Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stefanie Woodard | -- | 1601 | 2022-06-30 01:24:50 | | | |
| 2 | Conner Chen | -25 word(s) | 1576 | 2022-06-30 06:19:57 | | | | |
| 3 | Conner Chen | Meta information modification | 1576 | 2022-07-08 09:21:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bennett, C.; Carroll, C.; Wright, C.; Awad, B.; Park, J.M.; Farmer, M.; Brown, E.(.; Heatherly, A.; Woodard, S. Breast Cancer and Genetic Alterations. Encyclopedia. Available online: https://encyclopedia.pub/entry/24649 (accessed on 07 February 2026).
Bennett C, Carroll C, Wright C, Awad B, Park JM, Farmer M, et al. Breast Cancer and Genetic Alterations. Encyclopedia. Available at: https://encyclopedia.pub/entry/24649. Accessed February 07, 2026.
Bennett, Caroline, Caleb Carroll, Cooper Wright, Barbara Awad, Jeong Mi Park, Meagan Farmer, Elizabeth (Bryce) Brown, Alexis Heatherly, Stefanie Woodard. "Breast Cancer and Genetic Alterations" Encyclopedia, https://encyclopedia.pub/entry/24649 (accessed February 07, 2026).
Bennett, C., Carroll, C., Wright, C., Awad, B., Park, J.M., Farmer, M., Brown, E.(., Heatherly, A., & Woodard, S. (2022, June 30). Breast Cancer and Genetic Alterations. In Encyclopedia. https://encyclopedia.pub/entry/24649
Bennett, Caroline, et al. "Breast Cancer and Genetic Alterations." Encyclopedia. Web. 30 June, 2022.
Copy Citation
Breast cancer is a heterogeneous disease, and numerous associated genetic alterations have been identified.
primary breast cancer
metastatic breast cancer
driver mutations
1. Introduction
In 2021, breast cancer (BC) accounted for 30% of all cancers diagnosed in women and was the leading cause of cancer death in the United States among women aged 20–59 years [1]. In fact, one in eight US women will be diagnosed with invasive BC at some point in their life [1]. Although BC death rates have decreased by 41% from 1989 to 2018, its incidence continues to rise at a rate of 0.5% per year, partially due to increases in body weight and declines in fertility rate [1][2][3]. Implementation of more robust population-based screening mammography programs and the shift from film mammography to more sensitive digital mammography may have also contributed to this rise [4][5][6].
Although highly prevalent and heavily researched, many of the mechanisms underlying BC pathogenesis are still debated. Two models for BC pathogenesis, the sporadic clonal evolution model and the cancer stem cell model, have been proposed. In the sporadic clonal evolution model, random mutations can occur in any breast epithelial cell, and the cells that acquire advantageous genetic and epigenetic alterations are selected over time, eventually leading to tumor development. Alternatively, the cancer stem cell model argues that only a small subset of tumor cells, the stem and progenitor cells, can initiate and sustain tumor development. However, it has also been suggested that these two models may occur in concert via clonal evolution of stem cells [7]. A myriad of modifiable and non-modifiable risk factors may contribute to these changes. A few examples include obesity [8], chest radiation therapy, excessive alcohol consumption, diethylstilbestrol exposure, hormonal contraceptives, hormone replacement therapy after menopause, heritable gene mutations (such as inactivating mutations in TP53 in Li-Fraumeni syndrome and truncating PTEN mutations in Cowden syndrome), and even mutations in mitochondrial DNA [9][10]. Although most BC is considered sporadic, approximately 5–10% of BC is due to these underlying hereditary causes, most often resulting from pathogenic variants or mutations in the BRCA1 and BRCA2 genes [11].
Since the discovery of the role of BRCA1 and BRCA2 in hereditary BC, successful germline analysis of BRCA1, BRCA2, and other BC susceptibility genes has provided the foundation for genetics’ utility in screening, cancer risk reduction, and treatment [12]. With the advent of next generation sequencing, thousands of primary BC tumors have been sequenced, expanding knowledge of BC’s genomic landscape. This new information has helped to identify previously unrecognized mutations [13]. However, differentiating driver mutations, which impart growth advantage and promote tumorigenesis, from non-pathogenic passenger mutations (Figure 1) is necessary for the development of individualized treatment [14][15]. Given the scarcity of high-frequency driver mutations and the vast amount of genomic information now available, identifying clinically significant and targetable mutations is a primary challenge [16][17].
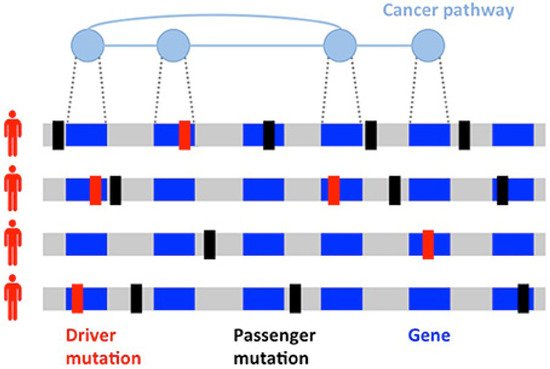
Figure 1. Heterogeneity of cancers. Driver (red) and passenger (black) mutations in the development of a tumor. Driver mutations are much less common than passenger mutations and involve genes implicated in cancer pathways.
Of those diagnosed with primary BC, 20–30% will develop distant metastasis. According to one SEER-based study, frequency of BC metastasis to the bone, lung, liver, and brain were 30–60% (bone), 21–32% (lung), 15–32% (liver), and 4–10% (brain) [18]. Moreover, the incidence of rare site BC metastasis continues to rise as a result of better imaging techniques and treatment advancements, which prolong survival [19]. Although metastasis is the leading cause of death for BC patients, BC diagnosed before distant metastasis occurs has a much better prognosis [13][20]. Metastases arise from primary cancer subclones and are more heterogenous, requiring specific treatment management and targets [13]. These statistics and knowledge underscore the importance in recognizing not only frequently implicated BC gene mutations but also lesser-known primary and metastatic BC driver mutations and their potential therapy targets.
2. Organotropism: Breast Cancer Molecular Subtypes
Efforts to better predict tumor behavior and improve BC treatment led to the classification of BC by molecular subtypes, which include luminal ER+ (luminal A and luminal B), human epidermal growth factor receptor 2 (HER2)-enriched, and basal-like [21][22]. Gene expression studies have shown that the different molecular subtypes vary markedly in not only prognosis but also in therapeutic targets [10]. In 1889, Stephen Paget postulated that BC metastasis was nonrandom, due to tumor cells (the seeds) having a predilection for specific organ sites (the soil) [23]. It has since been found that the different molecular subtypes demonstrate a propensity for specific organ sites [24]. Kennecke et al. also demonstrated that certain BC subtypes are associated with spread to specific organs, underscoring the critical role each “seed” plays in metastasis [25]. For example, bone metastasis is most common in luminal A and B subtypes, but the least common site in basal subtype [25]. The frequency of BC metastasis to the brain is most common in HER2-enriched (28.76%), basal-like (25.23%), and triple negative non-basal (22.06%) subtypes [25]. Liver metastasis is more common in the HER2-enriched compared to HER2-negative subtype [25]. One SEER-based study found that lung metastasis was more common in basal-like/triple-negative BCs (32.09%) compared to HR+/HER2−, HR+/HER2+, and HR−/HER+ BCs [18]. However, the factors influencing the timing and mechanisms involved in specific BC molecular subtype metastasis to the lung are not yet understood [26]. Aside from the molecular subtypes (Figure 2), the accumulation of mutations in cancer cells can alter critical genes and pathways and promote organ-specific metastasis [27]. As the genomic landscape of primary BC continues to be explored, the primary BC genetic alterations that promote organ-specific metastasis and survival in new immune and metabolic microenvironments continue to be elucidated.
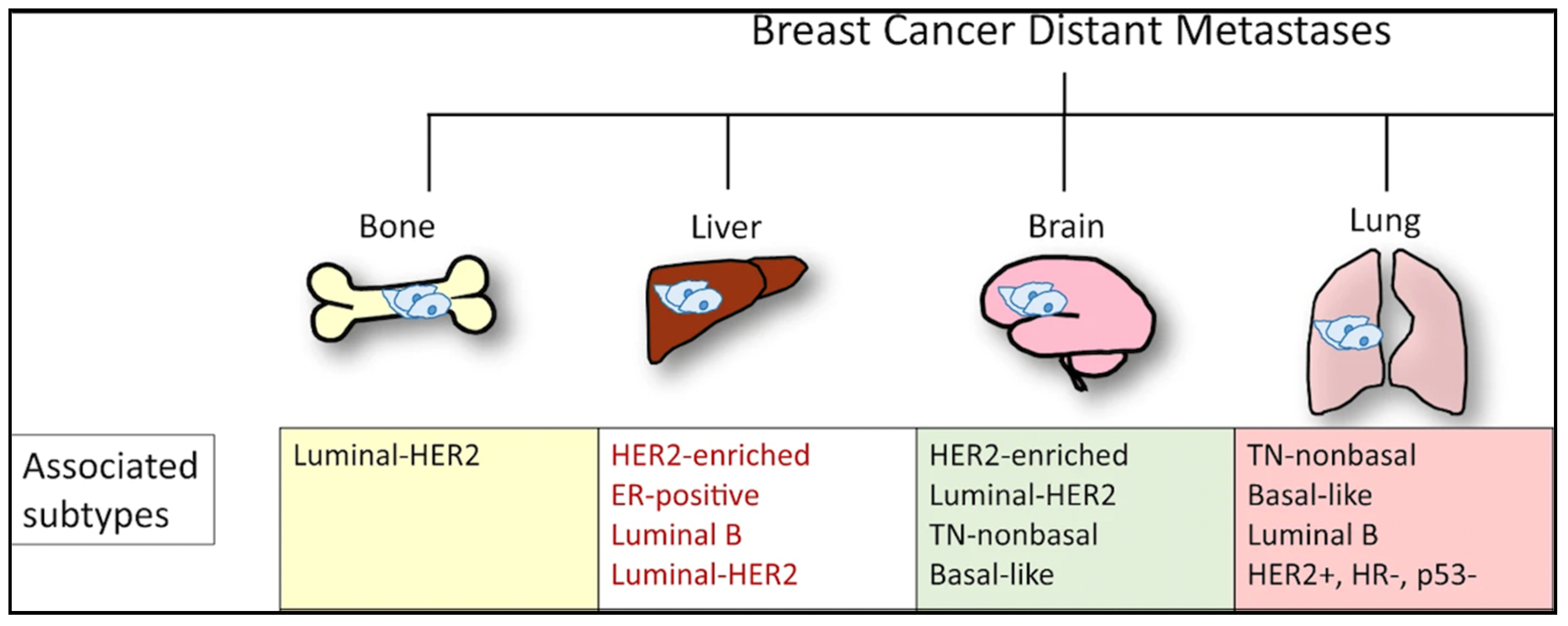
Figure 2. Molecular subtypes of BC and sites of the most commonly reported metastases.
3. Clinical Presentation and Detection of Breast Cancer
A palpable breast lump is the most commonly presenting symptom for BC, with one study finding that a breast lump was the presenting sign in 92.5% of the 8639 female BC patients evaluated [28]. Other signs and symptoms include nipple abnormalities (such as discharge or retraction), breast pain, skin changes, and, less commonly, back pain and weight loss [29][30]. Screen-detected BCs are associated with a strong survival advantage compared to symptomatic BC [31][32]. Even when accounting for lead-time and length-time bias, this advantage may be partly attributed to intrinsic biological differences between screen-detected and symptomatic BCs [32][33]. Screen-detected BCs are primarily of the less aggressive Luminal A subtype, whereas Luminal B, basal-like, and HER2-enriched subtypes are much less common [34]. Triple negative BCs, which comprise much of the basal-like BC molecular subtype, are more likely than other BCs to be detected symptomatically, in the interval between regular mammogram screenings [35][36]. These interval cancers tend to be more aggressive, larger in diameter, of higher stage and grade, and with a higher number of positive nodes compared to screen-detected cancers. They are also more likely to present with other unfavorable prognostic features such as hormone receptor negativity [37].
4. Clinical Implications: Successes of Targeted Therapy
Past and recent success of targeted molecular therapy heralds promise for the successful development and implementation of other therapies focused on specific genes, proteins, and pathways implicated in primary and metastatic BC. Prior to the approval of trastuzumab in 1998, the only available treatment for metastatic HER2+ invasive BC was traditional chemotherapy regimens [38]. Trastuzumab combined with chemotherapy has resulted in greatly improved survival rates in the historically aggressive HER2+ BC subtype [39][40]. Moreover, the α-specific PI3K inhibitor alpelisib combined with the estrogen receptor antagonist fulvestrant has demonstrated itself effective in the treatment of BC with PIK3CA mutations. These mutations are common in ER+/HER2- metastatic BC, with about 40% of HR+/HER2− BC patients having an activating mutation in the PIK3CA gene [41][42]. In a phase 3 clinical trial, patients with HR+/HER2−, PIK3CA-mutated BC who received alpelisib–fulvestrant demonstrated statistically significant and clinically meaningful progression-free survival, compared to those with HR+/HER2−, PIK3CA-mutated BC who received placebo–fulvestrant [43]. The development of alpelisib is critical, since many HR+ BCs develop endocrine resistance during treatment. The frequency of alterations in the MAPK signaling pathway (notably, loss-of-function mutations in NF1 and gain-of-function mutations in ERBB2) in addition to transcription-associated alterations (for example, MYC amplifications and CTCF and FOXA1 alterations) increase following endocrine treatment [44]. Mutation of the ligand-binding domain of Estrogen Receptor 1 (ESR1) encoding estrogen receptor α is the chief mechanism of resistance, particularly to aromatase inhibitors [45]. This recognition is important, considering that the majority of BCs are ER+ and, although many of these patients enter remission, half will relapse or progress to incurable metastatic disease. Compared to endocrine therapy alone, the CD4/6 inhibitors palbociclib, ribociclib, and abemaciclib, and the mTOR inhibitor, everolimus, show improved progression-free survival following metastasis when combined with endocrine therapy [46][47][48][49][50][51]. Additionally, utilization of the PARP inhibitors olaparib and talazoparib for germline BRCA mutations carriers with metastatic HER2− BC showed significant improvement in progression-free survival and health-related quality of life (although not overall survival), compared to chemotherapy alone [52][53][54]. With the success of these treatments, a precedent has been set for the discovery of other actionable and commonly shared BC drug targets [9]. Table 1 provides some of these therapies, mechanisms, targets, and advancements.
Table 1. Select successful targeted BC therapies, mechanisms, targets, and treatment advancements.
| Therapy | Mechanism | BC Molecular Subtype Target | Advancement |
|---|---|---|---|
| Trastuzumab | HER2 receptor inhibitor | HER2+ | Improved survival |
| Alpelisib | α-specific PI3K inhibitor | HR+/HER2− metastatic BCs with PIK3CA mutation | IPFS, when combined with fulvestrant |
| Palbociclib | CD4/6 inhibitor | HR+/HER2− | IPFS following metastasis, when combined with endocrine therapy |
| Ribociclib | CD4/6 inhibitor | HR+/HER2− | IPFS following metastasis, when combined with endocrine therapy |
| Abemaciclib | CD4/6 inhibitor | HR+/HER2− | IPFS following metastasis, when combined with endocrine therapy |
| Everolimus | mTOR inhibitor | HR+/HER2− metastatic BC | IPFS following metastasis, when combined with endocrine therapy |
| Olaparib | PARP inhibitor | HER2− metastatic BCs in germline BRCA mutation carriers | IPFS and health-related quality of life |
| Talazoparib | PARP inhibitor | HER2− metastatic BC (in germline BRCA mutation carriers) | IPFS and health-related quality of life |
IPFS = improved progression-free survival.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30.
- Pfeiffer, R.M.; Webb-Vargas, Y.; Wheeler, W.; Gail, M.H. Proportion of U.S. Trends in Breast Cancer Incidence Attributable to Long-term Changes in Risk Factor Distributions. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1214–1222.
- Harding, C.; Pompei, F.; Burmistrov, D.; Welch, H.G.; Abebe, R.; Wilson, R. Breast Cancer Screening, Incidence, and Mortality Across US Counties. JAMA Intern. Med. 2015, 175, 1483–1489.
- Bleyer, A.; Welch, H.G. Effect of three decades of screening mammography on breast-cancer incidence. N. Engl. J. Med. 2012, 367, 1998–2005.
- Fletcher, S.W. Breast cancer screening: A 35-year perspective. Epidemiol. Rev. 2011, 33, 165–175.
- Bombonati, A.; Sgroi, D.C. The molecular pathology of breast cancer progression. J. Pathol. 2011, 223, 307–317.
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Liu, J.; Reshetov, I.V.; Sukocheva, O.A. Advances in prevention and treatment of obesity-driven effects in breast cancers. Front. Oncol. 2021, 2663.
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2020, 83, 556–569.
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106.
- Liu, L.; Hao, X.; Song, Z.; Zhi, X.; Zhang, S.; Zhang, J. Correlation between family history and characteristics of breast cancer. Sci. Rep. 2021, 11, 6360.
- Couch, F.J.; Nathanson, K.L.; Offit, K. Two decades after BRCA: Setting paradigms in personalized cancer care and prevention. Science 2014, 343, 1466–1470.
- Yates, L.R.; Gerstung, M.; Knappskog, S.; Desmedt, C.; Gundem, G.; Van Loo, P.; Aas, T.; Alexandrov, L.B.; Larsimont, D.; Davies, H.; et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat. Med. 2015, 21, 751–759.
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352.
- Stratton, M.R.; Campbell, P.J.; Futreal, P.A. The cancer genome. Nature 2009, 458, 719–724.
- Low, S.K.; Zembutsu, H.; Nakamura, Y. Breast cancer: The translation of big genomic data to cancer precision medicine. Cancer Sci. 2018, 109, 497–506.
- Luen, S.; Virassamy, B.; Savas, P.; Salgado, R.; Loi, S. The genomic landscape of breast cancer and its interaction with host immunity. Breast 2016, 29, 241–250.
- Wu, Q.; Li, J.; Zhu, S.; Wu, J.; Chen, C.; Liu, Q.; Wei, W.; Zhang, Y.; Sun, S. Breast cancer subtypes predict the preferential site of distant metastases: A SEER based study. Oncotarget 2017, 8, 27990–27996.
- Di Micco, R.; Santurro, L.; Gasparri, M.L.; Zuber, V.; Fiacco, E.; Gazzetta, G.; Smart, C.E.; Valentini, A.; Gentilini, O.D. Rare sites of breast cancer metastasis: A review. Transl. Cancer Res. 2019, 8, S518–S552.
- Bertucci, F.; Ng, C.K.Y.; Patsouris, A.; Droin, N.; Piscuoglio, S.; Carbuccia, N.; Soria, J.C.; Dien, A.T.; Adnani, Y.; Kamal, M.; et al. Genomic characterization of metastatic breast cancers. Nature 2019, 569, 560–564.
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752.
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874.
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989, 8, 98–101.
- Wang, C.; Xu, K.; Wang, R.; Han, X.; Tang, J.; Guan, X. Heterogeneity of BCSCs contributes to the metastatic organotropism of breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 370.
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277.
- Medeiros, B.; Allan, A.L. Molecular Mechanisms of Breast Cancer Metastasis to the Lung: Clinical and Experimental Perspectives. Int. J. Mol. Sci. 2019, 20, 2272.
- Chen, W.; Hoffmann, A.D.; Liu, H.; Liu, X. Organotropism: New insights into molecular mechanisms of breast cancer metastasis. NPJ Precis. Oncol. 2018, 2, 4.
- Redaniel, M.T.; Martin, R.M.; Ridd, M.J.; Wade, J.; Jeffreys, M. Diagnostic intervals and its association with breast, prostate, lung and colorectal cancer survival in England: Historical cohort study using the Clinical Practice Research Datalink. PLoS ONE 2015, 10, e0126608.
- Koo, M.M.; von Wagner, C.; Abel, G.A.; McPhail, S.; Rubin, G.P.; Lyratzopoulos, G. Typical and atypical presenting symptoms of breast cancer and their associations with diagnostic intervals: Evidence from a national audit of cancer diagnosis. Cancer Epidemiol. 2017, 48, 140–146.
- NICE. 2021 Exceptional Surveillance of Suspected Cancer: Recognition and Referral (NICE Guideline NG12); NICE: London, UK, 2021.
- Mook, S.; Van’t Veer, L.J.; Rutgers, E.J.; Ravdin, P.M.; van de Velde, A.O.; van Leeuwen, F.E.; Visser, O.; Schmidt, M.K. Independent prognostic value of screen detection in invasive breast cancer. J. Natl. Cancer Inst. 2011, 103, 585–597.
- Wishart, G.C.; Greenberg, D.C.; Britton, P.D.; Chou, P.; Brown, C.H.; Purushotham, A.D.; Duffy, S.W. Screen-detected vs symptomatic breast cancer: Is improved survival due to stage migration alone? Br. J. Cancer 2008, 98, 1741–1744.
- Irvin, V.L.; Zhang, Z.; Simon, M.S.; Chlebowski, R.T.; Luoh, S.W.; Shadyab, A.H.; Krok-Schoen, J.L.; Tabung, F.K.; Qi, L.; Stefanick, M.L.; et al. Comparison of Mortality Among Participants of Women’s Health Initiative Trials With Screening-Detected Breast Cancers vs Interval Breast Cancers. JAMA Netw. Open 2020, 3, e207227.
- Farshid, G.; Walters, D. Molecular subtypes of screen-detected breast cancer. Breast Cancer Res. Treat. 2018, 172, 191–199.
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434.
- Collett, K.; Stefansson, I.M.; Eide, J.; Braaten, A.; Wang, H.; Eide, G.E.; Thoresen, S.O.; Foulkes, W.D.; Akslen, L.A. A basal epithelial phenotype is more frequent in interval breast cancers compared with screen detected tumors. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1108–1112.
- Kirsh, V.A.; Chiarelli, A.M.; Edwards, S.A.; O’Malley, F.P.; Shumak, R.S.; Yaffe, M.J.; Boyd, N.F. Tumor characteristics associated with mammographic detection of breast cancer in the Ontario breast screening program. J. Natl. Cancer Inst. 2011, 103, 942–950.
- Patel, A.; Unni, N.; Peng, Y. The Changing Paradigm for the Treatment of HER2-Positive Breast Cancer. Cancers 2020, 12, 2081.
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792.
- Martinez-Saez, O.; Prat, A. Current and Future Management of HER2-Positive Metastatic Breast Cancer. JCO Oncol. Pract. 2021, 17, 594–604.
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713.
- van Geelen, C.T.; Savas, P.; Teo, Z.L.; Luen, S.J.; Weng, C.F.; Ko, Y.A.; Kuykhoven, K.S.; Caramia, F.; Salgado, R.; Francis, P.A.; et al. Clinical implications of prospective genomic profiling of metastatic breast cancer patients. Breast Cancer Res. 2020, 22, 91.
- Andre, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217.
- Razavi, P.; Chang, M.T.; Xu, G.; Bandlamudi, C.; Ross, D.S.; Vasan, N.; Cai, Y.; Bielski, C.M.; Donoghue, M.T.A.; Jonsson, P.; et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell 2018, 34, 427–438.e426.
- Hermida-Prado, F.; Jeselsohn, R. The ESR1 Mutations: From Bedside to Bench to Bedside. Cancer Res. 2021, 81, 537–538.
- Finn, R.S.; Boer, K.; Bondarenko, I.; Patel, R.; Pinter, T.; Schmidt, M.; Shparyk, Y.V.; Thummala, A.; Voitko, N.; Bananis, E.; et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res. Treat. 2020, 183, 419–428.
- Rugo, H.S.; Finn, R.S.; Dieras, V.; Ettl, J.; Lipatov, O.; Joy, A.A.; Harbeck, N.; Castrellon, A.; Iyer, S.; Lu, D.R.; et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 2019, 174, 719–729.
- Turner, N.C.; Ro, J.; Andre, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219.
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950.
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019, 5, 5.
- Dustin, D.; Gu, G.; Fuqua, S.A.W. ESR1 mutations in breast cancer. Cancer 2019, 125, 3714–3728.
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Goncalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763.
- Robson, M.; Goessl, C.; Domchek, S. Olaparib for Metastatic Germline BRCA-Mutated Breast Cancer. N. Engl. J. Med. 2017, 377, 1792–1793.
- Tung, N.; Garber, J.E. PARP inhibition in breast cancer: Progress made and future hopes. Npj Breast Cancer 2022, 8, 47.
More
Information
Subjects:
Genetics & Heredity
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
3 times
(View History)
Update Date:
08 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




