You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Olivier Bousiges | -- | 3479 | 2022-06-29 13:15:41 | | | |
| 2 | Conner Chen | -22 word(s) | 3457 | 2022-06-30 07:47:03 | | | | |
| 3 | Conner Chen | + 8 word(s) | 3465 | 2022-07-01 08:01:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bousiges, O. Biomarkers of Dementia with Lewy Bodies. Encyclopedia. Available online: https://encyclopedia.pub/entry/24630 (accessed on 26 December 2025).
Bousiges O. Biomarkers of Dementia with Lewy Bodies. Encyclopedia. Available at: https://encyclopedia.pub/entry/24630. Accessed December 26, 2025.
Bousiges, Olivier. "Biomarkers of Dementia with Lewy Bodies" Encyclopedia, https://encyclopedia.pub/entry/24630 (accessed December 26, 2025).
Bousiges, O. (2022, June 29). Biomarkers of Dementia with Lewy Bodies. In Encyclopedia. https://encyclopedia.pub/entry/24630
Bousiges, Olivier. "Biomarkers of Dementia with Lewy Bodies." Encyclopedia. Web. 29 June, 2022.
Copy Citation
Dementia with Lewy Bodies (DLB) presents certain clinical specificities such as visual illusions or hallucinations and fluctuations in attention, but also a particular sensitivity to neuroleptics. Despite the very high diagnostic specificity of these criteria (the specificity of probable DLB is 95.1% in early stages and 88% in late stages), their sensitivity remains low (32%) in pure DLB or even lower (12%) when associated with Alzheimer’s disease (AD). In other words, DLB is still a largely underdiagnosed disease in more than two-thirds of cases. It is therefore essential to discover new biomarkers that can distinguish DLB from AD to improve differential diagnosis.
cerebro-spinal fluid
biomarkers
α-synuclein
1. Brain Imaging Diagnostic Biomarkers
1.1. Brain MRI
The prodromal stage of AD in the brain MRI group study shows greater hippocampal and parietal atrophy [1]. In addition, insular atrophy seems to be an interesting marker of prodromal DLB when compared to prodromal AD and healthy elderly controls (Figure 1) [1][2][3]. However, analysis with voxel-based morphometry (VBM) and cortical thickness on the groups of patients is not the same as visual and individual analysis. A recent study did not demonstrate differences in the insula and hippocampi between prodromal DLB and AD patients [4]. In this previous study, amyloid and tau biomarkers (CSF or PET) were not done for the two groups. However, when considering the differential diagnosis between prodromal DLB and prodromal AD, preserved hippocampal volumes are associated with an increased risk of probable DLB [5]. A result show that in DLB there is isolated atrophy of the anterosuperior part of the insulae, while in AD there is hippocampal and insular atrophy [6].
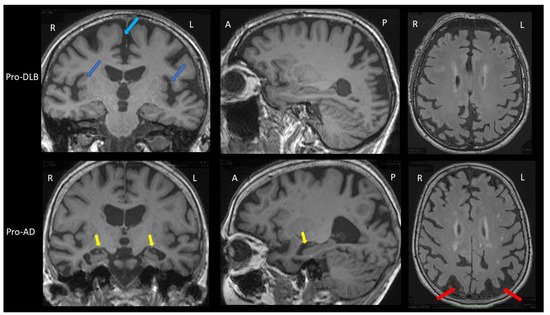
Figure 1. Brain MRI of patients with prodromal dementia with Lewy bodies and prodromal Alzheimer’s disease. Upper part: Brain MRI of a prodromal DLB (Pro-DLB) patient of 84 years with mild cognitive impairment (MMSE = 27/30), cognitive fluctuations, REM-sleep behavior disorder (RBD) and subtle Parkinsonism (UPDRS scale: rigidity 1/4 Froment’s maneuver, akinesia 1/4, attitude tremor). Top left image: T1 sequence, coronal section. The two dark blue arrows show the bilateral insular atrophy. Note the absence of hippocampal atrophy (Scheltens scale = 0/4) and the frontomesial atrophy (light blue arrow). Middle image at top: T1 sequence, sagittal section: absence of hippocampal atrophy and right parietal atrophy. Top right image: FLAIR sequence, axial section. There is no ischemic vascular lesion (Fazekas score = 0/3). Note the right parietal atrophy. Lower part: Brain MRI of a prodromal AD (Pro-AD) patient of 84 years old with mild cognitive impairment (MMSE = 29/30) with mainly verbal and visual memory storage disorders, and no Parkinsonism, no fluctuation, no RBD and no hallucination. Bottom left image: T1 sequence, coronal section. The two yellow arrows show the hippocampal atrophy (Scheltens scale = 3/4 for the left hippocampus and 2/4 for the right one). Note the subtle insular atrophy and frontomesial atrophy. Bottom middle image: T1 sequence, sagittal image. The yellow arrow shows the hippocampal atrophy. Note the parietal atrophy. Bottom right image: FLAIR sequence, axial section. The two red arrows show the bilateral parietal atrophy. There are few microvascular ischemic lesions as hypersignals (Fazekas score = 1/3). Comparison of the two patients shows greater hippocampal atrophy in the pro-AD patient, and greater insular atrophy in the pro-DLB patient.
The dementia stage of AD on brain MRI also shows greater hippocampal atrophy, with the possibility to differentiate AD from DLB with very good sensitivity (91%) and specificity (94%) [7]. However, this is valid for patients near death in moderately severe to severe stages (MMSE<15/30). At earlier stages (i.e., mild to moderate dementia with MMSE ≥ 15/30), hippocampal atrophy is less informative: a sensitivity of 64% and a specificity of 68% [8]. Recent studies have shown an interest to look into the loss of the swallow tail sign. SWI brain MRI usually detects a posterior hyperintense signal area in the substantia nigra of healthy control: the hyperintense signal in between the hypointense signal of the substantia nigra is named the swallow tail sign. Two studies with 15 [9] and 19 [10] DLB patients demonstrated different results when compared to AD patients. Rizzo et al. have shown a sensitivity of 80% and a specificity of 64% [9], Shams et al., a sensitivity 63% and a specificity of 75% [10]. Other studies are necessary to better know the interest of this loss of the swallow tail sign: the search for this sign can be difficult because a thinning of the substantia nigra can also appear in DLB (Figure 1B).
Thus, the clinician should look for insular atrophy without hippocampal atrophy in mild cognitive impairment (MCI) patients, and if present, the clinician must search for the cardinal symptoms of DLB, fluctuations, hallucinations, RBDs and Parkinsonism, including discrete ones [11]. In the case of suspected DLB at the dementia stage, the degree of atrophy is of little importance, apart from hippocampal atrophy, which is often less than in the rest of the brain.
1.2. Scintigraphy
1.2.1. FP-CIT SPECT
Nigral dopaminergic cell loss (and not synuclein) was associated with lower striatal I-2beta-carbomethoxy-3beta-(4-iodophenyl)-N-(3-fluoropropyl) nortropane ((123)I-FP-CIT) uptake [12]. FP-CIT SPECT has an excellent accuracy particularly at the stage of moderate dementia: 88% sensitivity and 100% specificity (neuropathologic analysis) [13]. A phase 3 study to validate FP-CIT SPECT for the diagnosis of DLB has demonstrated that in probable DLB (i.e., two core clinical signs), the sensitivity was 77.7% and the specificity was 90.4% [14]. These patients had a mean MMSE of 20 in favor of mild dementia. Interestingly, for possible DLB (i.e., one core clinical sign), a situation a biomarker would be of high interest and the sensitivity was lower (38.2%). Moreover, an abnormal FP-CIT SPECT does not exclude the diagnosis of FTLD. Thus, when FTLD is the control group, specificity for DLB is only 70%, and sensitivity remains excellent (90%) [15]. Despite the limitations cited, a recent meta-analysis confirms that FP-CIT has clear value as a biomarker of DLB at the stage of dementia [16].
The diagnostic value of FP-CIT SPECT for prodromal DLB is lower. In a study of 48 prodromal DLB, sensitivity was 54.2% for probable and possible prodromal DLB, and specificity was 89% [17]. For probable prodromal DLB, the sensitivity was better (61%). In other words, when the FP-CIT SPECT is abnormal in an MCI patient, it is of interest, but if normal, it is not useful in the diagnostic process.
For the clinician, the visual analysis of the FP-CIT SPECT must be deepened by looking at the different parts of the striatum, the caudate nucleus in the anterior part and the putamen in the posterior part (Figure 2A,B). The FP-CIT SPECT is of interest when it is abnormal, especially at the prodromal stage, but when it is normal, it does not eliminate the diagnosis of DLB.
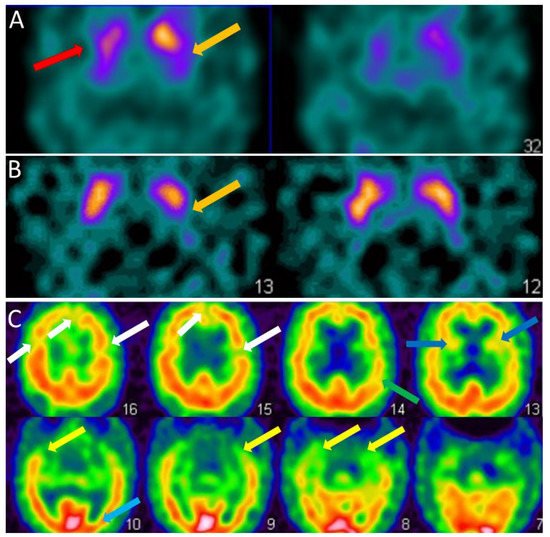
Figure 2. SPECT of patients with dementia with Lewy bodies. (A). Brain FP-CIT SPECT (DAT-scan) of a DLB patient of 81 years with mild dementia, cognitive fluctuations, and Capgras syndrome. Axial section FP-CIT SPECT shows lower right striatal, i.e., caudate and putamen, uptake (red arrow) and lower left putamen uptake (orange arrow). (B). Brain FP-CIT SPECT (DAT-scan) of a prodromal DLB patient of 74 years with mild cognitive impairment, cognitive fluctuations, and subtle Parkinsonism (UPDRS scale: rigidity 1/4, akinesia 1/4, no tremor). Axial section FP-CIT SPECT shows lower left putamen uptake (orange arrow). (C). Brain perfusion SPECT (neurolite) of the same prodromal DLB patient of 74 years with mild cognitive impairment, cognitive fluctuations, and subtle Parkinsonism (UPDRS scale: rigidity 1/4, akinesia 1/4, no tremor). Axial section perfusion SPECT showing a mity aspect with diffuse hypoperfusion involving frontal (white arrows), insular (dark blue arrows), parietal (green arrow), temporal (yellow arrow) and occipital lobes (subtle, light blue arrow).
1.2.2. Perfusion SPECT
Perfusion SPECT is an imaging technique for estimating brain perfusion. The main handicap of this technique is its low sensitivity in the context of DLB. Thus, the differentiation between DLB and AD obtained a sensitivity of 65% according to Lobotesis [18] and Pasquier [19] and 74% according to Hanyu [20], using occipital hypoperfusion as a biomarker of DLB. The specificity was better: 82% according to Hanyu. Using image processing with software SPECT Z-score maps with a focus on the cingulate island sign (CIS, see infra), the differential diagnosis between DLB and AD had a sensitivity and specificity of 92.3, and 76.9% [21]. However, these results were lower in a second more powerful study with sensitivity and specificity of 50 and 73% [22].
For the clinician, the hypoperfusion seen in DLB in the dementia stage is often diffuse and partial of the frontal, temporal, parietal, insular and occipital lobes (Figure 2C). Therefore, the clinician should look specifically for the existence of occipital hypoperfusion.
1.2.3. FDG-PET
As in perfusion SPECT occipital hypometabolism in DLB using FDG-PET is of interest and can help to discriminate DLB from AD [23]. The CIS has been proposed as a neuroimaging feature of DLB. The differences between DLB and AD were described in 1997 [24], and the concept of CIS was developed thereafter. Its definition is the hypometabolism of precuneus and cuneus but with sparing of the posterior cingulate cortex [25]. The CIS was found to be correlated with hippocampal atrophy, suggesting a link with AD pathology [26]. Lim et al. [25] reported that the sensitivities for using CIS to diagnose DLB ranged from 62 to 86% and only 43 to 50% for hypometabolism in the medial occipital lobe. Both (occipital hypometabolism and CIS) were demonstrated to have a better sensitivity of 77% and specificity of 80% [25]. Posterior cortical atrophy, one of the clinical subtypes of AD, and DLB show overlapping patterns of hypometabolism including occipital and posterior cingulate [27].
For the clinician, FDG-PET is better than perfusion SPECT for the diagnosis if the search for occipital hypometabolism and sparing of the posterior cingulate regions is performed, while considering the often diffused and decreased metabolism in PET-FDG.
1.2.4. Synuclein PET
There are many efforts to obtain a specific biomarker for synucleopathies in PET [28]. Alpha-synuclein PET is still missing. Many groups are trying to obtain it because it would be a kind of revolution to obtain such a biomarker. Recently, Kuebler et al. have demonstrated the capacity of MODAG-001 to bind to synuclein fibrils in a rat brain using in vivo PET [29]. Previously, Kikochi et al. had shown that synuclein PET could be used to visualize synuclein deposits in eight MSA patients [30]. More recently, Oskar Hansson presented at the ADPD congress 2022 synuclein PET (ACI 12589) results again in eight MSA patients compared to healthy controls, Parkinson’s disease patients and DLB patients. Only the MSA patients had significant labeling, especially in the cerebellar peduncles.
1.2.5. 123 I-Metaiodobenzylguanidine (MIBG) Myocardial Scintigraphy
MIBG myocardial scintigraphy is an imaging technique for estimating sympathetic nerve damage, which post-mortem studies show as reduced in DLB. These damages are seen in primary heart disease, diabetic neuropathy, and also PD and DLB [20]. For DLB at the stage of dementia, the sensitivity ranges from 68.9% [31] N = 61 DLB (ratio method) to 100% N = 19 DLB (ratio method) [20]. The specificity of MIBG scintigraphy when compared to healthy controls or AD is usually excellent: 87% [31], to 92% [20] N = 19 DLB, ratio method [20][31]. Although MIBG scintigraphy seems to be informative, there is a lack of phase 3 studies to validate this biomarker in its use as a differential diagnostic tool between DLB and AD [32].
As for FP-CIT SPECT, the diagnostic value of MIBG scintigraphy is lower in the context of prodromal DLB. In a study of 52 patients with prodromal DLB, sensitivity was 46.2% for probable and possible prodromal DLB, and specificity of 88% (controls: prodromal AD patients) [33].
Table 2 summarises the neuroimaging diagnosis of DLB patients.
Table 2. Neuroimaging and diagnosis of dementia with Lewy bodies.
| Prodromal DLB | Validity | DLB dementia | Validity | References | |
|---|---|---|---|---|---|
| Brain MRI T1 | Insular atrophy | Not demonstrated | No or mild hippocampal atrophy | Sensitivity = 64% Specificity = 68% (compared to AD) |
[8] |
| Brain MRI SWI | Loss of the swallow tail sign | Not demonstrated | Loss of the swallow tail sign | Sensitivity = 63% Specificity = 75% (compared to AD) |
[10] |
| FP-CIT SPECT (DAT-scan) | Presynaptic striatal dopaminergic decrease | Sensitivity = 54.2% Specificity = 89.0% (compared to prodromal AD) |
Presynaptic striatal dopaminergic decrease | Sensitivity = 77.7% Specificity = 90.4% (compared to AD) |
[14][17] |
| Perfusion SPECT | Occipital hypoperfusion | Not demonstrated | Occipital hypoperfusion | Sensitivity = 74.0% Specificity = 82.0% (compared to AD) |
[20] |
| FDG-PET | Occipital hypometabolism and Cingulate Island Sign | Not demonstrated | Occipital hypometabolism and Cingulate Island Sign | Sensitivity = 77.0% Specificity = 80.0% (compared to AD) |
[25] |
| Synuclein-PET | Cortical and basal ganglia accumulation? | Not existing | Cortical and basal ganglia accumulation? | Not existing | |
| MIBG scintigraphy | Decrease cardiac sympathetic activity | Sensitivity = 46.2% Specificity = 88.0% (compared to prodromal AD) |
Decrease cardiac sympathetic activity | Sensitivity = 68.9% Specificity = 87.0% (compared to AD) |
[31][33] |
2. CSF Biomarkers
CSF biomarkers in relation to AD biomarkers or total alpha-synuclein and its post-translational modifications have been addressed previously [34]. Here, the RT-QuIC alpha-synuclein technique is introduced, as well as biomarkers not directly related to the proteins aggregating in the diseases.
2.1. RT-QuIC Technique
RT-QuIC means “real-time quaking-induced conversion” and this technique, which was originally developed to detect the abnormal prion protein, is based on the “prion-like” effect of α-synuclein. It is important to know that from a pathophysiological point of view, physiological α-synuclein is transformed into “pathological” α-synuclein, i.e., it undergoes post-translational modifications, changes its conformation with the formation of beta-sheets, and aggregation phenomena leading to Lewy bodies formation. Moreover, the technique uses Thioflavin T which is a fluorophore that binds to these beta-sheets. Thus, the RT-QuIC technique consists of adding patient CSF to recombinant α-synuclein and Thioflavin T. In case of the presence of “pathological” α-synuclein in the sample, it will transform the recombinant α-synuclein into “pathological” α-synuclein. Thioflavin T binding increases as this transformation proceeds. The increase in fluorescence emitted following this binding will be read in real-time by a spectrometer. The transformation of recombinant α-synuclein into pathological α-synuclein can take several days, thus the reading is done under agitation at 42 °C for at least 5 days.
Thus, using this technique, research has shown a sensitivity of 92–93% and a specificity of 96–100% depending on the study between DLB and AD patients [35][36][37][38][39]. Thus the routine development of this technique could have a major impact on the diagnosis of synucleinopathies and DLB in particular.
2.2. Potentially Interesting Biomarkers in the Differential Diagnosis between AD and DLB
Some elements of the CSF, despite a lack of direct specificity with one or the other disease, are apparently able to differentiate them. Currently, some biomarkers may be of interest; however, for some of them, they were only found in a single study and further research is needed to confirm their discriminatory power.
2.2.1. Alpha-Synuclein Protease
Neurosin is one of the few proteins on the list that still has a link to alpha-synuclein since it is an alpha-synuclein-degrading protease. Neurosin levels appear to be significantly lowered in synucleinopathies (DLB, PD, PD dementia) compared to AD patients and controls [40].
2.2.2. Neuroinflammation
YKL-40, also called chitinase 3 like protein 1 (CHI3L1) or cartilage glycoprotein 39 (HC gp-39), is a secreted glycoprotein with glycosyl hydrolase functions. In the brain, YKL-40 is mainly expressed by astrocytes and plays a key role in inflammation, particularly in AD. In CSF, the YKL-40 level is significantly higher in AD patients compared to that observed in DLB patients or healthy controls [41][42].
Interleukin (IL)-6 has an important role in neuroinflammatory processes but also has a potential impact on cognitive function. Thus, it is not surprising that IL-6 secretion is impaired in neurodegenerative dementias involving neuroinflammation. In AD, activated glial cells induce elevated IL-6 expression, especially near senile plaques. Thus, IL-6 levels are significantly lower in the CSF of DLB patients than in AD patients and control subjects without dementia [43].
2.2.3. Neurotransmitter Metabolites
Because DLB is characterized by the impairment of different neurotransmitter systems, it would seem interesting to study dopamine and its metabolites such as homovanilic acid (HVA) and 3,4-Dihydroxyphenylacetic acid (DOPAC), serotonin metabolites such as 5-hydroxyindolacetic acid (5-HIAA), epinephrine/norepinephrine metabolites such as 3-methoxy-4-hydroxyphenylethylene glycol (MHPG) and gamma-aminobutyric acid (GABA). Thus, all these elements have been shown to be significantly lower in DLB than in AD [44].
2.2.4. Amino Acids and Neuropeptides
Cocaine and amphetamine-regulated transcript (CART) is a neuropeptide selectively expressed in hypothalamus neurons. These neurons project to regions thought to regulate mood, such as the prefrontal cortex, hippocampus and striatum. In humans, CART gene mutations are associated with depression and anxiety. It is interesting to note that in a recent MRI study, DLB patients had hypothalamic atrophy while this region was not affected in AD patients. This atrophy is associated with a significant reduction in CSF CART levels in DLB but not in AD [45].
Alterations in excitatory amino acid concentrations may be potentially involved in the pathogenesis of several neurodegenerative diseases, including AD, PD, amyotrophic lateral sclerosis, and MSA. Thus, patients with DLB have higher concentrations of asparagine (+25%) and glycine (+21%) in CSF compared to a control group [45]. It should be noted that this study did not include AD patients.
2.2.5. Minerals and Metals
Several neurodegenerative diseases, including AD and PD, are characterized by altered homeostasis of certain metals in the brain and CSF. These changes appear to contribute, directly or indirectly, to increased oxidative stress, an important factor of neuronal toxicity. DLB patients have elevated levels of calcium, magnesium, and copper in the CSF compared to control and AD patients. The combination of calcium and magnesium concentrations in the CSF distinguishes DLB from AD with a sensitivity of 93% and a specificity of 85% [45].
2.2.6. Synaptic Proteins
Chromogranin A (CgA) is a neuroactive glycoprotein present in neuroendocrine cells and in synaptic vesicles of neurons. CgA plays a role in the stabilization of secretory vesicles and the synaptic activity modulation. This protein seems to be significantly higher in DLB patients than in control, PD and PDD patients [46]. Note the absence of Alzheimer’s patients in this study, but it would seem that CgA is also increased because of the MCI stage of AD patients [47]. Further studies would therefore be necessary to determine whether CgA would discriminate between DLB and AD.
Neurogranin is a postsynaptic protein that binds to calmodulin and plays an important role in memory by regulating synaptic plasticity and learning. Thus, the neurogranin release into the CSF is a likely reflection of synaptic dysfunction or neuronal degeneration. Despite the presence of synaptic dysfunction in DLB, neurogranin levels in the CSF of AD patients are significantly higher than in those affected by DLB [41]; they are probably related to more extensive neuronal death processes during AD.
2.2.7. Others
Visinin-like protein 1 (VILIP-1) belongs to the family of neuronal calcium sensors. The entry of calcium cations into the cell induces a reversible translocation of VILIP-1 to membrane components of the cell. In this way, VILIP-1 modulates the signaling cascade in neurons, resulting in the regulation of neuronal ion channels, neuronal growth, synaptic plasticity, and the activation of cyclic adenosine monophosphate (cAMP) as well as cyclic guanine monophosphate (cGMP) signaling pathways. One study showed that the concentration of VILIP-1 in CSF was significantly increased in AD patients compared to normal controls and DLB patients [48][49].
A recent proteomic study has identified some biomarkers that may be of interest in the differential diagnosis of DLB [50]. The authors identified VGF, SCG2, NPTX2, NPTXR, PDYN and PCSK1N as possible biomarker candidates, with the NPTX2, VGF, SCG2, PDYN panels being the most relevant for differentiating DLB from AD [50][51]. All of these biomarker candidates are likely to be decreased in the CSF of DLB patients compared to controls and AD groups. However, several of them are also decreased in the CSF of AD patients compared to control patients, suggesting an even greater decrease for DLB patients. NPTX2 consistently shows decreased CSF concentrations in AD [52][53], Boiten and colleagues showed no difference in CSF NPTX2 levels between DLB and AD [54]. VGF and SCG2 also showed a decrease in CSF in AD patients [55][56], so among the potential biomarkers, only PDYN seems to remain unchanged in AD (no publications have been able to show any variation in the CSF levels of AD patients). VGF and SCG2 (but also PCSK1N) are members of the chromogranin/secretogranin family and play a role in the regulated secretory pathway of peptides, hormones, neurotransmitters and growth factors. VGF is a neurosecretory protein. VGF and peptides derived from its processing play many roles in neurotransmitter release, energy homeostasis, and regulation of gastrointestinal function but also in neurogenesis and neuroplasticity associated with learning, memory, depression and chronic pain [57][58]. The second biomarker candidate is SCG2, which is a neuroendocrine protein of the granin family that regulates the biogenesis of secretory granules playing a role in inflammatory responses and in the regulation of blood pressure [57]. NPTX2 (and NPTXR) are members of the neuronal pentraxin family [59]. NPTX2 plays role in the modification of cellular properties that underlie long-term plasticity promoting the formation of new excitatory synapses (through the glutamate-gated channels), synaptic homeostatic plasticity and regulation of AMPA-type receptors clustering at established synapses [60]. The last biomarker candidate identified is PDYN. This protein is a member of the enkephalin family that competes with and mimics the effects of opioid drugs. Enkephalins play a role in a number of physiologic functions, including pain perception and responses to stress [61][62].
Table 3 summarizes the variations in the levels of these different biomarkers between AD and DLB compared to control subjects.
Table 3. Summarizes the variations of potential biomarkers in AD and DLB CSF (- means no change, ↗ means increased compared to controls, ↘ means decreased compared to controls, ? means no data).
| Biomarkers | AD | DLB | References |
|---|---|---|---|
| YKL-40 | ↗ | - | [41][42] |
| neurogranin | ↗ | - | [41] |
| VILIP-1 | ↗ | - | [48][49] |
| Magnesium, calcium, copper | - | ↗ | [45] |
| Neurosin | - | ↘ | [40] |
| Il-6 | - | ↘ | [43] |
| CART | - | ↘ | [45] |
| Chromogranin A | ↗ | ↗ | [46][47] |
| Asparagine, glycine | ? | ↗ | [45] |
| HVA, 5-HIAA et MHPG | ↘ compared to AD | [44] | |
| NPTX2, VGF, SCG2, | ↘ | ↘(↘) | [50] |
| PDYN | ↘ | [50] | |
| RT-QuIC | - | ↗ | [35][36][37][38][39] |
References
- Blanc, F.; Colloby, S.J.; Philippi, N.; de Petigny, X.; Jung, B.; Demuynck, C.; Phillipps, C.; Anthony, P.; Thomas, A.; Bing, F.; et al. Cortical Thickness in Dementia with Lewy Bodies and Alzheimer’s Disease: A Comparison of Prodromal and Dementia Stages. PLoS ONE 2015, 10, e0127396.
- Roquet, D.; Noblet, V.; Anthony, P.; Philippi, N.; Demuynck, C.; Cretin, B.; Martin-Hunyadi, C.; Loureiro de Sousa, P.; Blanc, F. Insular atrophy at the prodromal stage of dementia with Lewy bodies: A VBM DARTEL study. Sci. Rep. 2017, 7, 9437.
- Blanc, F.; Colloby, S.J.; Cretin, B.; de Sousa, P.L.; Demuynck, C.; O’Brien, J.T.; Martin-Hunyadi, C.; McKeith, I.; Philippi, N.; Taylor, J.P. Grey matter atrophy in prodromal stage of dementia with Lewy bodies and Alzheimer’s disease. Alzheimer’s Res. Ther. 2016, 8, 31.
- Firbank, M.J.; Durcan, R.; O’Brien, J.T.; Allan, L.M.; Barker, S.; Ciafone, J.; Donaghy, P.C.; Hamilton, C.A.; Lawley, S.; Roberts, G.; et al. Hippocampal and insula volume in mild cognitive impairment with Lewy bodies. Parkinsonism Relat. Disord. 2021, 86, 27–33.
- Kantarci, K.; Lesnick, T.; Ferman, T.J.; Przybelski, S.A.; Boeve, B.F.; Smith, G.E.; Kremers, W.K.; Knopman, D.S.; Jack, C.R., Jr.; Petersen, R.C. Hippocampal volumes predict risk of dementia with Lewy bodies in mild cognitive impairment. Neurology 2016, 87, 2317–2323.
- Blanc, F.; Schulz, I.; Mondino, M.; Gobin, M.S.I.; Gudewiez, R.; Loureiro De Sousa, P.; Philippi, N.; Baloglu, S.; Cretin, B.; Demuynck, C.; et al. Atrophy of insulae and hippocampi in prodromal dementia with Lewy bodies and Alzheimer’s Disease: Visual assessment. ADPD 2019.
- Burton, E.J.; Barber, R.; Mukaetova-Ladinska, E.B.; Robson, J.; Perry, R.H.; Jaros, E.; Kalaria, R.N.; O’Brien, J.T. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: A prospective study with pathological verification of diagnosis. Brain A J. Neurol. 2009, 132 Pt 1, 195–203.
- Harper, L.; Fumagalli, G.G.; Barkhof, F.; Scheltens, P.; O’Brien, J.T.; Bouwman, F.; Burton, E.J.; Rohrer, J.D.; Fox, N.C.; Ridgway, G.R.; et al. MRI visual rating scales in the diagnosis of dementia: Evaluation in 184 post-mortem confirmed cases. Brain A J. Neurol. 2016, 139 Pt 4, 1211–1225.
- Rizzo, G.; De Blasi, R.; Capozzo, R.; Tortelli, R.; Barulli, M.R.; Liguori, R.; Grasso, D.; Logroscino, G. Loss of Swallow Tail Sign on Susceptibility-Weighted Imaging in Dementia with Lewy Bodies. J. Alzheimer’s Dis. JAD 2019, 67, 61–65.
- Shams, S.; Fallmar, D.; Schwarz, S.; Wahlund, L.O.; van Westen, D.; Hansson, O.; Larsson, E.M.; Haller, S. MRI of the Swallow Tail Sign: A Useful Marker in the Diagnosis of Lewy Body Dementia? AJNR Am. J. Neuroradiol. 2017, 38, 1737–1741.
- Blanc, F.; Verny, M. Prodromal stage of disease (dementia) with Lewy bodies, how to diagnose in practice? Geriatr. Psychol. Neuropsychiatr. Vieil. 2017, 15, 196–204.
- Colloby, S.J.; McParland, S.; O’Brien, J.T.; Attems, J. Neuropathological correlates of dopaminergic imaging in Alzheimer’s disease and Lewy body dementias. Brain A J. Neurol. 2012, 135 Pt 9, 2798–2808.
- Walker, Z.; Jaros, E.; Walker, R.W.; Lee, L.; Costa, D.C.; Livingston, G.; Ince, P.G.; Perry, R.; McKeith, I.; Katona, C.L. Dementia with Lewy bodies: A comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1176–1181.
- McKeith, I.; O’Brien, J.; Walker, Z.; Tatsch, K.; Booij, J.; Darcourt, J.; Padovani, A.; Giubbini, R.; Bonuccelli, U.; Volterrani, D.; et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: A phase III, multicentre study. Lancet Neurol. 2007, 6, 305–313.
- Morgan, S.; Kemp, P.; Booij, J.; Costa, D.C.; Padayachee, S.; Lee, L.; Barber, C.; Carter, J.; Walker, Z. Differentiation of frontotemporal dementia from dementia with Lewy bodies using FP-CIT SPECT. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1063–1070.
- Nihashi, T.; Ito, K.; Terasawa, T. Diagnostic accuracy of DAT-SPECT and MIBG scintigraphy for dementia with Lewy bodies: An updated systematic review and Bayesian latent class model meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1984–1997.
- Thomas, A.J.; Donaghy, P.; Roberts, G.; Colloby, S.J.; Barnett, N.A.; Petrides, G.; Lloyd, J.; Olsen, K.; Taylor, J.P.; McKeith, I.; et al. Diagnostic accuracy of dopaminergic imaging in prodromal dementia with Lewy bodies. Psychol. Med. 2019, 49, 396–402.
- Lobotesis, K.; Fenwick, J.D.; Phipps, A.; Ryman, A.; Swann, A.; Ballard, C.; McKeith, I.G.; O’Brien, J.T. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology 2001, 56, 643–649.
- Pasquier, J.; Michel, B.F.; Brenot-Rossi, I.; Hassan-Sebbag, N.; Sauvan, R.; Gastaut, J.L. Value of (99m)Tc-ECD SPET for the diagnosis of dementia with Lewy bodies. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1342–1348.
- Hanyu, H.; Shimizu, S.; Hirao, K.; Kanetaka, H.; Iwamoto, T.; Chikamori, T.; Usui, Y.; Yamashina, A.; Koizumi, K.; Abe, K. Comparative value of brain perfusion SPECT and MIBG myocardial scintigraphy in distinguishing between dementia with Lewy bodies and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 248–253.
- Imabayashi, E.; Soma, T.; Sone, D.; Tsukamoto, T.; Kimura, Y.; Sato, N.; Murata, M.; Matsuda, H. Validation of the cingulate island sign with optimized ratios for discriminating dementia with Lewy bodies from Alzheimer’s disease using brain perfusion SPECT. Ann. Nucl. Med. 2017, 31, 536–543.
- Kanetaka, H.; Shimizu, S.; Inagawa, Y.; Hirose, D.; Takenoshita, N.; Sakurai, H.; Hanyu, H. Differentiating Mild Cognitive Impairment, Alzheimer’s Disease, and Dementia with Lewy Bodies Using Cingulate Island Sign on Perfusion IMP-SPECT. Front. Neurol. 2020, 11, 568438.
- O’Brien, J.T.; Firbank, M.J.; Davison, C.; Barnett, N.; Bamford, C.; Donaldson, C.; Olsen, K.; Herholz, K.; Williams, D.; Lloyd, J. 18F-FDG PET and perfusion SPECT in the diagnosis of Alzheimer and Lewy body dementias. J. Nucl. Med. 2014, 55, 1959–1965.
- Imamura, T.; Ishii, K.; Sasaki, M.; Kitagaki, H.; Yamaji, S.; Hirono, N.; Shimomura, T.; Hashimoto, M.; Tanimukai, S.; Kazui, H.; et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease: A comparative study using positron emission tomography. Neurosci. Lett. 1997, 235, 49–52.
- Lim, S.M.; Katsifis, A.; Villemagne, V.L.; Best, R.; Jones, G.; Saling, M.; Bradshaw, J.; Merory, J.; Woodward, M.; Hopwood, M.; et al. The 18F-FDG PET Cingulate Island Sign and Comparison to 123I-β-CIT SPECT for Diagnosis of Dementia with Lewy Bodies. J. Nucl. Med. 2009, 50, 1638–1645.
- Iizuka, T.; Kameyama, M. Cingulate island sign on FDG-PET is associated with medial temporal lobe atrophy in dementia with Lewy bodies. Ann. Nucl. Med. 2016, 30, 421–429.
- Whitwell, J.L.; Graff-Radford, J.; Singh, T.D.; Drubach, D.A.; Senjem, M.L.; Spychalla, A.J.; Tosakulwong, N.; Lowe, V.J.; Josephs, K.A. (18)F-FDG PET in Posterior Cortical Atrophy and Dementia with Lewy Bodies. J. Nucl. Med. 2017, 58, 632–638.
- Korat, Š.; Bidesi, N.S.R.; Bonanno, F.; Di Nanni, A.; Hoàng, A.N.N.; Herfert, K.; Maurer, A.; Battisti, U.M.; Bowden, G.D.; Thonon, D.; et al. Alpha-Synuclein PET Tracer Development—An Overview about Current Efforts. Pharmaceuticals 2021, 14, 847.
- Kuebler, L.; Buss, S.; Leonov, A.; Ryazanov, S.; Schmidt, F.; Maurer, A.; Weckbecker, D.; Landau, A.M.; Lillethorup, T.P.; Bleher, D.; et al. MODAG-001-towards a PET tracer targeting alpha-synuclein aggregates. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1759–1772.
- Kikuchi, A.; Takeda, A.; Okamura, N.; Tashiro, M.; Hasegawa, T.; Furumoto, S.; Kobayashi, M.; Sugeno, N.; Baba, T.; Miki, Y.; et al. In vivo visualization of alpha-synuclein deposition by carbon-11-labelled 2--6-benzoxazole positron emission tomography in multiple system atrophy. Brain A J. Neurol. 2010, 133 Pt 6, 1772–1778.
- Yoshita, M.; Arai, H.; Arai, H.; Arai, T.; Asada, T.; Fujishiro, H.; Hanyu, H.; Iizuka, O.; Iseki, E.; Kashihara, K.; et al. Diagnostic accuracy of 123I-meta-iodobenzylguanidine myocardial scintigraphy in dementia with Lewy bodies: A multicenter study. PLoS ONE 2015, 10, e0120540.
- Sonni, I.; Ratib, O.; Boccardi, M.; Picco, A.; Herholz, K.; Nobili, F.; Varrone, A. Clinical validity of presynaptic dopaminergic imaging with (123)I-ioflupane and noradrenergic imaging with (123)I-MIBG in the differential diagnosis between Alzheimer’s disease and dementia with Lewy bodies in the context of a structured 5-phase development framework. Neurobiol. Aging 2017, 52, 228–242.
- Roberts, G.; Durcan, R.; Donaghy, P.C.; Lawley, S.; Ciafone, J.; Hamilton, C.A.; Colloby, S.J.; Firbank, M.J.; Allan, L.; Barnett, N.; et al. Accuracy of Cardiac Innervation Scintigraphy for Mild Cognitive Impairment with Lewy Bodies. Neurology 2021, 96, e2801–e2811.
- Bousiges, O.; Blanc, F. Diagnostic value of cerebro-spinal fluid biomarkers in dementia with lewy bodies. Clin. Chim. Acta 2019, 490, 222–228.
- Fairfoul, G.; McGuire, L.I.; Pal, S.; Ironside, J.W.; Neumann, J.; Christie, S.; Joachim, C.; Esiri, M.; Evetts, S.G.; Rolinski, M.; et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 2016, 3, 812–818.
- Bongianni, M.; Ladogana, A.; Capaldi, S.; Klotz, S.; Baiardi, S.; Cagnin, A.; Perra, D.; Fiorini, M.; Poleggi, A.; Legname, G.; et al. alpha-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann. Clin. Transl. Neurol. 2019, 6, 2120–2126.
- Bargar, C.; Wang, W.; Gunzler, S.A.; LeFevre, A.; Wang, Z.; Lerner, A.J.; Singh, N.; Tatsuoka, C.; Appleby, B.; Zhu, X.; et al. Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 2021, 9, 62.
- Groveman, B.R.; Orru, C.D.; Hughson, A.G.; Raymond, L.D.; Zanusso, G.; Ghetti, B.; Campbell, K.J.; Safar, J.; Galasko, D.; Caughey, B. Rapid and ultra-sensitive quantitation of disease-associated alpha-synuclein seeds in brain and cerebrospinal fluid by alphaSyn RT-QuIC. Acta Neuropathol. Commun. 2018, 6, 7.
- Rossi, M.; Candelise, N.; Baiardi, S.; Capellari, S.; Giannini, G.; Orru, C.D.; Antelmi, E.; Mammana, A.; Hughson, A.G.; Calandra-Buonaura, G.; et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020, 140, 49–62.
- Wennstrom, M.; Surova, Y.; Hall, S.; Nilsson, C.; Minthon, L.; Bostrom, F.; Hansson, O.; Nielsen, H.M. Low CSF levels of both alpha-synuclein and the alpha-synuclein cleaving enzyme neurosin in patients with synucleinopathy. PLoS ONE 2013, 8, e53250.
- McGrowder, D.A.; Miller, F.; Vaz, K.; Nwokocha, C.; Wilson-Clarke, C.; Anderson-Cross, M.; Brown, J.; Anderson-Jackson, L.; Williams, L.; Latore, L.; et al. Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease: Current Evidence and Future Perspectives. Brain Sci. 2021, 11, 215.
- Morenas-Rodriguez, E.; Alcolea, D.; Suarez-Calvet, M.; Munoz-Llahuna, L.; Vilaplana, E.; Sala, I.; Subirana, A.; Querol-Vilaseca, M.; Carmona-Iragui, M.; Illán-Gala, I.; et al. Different pattern of CSF glial markers between dementia with Lewy bodies and Alzheimer’s disease. Sci. Rep. 2019, 9, 7803.
- Wennstrom, M.; Hall, S.; Nagga, K.; Londos, E.; Minthon, L.; Hansson, O. Cerebrospinal fluid levels of IL-6 are decreased and correlate with cognitive status in DLB patients. Alzheimer’s Res. Ther. 2015, 7, 63.
- Lourenco, M.V.; Ribeiro, F.C.; Santos, L.E.; Beckman, D.; Melo, H.M.; Sudo, F.K.; Drummond, C.; Assunção, N.; Vanderborght, B.; Tovar-Moll, F.; et al. Cerebrospinal Fluid Neurotransmitters, Cytokines, and Chemokines in Alzheimer’s and Lewy Body Diseases. J. Alzheimer’s Dis. JAD 2021, 82, 1067–1074.
- Mukaetova-Ladinska, E.B.; Monteith, R.; Perry, E.K. Cerebrospinal fluid biomarkers for dementia with lewy bodies. Int. J. Alzheimer’s Dis. 2010, 2010, 536538.
- Gmitterova, K.; Varges, D.; Schmitz, M.; Zafar, S.; Maass, F.; Lingor, P.; Zerr, I. Chromogranin A Analysis in the Differential Diagnosis Across Lewy Body Disorders. J. Alzheimer’s Dis. JAD 2020, 73, 1355–1361.
- Duits, F.H.; Brinkmalm, G.; Teunissen, C.E.; Brinkmalm, A.; Scheltens, P.; Van der Flier, W.M.; Zetterberg, H.; Blennow, K. Synaptic proteins in CSF as potential novel biomarkers for prognosis in prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 5.
- Luo, X.; Hou, L.; Shi, H.; Zhong, X.; Zhang, Y.; Zheng, D.; Tan, Y.; Hu, G.; Mu, N.; Chan, J.; et al. CSF levels of the neuronal injury biomarker visinin-like protein-1 in Alzheimer’s disease and dementia with Lewy bodies. J. Neurochem. 2013, 127, 681–690.
- Zou, K.; Abdullah, M.; Michikawa, M. Current Biomarkers for Alzheimer’s Disease: From CSF to Blood. J. Pers. Med. 2020, 10, 85.
- Van Steenoven, I.; Koel-Simmelink, M.J.A.; Vergouw, L.J.M.; Tijms, B.M.; Piersma, S.R.; Pham, T.V.; Bridel, C.; Ferri, G.L.; Cocco, C.; Noli, B.; et al. Identification of novel cerebrospinal fluid biomarker candidates for dementia with Lewy bodies: A proteomic approach. Mol. Neurodegener. 2020, 15, 36.
- Van Steenoven, I.; Noli, B.; Cocco, C.; Ferri, G.L.; Oeckl, P.; Otto, M.; Koel-Simmelink, M.J.A.; Bridel, C.; van der Flier, W.M.; Lemstra, A.W.; et al. VGF Peptides in Cerebrospinal Fluid of Patients with Dementia with Lewy Bodies. Int. J. Mol. Sci. 2019, 20, 4674.
- Nilsson, J.; Gobom, J.; Sjodin, S.; Brinkmalm, G.; Ashton, N.J.; Svensson, J.; Johansson, P.; Portelius, E.; Zetterberg, H.; Blennow, K.; et al. Cerebrospinal fluid biomarker panel for synaptic dysfunction in Alzheimer’s disease. Alzheimers Dement. 2021, 13, e12179.
- Libiger, O.; Shaw, L.M.; Watson, M.H.; Nairn, A.C.; Umana, K.L.; Biarnes, M.C.; Canet-Avilés, R.M.; Jack, C.R., Jr.; Breton, Y.A.; Cortes, L.; et al. Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer’s disease. Alzheimers Dement. 2021, 17, 1976–1987.
- Boiten, W.A.; van Steenoven, I.; Xiao, M.; Worley, P.F.; Lemstra, A.W.; Teunissen, C.E. Pathologically Decreased CSF Levels of Synaptic Marker NPTX2 in DLB Are Correlated with Levels of Alpha-Synuclein and VGF. Cells 2020, 10, 38.
- Brinkmalm, G.; Sjodin, S.; Simonsen, A.H.; Hasselbalch, S.G.; Zetterberg, H.; Brinkmalm, A.; Blennow, K. A Parallel Reaction Monitoring Mass Spectrometric Method for Analysis of Potential CSF Biomarkers for Alzheimer’s Disease. PROTEOMICS Clin. Appl. 2018, 12, 1700131.
- Llano, D.A.; Devanarayan, P.; Devanarayan, V.; Alzheimer’s Disease Neuroimaging Initiative (ADNI). VGF in Cerebrospinal Fluid Combined With Conventional Biomarkers Enhances Prediction of Conversion from MCI to AD. Alzheimer Dis. Assoc. Disord. 2019, 33, 307–314.
- Bartolomucci, A.; Possenti, R.; Mahata, S.K.; Fischer-Colbrie, R.; Loh, Y.P.; Salton, S.R. The extended granin family: Structure, function, and biomedical implications. Endocr. Rev. 2011, 32, 755–797.
- Ferri, G.L.; Noli, B.; Brancia, C.; D’Amato, F.; Cocco, C. VGF: An inducible gene product, precursor of a diverse array of neuro-endocrine peptides and tissue-specific disease biomarkers. J. Chem. Neuroanat. 2011, 42, 249–261.
- Xu, D.; Hopf, C.; Reddy, R.; Cho, R.W.; Guo, L.; Lanahan, A.; Petralia, R.S.; Wenthold, R.J.; O’Brien, R.J.; Worley, P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron 2003, 39, 513–528.
- O’Brien, R.J.; Xu, D.; Petralia, R.S.; Steward, O.; Huganir, R.L.; Worley, P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron 1999, 23, 309–323.
- Schwarzer, C. 30 years of dynorphins—New insights on their functions in neuropsychiatric diseases. Pharmacol. Ther. 2009, 123, 353–370.
- Tejeda, H.A.; Shippenberg, T.S.; Henriksson, R. The dynorphin/kappa-opioid receptor system and its role in psychiatric disorders. Cell. Mol. Life Sci. 2012, 69, 857–896.
More
Information
Subjects:
Neurosciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
646
Revisions:
3 times
(View History)
Update Date:
01 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




