Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jack Chen | -- | 2075 | 2022-06-29 18:05:09 | | | |
| 2 | Catherine Yang | Meta information modification | 2075 | 2022-06-30 03:01:35 | | | | |
| 3 | Catherine Yang | Meta information modification | 2075 | 2022-07-01 05:04:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, J.; Lynn, E.G.; Yousof, T.R.; Sharma, H.; Macdonald, M.E.; Byun, J.H.; Shayegan, B.; Austin, R.C. Targeting Cell Surface GRP78 in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/24646 (accessed on 08 March 2026).
Chen J, Lynn EG, Yousof TR, Sharma H, Macdonald ME, Byun JH, et al. Targeting Cell Surface GRP78 in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/24646. Accessed March 08, 2026.
Chen, Jack, Edward G. Lynn, Tamana R. Yousof, Hitesh Sharma, Melissa E. Macdonald, Jae Hyun Byun, Bobby Shayegan, Richard C. Austin. "Targeting Cell Surface GRP78 in Cancer" Encyclopedia, https://encyclopedia.pub/entry/24646 (accessed March 08, 2026).
Chen, J., Lynn, E.G., Yousof, T.R., Sharma, H., Macdonald, M.E., Byun, J.H., Shayegan, B., & Austin, R.C. (2022, June 29). Targeting Cell Surface GRP78 in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/24646
Chen, Jack, et al. "Targeting Cell Surface GRP78 in Cancer." Encyclopedia. Web. 29 June, 2022.
Copy Citation
The 78-kDa glucose-regulated protein (GRP78) is an endoplasmic reticulum (ER)-resident molecular chaperone that plays a crucial role in protein folding homeostasis by regulating the unfolded protein response (UPR). In tumour cells, GRP78 is present at the cell surface, where it functions as a signalling receptor involved in numerous proapoptotic and apoptotic pathways that contribute to cancer cell proliferation and metastasis. As such, novel therapeutic strategies that target cell surface GRP78 in the treatment of several human cancers is highlighted.
GRP78
cancer
autoantibody
cell surface
tumour
1. Introduction
The endoplasmic reticulum (ER) is a complex organelle responsible for protein synthesis and folding, the storage of intracellular Ca2+ and lipid metabolism [1][2]. ER-resident chaperones facilitate the capacity of the ER for protein folding and prevent the aggregation of misfolded polypeptides [3]. Within the ER, the 78-kDa glucose-regulated protein (GRP78, also known as BiP; HSPA5 gene) assists in folding and the quality control of nascent polypeptides by binding to exposed hydrophobic motifs on misfolded polypeptides in an ATP-dependent manner (Figure 1) [4]. Furthermore, ER-resident GRP78 is a vital modulator of oxidative stress, lipotoxicity, inflammation, ER Ca2+ depletion, glucose deprivation, hypoxia and viral infection, which all can disrupt ER homeostasis and lead to an accumulation of misfolded and unfolded proteins, a condition known as ER stress [5][6][7][8][9][10][11]. To mitigate ER stress, GRP78 dissociates from protein kinase RNA-like ER kinase (PERK), inositol-requiring protein 1α (IRE1α) and activating transcription factor 6 (ATF6), provoking a dynamic signalling cascade known as the unfolded protein response (UPR) [10][11]. Activation of the UPR leads to the inhibition of de novo protein synthesis, the degradation of misfolded ER proteins and the upregulation of protein chaperone expression [12]. In cases where ER stress is insufficiently mitigated, chronic UPR activation can lead to the upregulation of proapoptotic signalling [13].
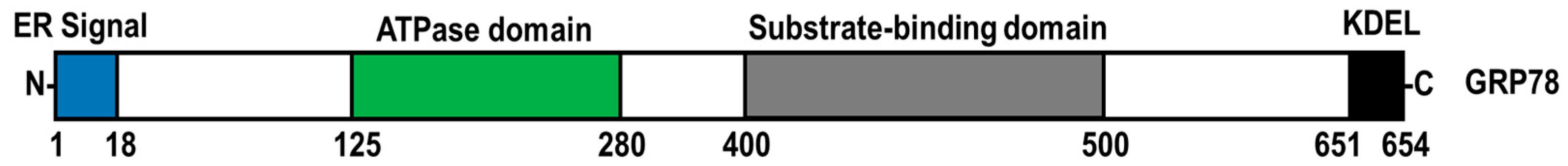
Figure 1. Functional domain structure of GRP78. GRP78 is composed of an ER signal sequence, ATPase domain, substrate-binding domain, and a C-terminal KDEL tetrapeptide sequence.
Highly proliferative tumour microenvironments are hypoxic and glucose-deprived, negatively impacting protein folding and resulting in ER stress and elevated GRP78 expression [14][15]. Upregulated GRP78 expression is associated with tumour proliferation, metastasis, neovascularization, and poorer prognosis in cancer patients [16][17]. Although GRP78 has classically been viewed as an ER-resident molecular chaperone, it has been reported in several other cellular organelles, including the nucleus and the mitochondria [18][19]. Additionally, GRP78 has been observed on the cell surface of several human cancers, including prostate cancer, breast cancer, ovarian cancer, brain cancer, melanoma cancer, leukemia and lymphoma, where it functions as a signalling receptor in cell proliferation as well as apoptotic pathways [20][21][22][23][24]. Furthermore, the expression of cell surface GRP78 (csGRP78) induces a humoral response that leads to the generation of anti-GRP78 autoantibodies in patients with cancer, which is associated with disease progression, elevated risk of metastasis, and reduced overall survival [25].
2. csGRP78 as a Therapeutic Target
Targeting tumour-specific surface antigens is a promising therapeutic approach that has been used in anti-cancer treatment due to its potential to mitigate non-specific side effects associated with contemporary cancer therapies [26]. csGRP78 is expressed predominantly in malignant tumour cells and not normal cells; therefore, targeting csGRP78 can be exploited as a novel therapeutic strategy for cancer treatment. Targeting csGRP78 has been studied in cancer imaging as a means to improve cancer diagnoses. Several promising small peptides, chimeric antigen receptor (CAR) T cells, monoclonal antibodies (MAb), and single-chain variable fragments (scFvs) against csGRP78 have been shown to target tumour cells and attenuate tumour cell progression [27].
2.1. GRP78-Binding Small Peptides
The use of small peptides is an innovative approach for the treatment of cancer because short amino acid chains can easily be synthesized with high specificity for cancer-specific targets such as csGRP78 [28]. Arap and colleagues identified two GRP78-binding peptides (WIFPWIQL and WDLAWMFRLPVG) against csGRP78 found on DU145 tumour xenografts [27]. Chimeric fusion peptides of either WIFPWIQL or WDLAWMFRLPVG to the proapoptotic motif D(KLAKLAK)2 showed a dose-dependent reduction in cell viability in DU145 cells [27]. In support of this concept, both WIFPWIQL-GG-D(KLAKLAK)2 and WDLAWMFRLPVG-GG-D(KLAKLAK)2 inhibited DU145 tumour growth in both nude mice and Balb/c mice bearing EF43-fgf4 breast carcinoma tumours [27]. WIFPWIQL-GG-D(KLAKLAK)2, later termed bone-metastasis-targeting peptidomimetic-78 (BMTP-78), was further investigated due to its effects in mammary tumour metastasis [29]. BTMP-78 was demonstrated to dose-dependently inhibit the viability of metastatic 4T1.2 breast cancer cells, and such an effect was blocked by treatment with an anti-GRP78 antibody [29]. BMTP-78 also reduced tumour weight in mice bearing 4T1.2 mammary tumours but did not affect tumour volume in the less metastatic tumour xenografts 67NR or 66cl4 [29]. Notably, BMTP-78-treated mice bearing 4T1.2 tumours exhibited an extended period of disease-free survival compared with mice treated with either saline or control peptide [29]. BMTP-78 was also investigated for its therapeutic use in treating acute myeloid leukemia (AML) [30]. FACS analysis revealed the expression of csGRP78 in mononuclear cells in patients with AML [30]. Moreover, BMTP-78 was demonstrated to dose-dependently reduce cell viability among several human-derived leukemia and lymphoma cell lines and in AML-patient-derived peripheral blood cells [30]. Although this study showed acceptable toxicity levels in small rodents treated with BMTP-78, the use of BMTP-78 in the treatment of AML was halted due to the development of lesions at the injection site, kidney lesions, and cardiac lesions in non-human primates [30]. Furthermore, cardiac arrhythmias were also observed in a separate cohort of female rhesus monkeys intravenously infused with BTMP-78 [30].
GRP78-binding peptides may also be utilized as a diagnostic tool in cancer. One previous study examined the use of the GRP78-binding peptide, WIFPWIQL, with radiolabelled polymeric micelles in the nuclear imaging of MKN45 tumour xenografts [31]. Nude mice injected with WIFPWIQL-111In-labelled polymeric micelles exhibited elevated radioactive intensity in the tumour compared with mice injected with 111In-labelled polymeric micelles alone, demonstrating that targeting csGRP78 can improve cancer imaging. Consistent with these findings, GIRLRG, a synthetic peptide generated based on computational modelling of the ATPase domain of GRP78, was confirmed to bind to GRP78 using surface plasmon resonance [32]. Additionally, the injection of radiolabelled 111In-PEG-GIRLRG was shown to enhance the resolution of single-photon emission computerized tomography (SPECT) scans of nude mice bearing either A549 lung, BXPC3 pancreatic, or D54 brain tumours [32].
2.2. CAR T Cell Therapy
CAR T cell therapy is a revolutionary cancer treatment approach where patient-derived T lymphocytes are reprogrammed to express synthetically designed receptors that recognize the surface antigens of tumour cells [33]. Hebbar et al. recently generated CAR T cells that specifically target csGRP78 (GRP78.1x, GRP78.2x, and GRP78.3x CAR T cells) found on AML cells [22]. Coincubation of GRP78.1x CAR T cells with MOLM13 human leukemia cells expressing csGRP78 was shown to enhance the levels of anti-tumour cytokines interferon (IFN)-γ, interleukin (IL)-2, tumour necrosis factor α (TNFα), granulocyte-macrophage colony-stimulating factor (GM-CSF), and to a lesser extent, IL-4, IL-5, IL-6, IL-10 and IL-13 [22]. Importantly, all three GRP78–CAR T cell variants were shown to potently suppress MOLM13 xenograft tumour progression in NSG immunodeficient mice [22]. However, the authors indicated that the anti-tumourigenic effect of GRP78 CAR T cells became limited over time due to the depletion of circulating GRP78 CAR T cells [22]. Remarkably, dasatinib-treated GRP78.1x CAR T cells were shown to induce complete remission and extend the overall survival of NSG mice bearing THP-1 tumour cells, compared with mice treated with GRP78.1x CAR T cells alone [22]. The authors indicated that dasatinib prevented early T cell activation by suppressing csGRP78 expression in the CAR T cells, which enhanced GRP78–CAR T cell viability [22].
2.3. Anti-GRP78 Antibodies
The use of MAb is an emerging treatment strategy for cancer by targeting tumour cell-specific cell surface proteins [34]. Furthermore, several MAb that recognize csGRP78 have been identified [27]. It is well-established that targeting the C-terminal domain of csGRP78 promotes p53 activation and cell death in cancer cells [35]. Two anti-GRP78 antibodies, C38 and C107, were recently shown to bind to a region near the C-terminus of GRP78 [35]. Furthermore, it was shown that the C38 antibody blocked α2M and N-terminal-specific anti-GRP78 antibody-induced activation of AKT [35]. However, the C38 antibody alone did not induce chromatin fragmentation or inhibit B16F1 tumour growth, suggesting that the C38 antibody primarily acts as a steric inhibitor of N-terminal agonists to csGRP78 [35]. In contrast, engagement of the C107 antibody to csGRP78-induced chromatin fragmentation and inhibited B16F1 tumour growth [35]. MAb159 is another C-terminal anti-GRP78 MAb that has been shown to induce csGRP78 internalization and suppress HT29, H249, and A549 tumour progression [36]. Interestingly, A549 tumours treated with MAb159 exhibited enhanced apoptotic TUNEL staining and reduced Ki67 cell proliferation staining [36]. In addition, MAb159 attenuated PI3K activation with a modest effect on ERK1/2 and Src signaling [36]. Moreover, MAb159 suppressed 4T1 tumour metastasis in the liver and lung, compared with IgG-treated mice [36].
PAT-SM6 (formerly known as SAM-6) is an anti-GRP78 IgM antibody that was first isolated from a gastric cancer patient [37][38]. Interestingly, the interaction of PAT-SM6 with csGRP78 was shown to induce the accumulation of intracellular lipids, which, in turn, induced apoptosis in 23132/87 gastric carcinoma cell lines [38]. PAT-SM6 has previously been evaluated in the treatment of multiple myeloma [39]. It was shown that PAT-SM6 alone induced cytotoxicity in primary and cultured myeloma cells [39]. Moreover, it was demonstrated that PAT-SM6 also contributes to the complement-dependent cytotoxicity of primary and cultured multiple myeloma cell lines [39]. Promising phase I clinical trial results have indicated that PAT-SM6 is safe and well-tolerated in patients with relapsed or refractory multiple myeloma, with satisfactory pharmacokinetic parameters [40]. Furthermore, PAT-SM6 was recently shown to have synergistic effects with existing anti-multiple myeloma combination therapies such as bortezomib and lenalidomide.
Unlike MAb, scFvs are engineered antibodies that contain a single variable light and heavy chain connected by a flexible peptide linker [41]. Furthermore, an advantage of scFv over MAb is that scFvs can be generated in recombinant protein bacterial expression systems, allowing for the rapid production of scFvs without the need for expensive hybridomas [41]. Anti-GRP78 scFvs conjugated to quantum dot (Qdot) nanobeads have effectively been used to fluorescently label MDA-MB-231 breast cancer and LNCaP prostate cancer cells, which express csGRP78. Intriguingly, incubation of the anti-GRP78 scFv/Qdot conjugates induced apoptosis in MDA-MB-231 cells. In addition, anti-GRP78 scFv/Qdot conjugates attenuated MDA-MB-231 tumour growth, compared with unlabelled nanobeads [42]. Although these findings indicate that scFvs which target GRP78 are a promising diagnostic and therapeutic tool, further investigation of the mechanisms by which the anti-GRP78 scFv/Qdot conjugate induces apoptosis and inhibits tumour growth is required. GSF3, an scFv that specifically targets the C-terminal domain of GRP78, was recently identified using a ribosome display panning method [43]. Given its specificity for the C-terminus of GRP78, GSF3 may exhibit similar anti-tumourigenic effects to the MAb that target C-terminal GRP78.
2.4. Clinical Trials, Limitations and Challenges
PAT-SM6, an anti-GRP78 monoclonal antibody used in a Phase I trial, was well-tolerated with modest clinical benefit in multiple myeloma (NCT01727778). Furthermore, IT-139 (also known as NKP1339, BOLD-100), a ruthenium-based anti-cancer compound that targets GRP78, was evaluated in a Phase I trial (NCT01415297) in forty-six patients with solid tumours. Burris et al. reported that overall IT-139 monotherapy was well tolerated, with modest anti-tumour effects [44]. The results from these initial clinical studies suggest that therapies targeting GRP78 have clinical value and require continued investigation. Currently, a Phase I trial (NCT04421820) is recruiting participants to evaluate IT-139 in combination with chemotherapy. Although csGRP78 is a promising therapeutic target in cancer treatment, there are several limitations, such as potential off-target effects. Several studies have reported the expression and function of csGRP78 in non-cancer pathologies, including atherosclerosis, thrombotic disease, rheumatoid arthritis, and diabetic nephropathy, thereby potentially complicating the utility of csGRP78-based therapies in cancer patients with co-morbidities [45][46][47].
3. Targeting Cell Surface GRP78/Anti-GRP78 Autoantibody Complex
The binding of anti-GRP78 autoantibodies to the N-terminal region of csGRP78 promotes malignant tumour growth and metastasis. Hence, disrupting the csGRP78/anti-GRP78 autoantibody complex may lead to novel therapeutics [48][49]. Our previous studies have demonstrated that the low-molecular-weight heparin, enoxaparin, blocked the binding of the anti-GRP78 autoantibody to csGRP78, resulting in the attenuation of DU145 xenograft growth in mice [48]. Mechanistically, because a heparin-binding domain has been identified near the anti-GRP78 autoantibody epitope on csGRP78 [50], heparin-like molecules may function as competitive antagonists that disrupt the binding of autoantibodies to csGRP78 [50]. An alternative strategy to disrupt the anti-GRP78 autoantibody complex is to identify small molecules that bind to GRP78 and hinder its interaction with the autoantibody. Furthermore, MAb that binds to alternative epitopes on csGRP78 may interfere with pro-tumourigenic GRP78 N-terminal antibodies. Interestingly, antibodies targeting the C-terminal region of GRP78 have been shown to inhibit the interaction of the N-terminal-specific anti-GRP78 antibody in mice [49]. Further investigations should be conducted to examine whether a similar effect occurs with cancer-patient-derived anti-GRP78 autoantibodies.
References
- Dandekar, A.; Mendez, R.; Zhang, K. Cross Talk Between Er Stress, Oxidative Stress, and Inflammation in Health and Disease. In Stress Responses; Springer: New York, NY, USA, 2015; pp. 205–214.
- Lebeau, P.; Al-Hashimi, A.; Sood, S.; Lhoták, Š.; Yu, P.; Gyulay, G.; Paré, G.; Chen, S.R.W.; Trigatti, B.; Prat, A.; et al. Endoplasmic reticulum stress and Ca2+ depletion differentially modulate the sterol regulatory protein PCSK9 to control lipid metabolism. J. Biol. Chem. 2017, 292, 1510–1523.
- Austin, R.C. The unfolded protein response in health and disease. Antioxid Redox Signal 2009, 11, 2279–2287.
- Knarr, G.; Modrow, S.; Todd, A.; Gething, M.-J.; Buchner, J. BiP-binding sequences in HIV gp160. J. Biol. Chem. 1999, 274, 29850–29857.
- Cao, S.S.; Kaufman, R.J. Unfolded protein response. Curr. Biol. 2012, 22, R622–R626.
- Chakrabarti, A.; Chen, A.W.; Varner, J.D. A review of the mammalian unfolded protein response. Biotechnol. Bioeng. 2011, 108, 2777–2793.
- Han, J.; Kaufman, R.J. The role of ER stress in lipid metabolism and lipotoxicity. J. Lipid. Res. 2016, 57, 1329–1338.
- Lin, J.H.; Walter, P.; Yen, T.S.B. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol Mech Dis 2008, 3, 399–425.
- Wang, M.; Wey, S.; Zhang, Y.; Ye, R.; Lee, A.S. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal 2009, 11, 2307–2316.
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086.
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438.
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381.
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell Mol. Life Sci 2016, 73, 79–94.
- Brown, J.M.; Giaccia, A.J. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res 1998, 58, 1408–1416.
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell 2017, 168, 692–706.
- Dong, D.; Stapleton, C.; Luo, B.; Xiong, S.; Ye, W.; Zhang, Y.; Jhaveri, N.; Zhu, G.; Ye, R.; Liu, Z.; et al. A critical role for GRP78/BiP in the tumor microenvironment for neovascularization during tumor growth and metastasis. Cancer Res. 2011, 71, 2848–2857.
- Niu, Z.; Wang, M.; Zhou, L.; Yao, L.; Liao, Q.; Zhao, Y. Elevated GRP78 expression is associated with poor prognosis in patients with pancreatic cancer. Sci. Rep. 2015, 5, 16067.
- Huang, S.-P.; Chen, J.-C.; Wu, C.-C.; Chen, C.-T.; Tang, N.-Y.; Ho, Y.-T.; Lo, C.; Lin, J.-P.J.-G.; Chung, J.-G.; Lin, J.-P.J.-G. Capsaicin-induced apoptosis in human hepatoma HepG2 cells. Anticancer Res. 2009, 29, 165–174.
- Sun, F.-C.; Wei, S.; Li, C.-W.; Chang, Y.-S.; Chao, C.-C.; Lai, Y.-K. Localization of GRP78 to mitochondria under the unfolded protein response. Biochem. J. 2006, 396, 31–39.
- Conner, C.; Lager, T.W.; Guldner, I.H.; Wu, M.-Z.; Hishida, Y.; Hishida, T.; Ruiz, S.; Yamasaki, A.E.; Gilson, R.C.; Belmonte, J.C.I.; et al. Cell surface GRP78 promotes stemness in normal and neoplastic cells. Sci. Rep. 2020, 10, 3474.
- Misra, U.K.; Payne, S.; Pizzo, S.V. Ligation of prostate cancer cell surface GRP78 activates a proproliferative and antiapoptotic feedback loop. J. Biol. Chem. 2011, 286, 1248–1259.
- Hebbar, N.; Epperly, R.; Vaidya, A.; Thanekar, U.; Moore, S.E.; Umeda, M.; Ma, J.; Patil, S.L.; Langfitt, D.; Huang, S.; et al. CAR T cells redirected to cell surface GRP78 display robust anti-acute myeloid leukemia activity and do not target hematopoietic progenitor cells. Nat. Commun. 2022, 13, 587.
- Lin, M.-L.; Chen, S.-S.; Ng, S.-H. CHM-1 suppresses formation of cell surface-associated GRP78-p85α complexes, inhibiting PI3K-AKT Signaling and inducing apoptosis of human nasopharyngeal carcinoma cells. Anticancer Res. 2015, 35, 5359–5368.
- Kang, B.R.; Yang, S.-H.; Chung, B.-R.; Kim, W.; Kim, Y. Cell surface GRP78 as a biomarker and target for suppressing glioma cells. Sci. Rep. 2016, 6, 34922.
- Mintz, P.J.; Kim, J.; Do, K.-A.; Wang, X.; Zinner, R.G.; Cristofanilli, M.; Arap, M.A.; Hong, W.K.; Troncoso, P.; Logothetis, C.J.; et al. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat. Biotechnol. 2003, 21, 57–63.
- Crisci, S.; Amitrano, F.; Saggese, M.; Muto, T.; Sarno, S.; Mele, S.; Vitale, P.; Ronga, G.; Berretta, M.; Di Francia, R. Overview of current targeted anti-cancer drugs for therapy in onco-hematology. Medicina 2019, 55, 414.
- Arap, M.A.; Lahdenranta, J.; Mintz, P.J.; Hajitou, A.; Sarkis, Á.S.; Arap, W.; Pasqualini, R. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell 2004, 6, 275–284.
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21.
- Miao, Y.R.; Eckhardt, B.L.; Cao, Y.; Pasqualini, R.; Argani, P.; Arap, W.; Ramsay, R.G.; Anderson, R.L. Inhibition of established micrometastases by targeted drug delivery via cell surface-associated GRP78. Clin. Cancer Res. 2013, 19, 2107–2116.
- Staquicini, D.I.; D’Angelo, S.; Ferrara, F.; Karjalainen, K.; Sharma, G.; Smith, T.L.; Tarleton, C.A.; Jaalouk, D.E.; Kuniyasu, A.; Baze, W.B.; et al. Therapeutic targeting of membrane-associated GRP78 in leukemia and lymphoma: Preclinical efficacy in vitro and formal toxicity study of BMTP-78 in rodents and primates. Pharm. J. 2018, 18, 436–443.
- Cheng, C.-C.; Huang, C.-F.; Ho, A.-S.; Peng, C.-L.; Chang, C.-C.; Mai, F.-D.; Chen, L.-Y.; Luo, T.-Y.; Chang, J. Novel targeted nuclear imaging agent for gastric cancer diagnosis: Glucose-regulated protein 78 binding peptide-guided 111In-labeled polymeric micelles. Int. J. Nanomed. 1385, 2013.
- Kapoor, V.; Dadey, D.Y.A.; Nguyen, K.; Wildman, S.A.; Hoye, K.; Khudanyan, A.; Bandara, N.; Rogers, B.E.; Thotala, D.; Hallahan, D.E. Tumor-specific binding of radiolabeled PEGylated GIRLRG peptide: A novel agent for targeting cancers. J. Nucl. Med. 2016, 57, 1991–1997.
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69.
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34.
- de Ridder, G.G.; Ray, R.; Pizzo, S.V. A murine monoclonal antibody directed against the carboxyl-terminal domain of GRP78 suppresses melanoma growth in mice. Melanoma Res. 2012, 22, 225–235.
- Liu, R.; Li, X.; Gao, W.; Zhou, Y.; Wey, S.; Mitra, S.K.; Krasnoperov, V.; Dong, D.; Liu, S.; Li, D.; et al. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth, and metastasis. Clin. Cancer Res 2013, 19, 6802–6811.
- Rauschert, N.; Brändlein, S.; Holzinger, E.; Hensel, F.; Müller-Hermelink, H.-K.; Vollmers, H.P. A new tumor-specific variant of GRP78 as target for antibody-based therapy. Lab. Investig.. 2008, 88, 375–386.
- Pohle, T.; Brändlein, S.; Ruoff, N.; Müller-Hermelink, H.K.; Vollmers, H.P. Lipoptosis: Tumor-specific cell death by antibody-induced intracellular lipid accumulation. Cancer Res 2004, 64, 3900–3906.
- Rasche, L.; Duell, J.; Morgner, C.; Chatterjee, M.; Hensel, F.; Rosenwald, A.; Einsele, H.; Topp, M.S.; Brändlein, S. The natural human IgM antibody PAT-SM6 induces apoptosis in primary human multiple myeloma cells by targeting heat shock protein GRP78. PLoS ONE 2013, 8, e63414.
- Rasche, L.; Duell, J.; Castro, I.C.; Dubljevic, V.; Chatterjee, M.; Knop, S.; Hensel, F.; Rosenwald, A.; Einsele, H.; Topp, M.S.; et al. GRP78-directed immunotherapy in relapsed or refractory multiple myeloma - results from a phase 1 trial with the monoclonal immunoglobulin M antibody PAT-SM6. Haematologica 2015, 100, 377–384.
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.M.; Hamid, M. scFv antibody: Principles and clinical application. Clin. Dev. Immunol. 2012, 2012, 980250.
- Xu, W.; Liu, L.; Brown, N.J.; Christian, S.; Hornby, D. Quantum dot-conjugated Anti-GRP78 scFv inhibits cancer growth in mice. Molecules 2012, 17, 796–808.
- Shabani, S.; Moghadam, M.F.; Gargari, S.L.M. Isolation and characterization of a novel GRP78-specific single-chain variable fragment (scFv) using ribosome display method. Med. Oncol. 2021, 38, 115.
- Burris, H.A.; Bakewell, S.; Bendell, J.C.; Infante, J.; Jones, S.F.; Spigel, D.R.; Weiss, G.J.; Ramanathan, R.K.; Ogden, A.; Von Hoff, D. Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: A first-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open 2016, 1, e000154.
- Yoo, S.-A.; You, S.; Yoon, H.-J.; Kim, D.-H.; Kim, H.-S.; Lee, K.; Ahn, J.H.; Hwang, D.; Lee, A.S.; Kim, K.-J.; et al. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J. Exp. Med. 2012, 209, 871–886.
- Crane, E.D.; Al-Hashimi, A.A.; Chen, J.; Lynn, E.G.; Won, K.D.; Lhoták, Š.; Naeim, M.; Platko, K.; Lebeau, P.; Byun, J.H.; et al. Anti-GRP78 autoantibodies induce endothelial cell activation and accelerate the development of atherosclerotic lesions. JCI Insight 2018, 3, e99363.
- Liu, C.; Bhattacharjee, G.; Boisvert, W.; Dilley, R.; Edgington, T. In vivo interrogation of the molecular display of atherosclerotic lesion surfaces. Am. J. Pathol. 2003, 163, 1859–1871.
- Al-Hashimi, A.A.; Lebeau, P.; Majeed, F.; Polena, E.; Lhotak, Š.; Collins, C.A.F.; Pinthus, J.H.; Gonzalez-Gronow, M.; Hoogenes, J.; Pizzo, S.V.; et al. Autoantibodies against the cell surface–associated chaperone GRP78 stimulate tumor growth via tissue factor. J. Biol. Chem. 2017, 292, 21180–21192.
- de Ridder, G.G.; Gonzalez-Gronow, M.; Ray, R.; Pizzo, S.V. Autoantibodies against cell surface GRP78 promote tumor growth in a murine model of melanoma. Melanoma Res. 2011, 21, 35–43.
- Gonzalez-Gronow, M.; Cuchacovich, M.; Llanos, C.; Urzua, C.; Gawdi, G.; Pizzo, S.V. Prostate cancer cell proliferation in vitro Is modulated by antibodies against glucose-regulated protein 78 isolated from patient serum. Cancer Res. 2006, 66, 11424–11431.
More
Information
Subjects:
Oncology; Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
826
Revisions:
3 times
(View History)
Update Date:
01 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





