthritis: Modulatory effects on fibrotic and inflammatory biomarkers in a real-life setting. Int. Immunopharmacol. 2020, 86, 106748.
- Stebbing, J.; Sanchez Nievas, G.; Falcone, M.; Youhanna, S.; Richardson, P.; Ottaviani, S.; Shen, J.X.; Sommerauer, C.; Tiseo, G.; Ghiadoni, L.; et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci. Adv. 2021, 7.
- Bronte, V.; Ugel, S.; Tinazzi, E.; Vella, A.; De Sanctis, F.; Canè, S.; Batani, V.; Trovato, R.; Fiore, A.; Petrova, V.; et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J. Clin. Investig. 2020, 130, 6409–6416.
- Cantini, F.; Niccoli, L.; Nannini, C.; Matarrese, D.; Di Natale, M.E.; Lotti, P.; Aquilini, D.; Landini, G.; Cimolato, B.; Di Pietro, M.A.; et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 2020, 81, 647–679.
- Titanji, B.K.; Farley, M.M.; Mehta, A.; Connor-Schuler, R.; Moanna, A.; Cribbs, S.K.; O’Shea, J.; DeSilva, K.; Chan, B.; Edwards, A.; et al. Use of Baricitinib in Patients with Moderate and Severe COVID-19. Clin. Infect. Dis. 2020, 72, ciaa879.
- Rodriguez-Garcia, J.L.; Sanchez-Nievas, G.; Arevalo-Serrano, J.; Garcia-Gomez, C.; Jimenez-Vizuete, J.M.; Martinez-Alfaro, E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: An observational cohort study. Rheumatology 2021, 60, 399–407.
- Abizanda, P.; Mayo, J.M.C.; Marta Mas Romer, M.M.; Cortés Zamora, E.B.; Tabernero Sahuquillo, M.T.; Romero Rizos, L.; Sánchez-Jurado, P.M.; Sánchez-Nievas, G.; Campayo Escolano, C.; Ochoa Serrano, A.; et al. Baricitinib reduces 30-day mortality in older adults with moderate-to-severe COVID-19 pneumonia. J. Am. Geriatr. Soc. 2021, 69, 2752–2758.
- Beigel, J.H.; Tomashek, K.M.; Lori E Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826.
- Piscoya, A.; Ng-Sueng, L.F.; Parra Del Riego, A.; Cerna-Viacava, R.; Pasupuleti, V.; Roman, Y.M.; Thota, P.; White, C.M.; Hernandez, A.V. Efficacy and harms of remdesivir for the treatment of COVID-19: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0243705.
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. ACTT-2 Study Group Members. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N. Engl. J. Med. 2021, 384, 795–807.
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; Kartman, C.E.; Krishnan, V.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Alatorre-Alexander, J.; de Cassia Pellegrini, R.; et al. COV-BARRIER Study Group. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 1407–1418.
- Ely, E.W.; Ramanan, A.V.; Kartman, C.E.; de Bono, S.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Saraiva, J.F.K.; Chakladar, S.; Marconi, V.C.; et al. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: An exploratory, randomised, placebo-controlled trial. Lancet Respir. Med. 2022, 10, 327–336.
- Kalil, A.C.; Stebbing, J. Baricitinib: The first immunomodulatory treatment to reduce COVID-19 mortality in a placebo-controlled trial. Lancet Respir. Med. 2021, 9, 1349–1351.
- Horby, P.W.; Emberson, J.R.; Mafham, M.; Campbell, M.; Peto, L.; Pessoa-Amorim, G.; Spata, E.; Staplin, N.; Lowe, C.; Chadwick, D.R.; et al. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial and updated meta-analysis. MedRxiv 2022.
- Robinson, B.W.S.; Tai, A.; Springer, K. Why we still need drugs for COVID-19 and can’t just rely on vaccines. Respirology 2022, 27, 109–111.
- Blaas, D. Viral entry pathways: The example of common cold viruses. Wien Med. Wochenschr. 2016, 166, 211–226.
- Sunzini, F.; McInnes, I.; Siebert, S. JAK inhibitors and infections risk: Focus on herpes zoster. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20936059.
- European Medicines Agency. Olumiant: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/productinformation/olumiant-epar-product-information_en.pdf (accessed on 24 February 2020).
- Lin, Z.; Niu, J.; Xu, Y.; Qin, L.; Ding, J.; Zhou, L. Clinical efficacy and adverse events of baricitinib treatment for coronavirus disease-2019 (COVID-19): A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 1523–1534.
- Wolfe, C.R.; Tomashek, K.M.; Patterson, T.F.; Gomez, C.A.; Marconi, V.C.; Jain, M.K.; Yang, O.O.; Paules, C.I.; Palacios, G.M.R.; Grossberg, R.; et al. Baricitinib versus dexamethasone for adults hospitalised with COVID-19 (ACTT-4): A randomised, double-blind, double placebo-controlled trial. Lancet Resp. Med. 2022.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
922
Entry Collection:
COVID-19
Revisions:
2 times
(View History)
Update Date:
01 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




