
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mayra G. Álvarez | -- | 1558 | 2022-06-28 17:36:43 | | | |
| 2 | Conner Chen | Meta information modification | 1558 | 2022-06-29 02:55:48 | | |
Video Upload Options
The synthesis and applications of composites based on layered double hydroxides (LDHs) and nanocarbons have recently seen great development. On the one hand, LDHs are versatile 2D compounds that present a plethora of applications, from medicine to energy conversion, environmental remediation, and heterogeneous catalysis. On the other, nanocarbons present unique physical and chemical properties owing to their low-dimensional structure and sp2 hybridization of carbon atoms, which endows them with excellent charge carrier mobility, outstanding mechanical strength, and high thermal conductivity.
1. Introduction
2. LDHs and Carbon Materials
2.1. LDHs
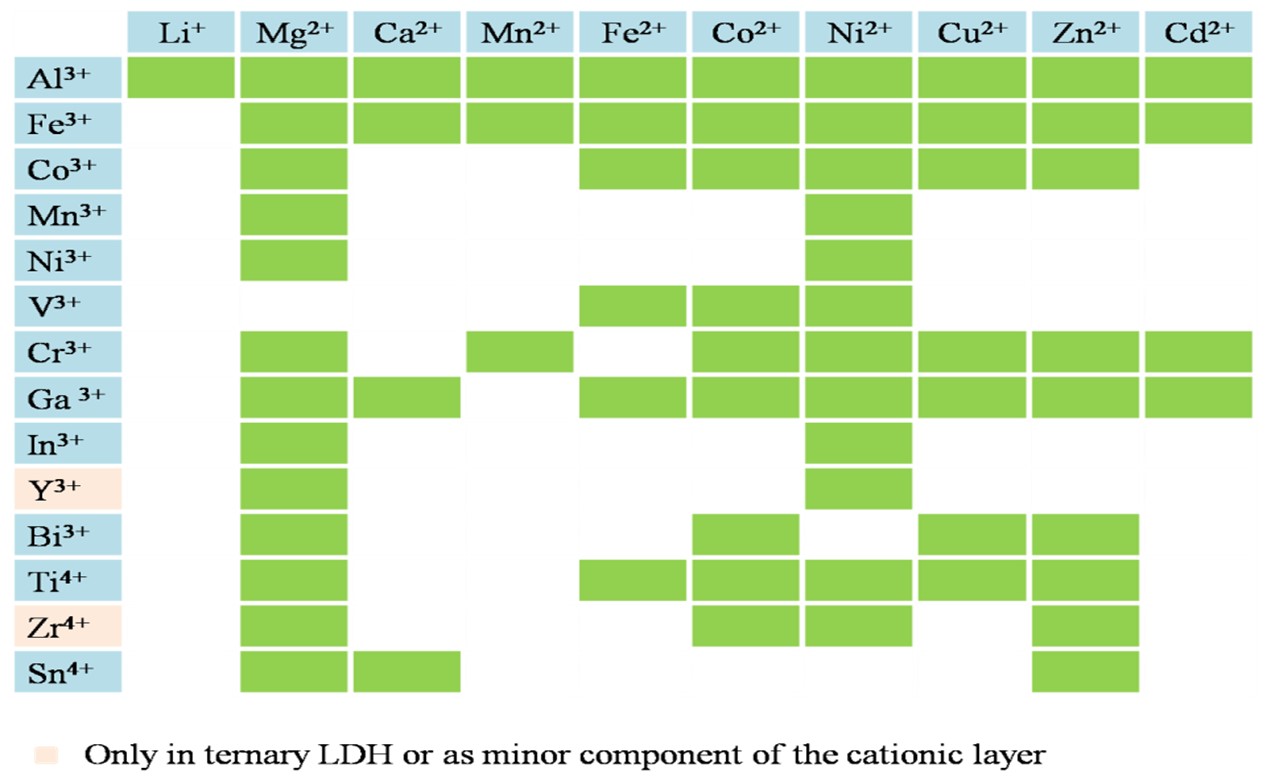
Figure 1. Possible cations combination in LDH.
|
Synthesis of LDH |
Applications of LDH |
|
Common methods Coprecipitation at low or high supersaturation Urea hydrolysis method Sol-gel method Ion-exchange method Rehydration/reconstruction Miscellaneous methods Salt-oxide method Surface synthesis Templated synthesis |
Catalyst support: Ziegler-Natta, metal complexes, etc. Catalysts: Hydrogenation, polymerization, polyalkoxilation, transesterification, condensation reactions, epoxidation, reduction, esterification, oxidation, oxidative dehydrogenation, water splitting, photocatalysis, etc. Medicine: Antiacid, stabilizer, molecular container, etc. Industry: Flame retardant, molecular sieve, ion exchanger, etc Adsorbent: Anion scavenger, wastewater treatment, CO2 adsorption |
2.2. Carbon Materials
| Nanocarbon | Properties | Synthesis Methods |
|---|---|---|
| Graphene-like materials | sp2-sp3 electronic configuration with free π-electrons Semiconductor, fast electron transfer Highly functionalizable Strong hybridization with electronic state of catalyst species, strong interfacial coupling π-π conjugation interaction of reactants Extremely high theoretical surface area |
Confined self-assembly Chemical vapor deposition Arc discharge Epitaxial growth on SiC layer Unzipping of carbon nanotubes Mechanical exfoliation of graphite Sonication of graphite Electrochemical exfoliation/functionalization of graphene Chemical synthesis/exfoliation |
| CNT | sp2 electronic configuration with free π-electrons Highly graphitic Enhanced charge transport Tailorable acid/basicity and easy functionalization High surface area Good thermal stability |
Arc discharge Laser ablation Chemical vapor deposition |
| CNF | Facile and eco-friendly preparation Semiconductive, electronic structure similar to graphite Chemically active edges, easy functionalization Excellent thermal resistance High surface area |
Chemical vapor deposition Floating catalyst method Electrospinning/carbonization |
| CD | sp2 hybridization Water solubility, easy functionalization Low cost and toxicity Quantum confinement properties, semiconductor |
Microwave-assisted Combustion/hydrothermal Supporting synthesis method Arc discharge Laser ablation Electrochemical synthesis Chemical oxidation |
References
- Varadwaj, G.B.B.; Nyamori, V.O. Layered double hydroxide- and graphene-based hierarchical nanocomposites: Synthetic strategies and promising applications in energy conversion and conservation. Nano Res. 2016, 9, 3598–3621.
- Zhao, M.; Zhao, Q.; Li, B.; Xue, H.; Pang, H.; Chen, C. Recent progress in layered double hydroxide based materials for electrochemical capacitors: Design, synthesis and performance. Nanoscale 2017, 9, 15206–15225.
- Zhao, M.Q.; Zhang, Q.; Huang, J.Q.; Wei, F. Hierarchical Nanocomposites Derived from Nanocarbons and Layered Double Hydroxides–Properties, Synthesis, and Applications. Adv. Func. Mater. 2012, 22, 675.
- Kulandaivalu, S.; Azman, N.H.N.; Sulaiman, Y. Advances in Layered Double Hydroxide/Carbon Nanocomposites Containing Ni2+ and Co2+/3+ for Supercapacitors. Front. Mater. 2020, 7, 147.
- Tang, C.; Titirici, M.M.; Zhang, Q. A review of nanocarbons in energy electrocatalysis: Multifunctional substrates and highly active sites. J. Energy Chem. 2017, 26, 1077–1093.
- Tang, C.; Wang, H.F.; Zhu, X.L.; Li, B.Q.; Zhang, Q. Advances in Hybrid Electrocatalysts for Oxygen Evolution Reactions: Rational Integration of NiFe Layered Double Hydroxides and Nanocarbon. Part. Part. Syst. Charact. 2016, 33, 473–486.
- Song, B.; Zeng, Z.; Zeng, G.; Gong, J.; Xiao, R.; Ye, S.; Chen, M.; Lai, C.; Xu, P.; Tang, X. Powerful combination of g-C3N4 and LDHs for enhanced photocatalytic performance: A review of strategy, synthesis, and applications. Adv. Colloid Interface Sci. 2019, 272, 101999.
- Daud, M.; Kamal, M.S.; Shehzad, F.; Al Harthi, M. Graphene/Layered Double Hydroxides Nanocomposites: A Review of Recent Progress in Synthesis and Applications. Carbon 2016, 104, 241–252.
- Gu, P.; Zhang, S.; Li, X.; Wang, X.; Wen, T.; Jehan, R.; Alsaedi, A.; Hayat, T.; Wang, X. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 2018, 240, 493–505.
- Pang, H.; Wu, Y.; Wang, X.; Hu, B.; Wang, X. Recent Advances in Composites of Graphene and Layered Double Hydroxides for Water Remediation: A Review. Chem. Asian J. 2019, 14, 2542–2552.
- Cao, Y.; Li, G.; Li, X. Graphene/layered double hydroxide nanocomposite: Properties, synthesis, and applications. Chem. Eng. J. 2016, 292, 207–223.
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066.
- Winter, F.; Van Dillen, A.J.; de Jong, K.P. Supported hydrotalcites as highly active solid base catalysts. Chem. Commun. 2005, 31, 3977–3979.
- Winter, F.; Koot, V.; van Dillen, A.J.; Geus, J.W.; de Jong, K.P. Hydrotalcites supported on carbon nanofibers as solid base catalysts for the synthesis of MIBK. J. Catal. 2005, 236, 91–100.
- Liang, Y.N.; Oh, W.D.; Li, Y.; Hu, X. Nanocarbons as platforms for developing novel catalytic composites: Overview and prospects. Appl. Catal. A Gen. 2018, 562, 94–105.
- Bhuyan, M.S.A.; Uddin, M.N.M.; Islam, M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83.
- Mallakpour, S.; Khadem, E. Carbon nanotube–metal oxide nanocomposites: Fabrication, properties and applications. Chem. Eng. J. 2016, 302, 344–367.
- Sousa, H.B.A.; Martins, C.S.M.; Prior, J.A.V. You Don’t Learn That in School: An Updated Practical Guide to Carbon Quantum Dots. Nanomaterials 2021, 11, 611.
- Feng, L.; Xie, N.; Zhong, J. Carbon Nanofibers and Their Composites: A Review of Synthesizing, Properties and Applications. Materials 2014, 7, 3919–3945.
- Álvarez, M.G.; Tichit, D.; Medina, F.; Llorca, J. Role of the synthesis route on the properties of hybrid LDH-graphene as basic catalysts. Appl. Surf. Sci. 2017, 396, 821–831.
- Ahmed, N.S.; Menzel, R.; Wang, Y.; Garcia-Gallastegui, A.; Bawaked, S.M.; Obaid, A.Y.; Basahel, S.N.; Mokhtar, M. Graphene-oxide-supported CuAl and CoAl layered double hydroxides as enhanced catalysts for carbon-carbon coupling via Ullmann reaction. J. Solid State Chem. 2017, 246, 130–137.
- Yang, Y.; Zhu, W.; Cui, D.; Lü, C. Mussel-inspired preparation of temperature-responsive polymer brushes modified layered double /carbon dots hybrid for catalytic applications. Appl. Clay Sci. 2021, 200, 105958.
- Tichit, D.; Coq, B. Catalysis by Hydrotalcites and Related Materials. Cattech 2003, 7, 206–217.
- Takehira, K. Recent development of layered double hydroxide-derived catalysts − Rehydration, reconstitution, and supporting, aiming at commercial application. Appl. Clay Sci. 2017, 136, 112–141.
- Debecker, D.P.; Gaigneaux, E.M.; Busca, G. Exploring, Tuning, and Exploiting the Basicity of Hydrotalcites for Applications in Heterogeneous Catalysis. Chem. Eur. J. 2009, 15, 3920–3935.
- Li, C.; Wei, M.; Evans, D.G.; Duan, X. Layered Double Hydroxide-based Nanomaterials as Highly Efficient Catalysts and Adsorbents. Small 2014, 10, 4469.
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339.
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–391.
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic carbon nitrides (g-C3N4) with comparative discussion to carbon materials. Carbon 2019, 141, 580–607.
- Álvarez, M.G.; Marcu, I.C.; Tichit, D. Progress in Layered Double Hydroxides—From Synthesis to New Applications; Nocchetti, M., Costantino, U., Eds.; World Scientific Publishing Ltd.: Singapore, 2022; pp. 189–362.
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S.Z. Graphitic Carbon Nitride Materials: Controllable Synthesis and Applications in Fuel Cells and Photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731.




